








生物技术通报 ›› 2024, Vol. 40 ›› Issue (9): 104-112.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0243
• 薯类作物生物技术专题(专题主编:徐建飞,尚轶) • 上一篇 下一篇
宋兵芳( ), 柳宁, 程新艳, 徐晓斌, 田文茂, 高悦, 毕阳, 王毅(
), 柳宁, 程新艳, 徐晓斌, 田文茂, 高悦, 毕阳, 王毅( )
)
收稿日期:2024-03-12
出版日期:2024-09-26
发布日期:2024-07-31
通讯作者:
王毅,硕士,正高级实验师,研究方向:果蔬采后生物学与技术;E-mail: wangyi@gsau.edu.cn作者简介:宋兵芳,女,硕士研究生,研究方向:果蔬采后生物学与技术;E-mail: 3132897396@qq.com
基金资助:
SONG Bing-fang( ), LIU Ning, CHENG Xin-yan, XU Xiao-bin, TIAN Wen-mao, GAO Yue, BI Yang, WANG Yi(
), LIU Ning, CHENG Xin-yan, XU Xiao-bin, TIAN Wen-mao, GAO Yue, BI Yang, WANG Yi( )
)
Received:2024-03-12
Published:2024-09-26
Online:2024-07-31
摘要:
【目的】葡萄糖-6-磷酸脱氢酶(G6PDH)在植物响应非生物胁迫中发挥重要作用,鉴定马铃薯中G6PDH基因家族,并分析其在损伤块茎的表达模式,为深入研究马铃薯G6PDH基因在损伤胁迫中的作用提供参考。【方法】利用生物信息学对马铃薯G6PDH基因家族进行鉴定,并对该基因家族成员编码蛋白的染色体分布、蛋白理化性质和二级结构、进化关系、基因结构、保守基序和启动子顺式作用元件,以及在不同器官和损伤块茎中的表达模式进行分析。【结果】在马铃薯基因组中共鉴定到4个StG6PDHs家族成员,分别分布在4条染色体上,命名为StG6PDH1-StG6PDH4。根据亚细胞定位和系统进化分析,StG6PDH1、StG6PDH3和StG6PDH4位于叶绿体,属于质体型;StG6PDH2位于细胞质,属于胞质型。马铃薯G6PDH蛋白的氨基酸个数介于511-596 aa,分子量为58.48-66.65 kD,等电点为5.83-8.57,不稳定系数为39.79-47.53。蛋白二级结构以α-螺旋和无规则卷曲占比最多,β-转角最少。此外,StG6PDHs启动子含大量植物激素、光和胁迫响应元件。4个StG6PDHs在马铃薯根、茎、叶和块茎均有表达,且在叶片中的表达高于其他组织。StG6PDHs各成员共同参与马铃薯块茎对损伤胁迫的响应,其中,StG6PDH1、StG6PDH2和StG6PDH3在块茎损伤后36 h内上调表达,StG6PDH4在损伤后下调表达。【结论】在马铃薯中共鉴定出4个StG6PDHs基因家族成员,不均匀地分布于4条染色体上,其中,1个为胞质型,3个为质体型。StG6PDHs启动子区有光、激素和胁迫响应元件。损伤块茎中StG6PDHs的表达具有差异性,各成员协同调控了马铃薯块茎对损伤胁迫的应答。
宋兵芳, 柳宁, 程新艳, 徐晓斌, 田文茂, 高悦, 毕阳, 王毅. 马铃薯G6PDH基因家族鉴定及其在损伤块茎的表达分析[J]. 生物技术通报, 2024, 40(9): 104-112.
SONG Bing-fang, LIU Ning, CHENG Xin-yan, XU Xiao-bin, TIAN Wen-mao, GAO Yue, BI Yang, WANG Yi. Identification of Potato G6PDH Gene Family and Its Expression Analysis in Damaged Tubers[J]. Biotechnology Bulletin, 2024, 40(9): 104-112.
| 基因名称 Gene name | 基因ID Gene ID | 引物序列 Primer sequence(5'-3') |
|---|---|---|
| StG6PDH1 | Soltu.DM.01G040700 | F: GCTTTCACCAGTATCGCTATCA |
| R: CGGACAACAACGGTAGGTAAT | ||
| StG6PDH2 | Soltu.DM.02G033900 | F: CAAGAAACCTGGGCTTGAAATG |
| R: CGTTCATAAGCCTCTGGAATGA | ||
| StG6PDH3 | Soltu.DM.05G012290 | F: ATACCAGGAGTGCGGAAATAAG |
| R: CGTCAGGTTGAACACGGATAA | ||
| StG6PDH4 | Soltu.DM.07G015360 | F: GCTGACCAGATCCCTAAAGAAG |
| R: GACAAGATTCGAGAACCGAAGA | ||
| StEF1α | Soltu.DM.06G005580 | F: ATTGATGCCCCTGGTCACAG |
| R: CATGTTCACGGGTCTGACCA |
表1 RT-qPCR引物序列
Table 1 Primers’ sequences for RT-qPCR
| 基因名称 Gene name | 基因ID Gene ID | 引物序列 Primer sequence(5'-3') |
|---|---|---|
| StG6PDH1 | Soltu.DM.01G040700 | F: GCTTTCACCAGTATCGCTATCA |
| R: CGGACAACAACGGTAGGTAAT | ||
| StG6PDH2 | Soltu.DM.02G033900 | F: CAAGAAACCTGGGCTTGAAATG |
| R: CGTTCATAAGCCTCTGGAATGA | ||
| StG6PDH3 | Soltu.DM.05G012290 | F: ATACCAGGAGTGCGGAAATAAG |
| R: CGTCAGGTTGAACACGGATAA | ||
| StG6PDH4 | Soltu.DM.07G015360 | F: GCTGACCAGATCCCTAAAGAAG |
| R: GACAAGATTCGAGAACCGAAGA | ||
| StEF1α | Soltu.DM.06G005580 | F: ATTGATGCCCCTGGTCACAG |
| R: CATGTTCACGGGTCTGACCA |
| 基因名称 Gene name | 基因ID Gene ID | 染色体位置 Chromosome location | 亚细胞定位 Subcellular location |
|---|---|---|---|
| StG6PDH1 | Soltu.DM.01G040700 | chr.01 | 叶绿体Chloroplast |
| StG6PDH2 | Soltu.DM.02G033900 | chr.02 | 细胞质Cytoplasmic |
| StG6PDH3 | Soltu.DM.05G012290 | chr.05 | 叶绿体Chloroplast |
| StG6PDH4 | Soltu.DM.07G015360 | chr.07 | 叶绿体Chloroplast |
表2 马铃薯G6PDH蛋白亚细胞定位预测
Table 2 Prediction of subcellular localization of G6PDH protein in potato
| 基因名称 Gene name | 基因ID Gene ID | 染色体位置 Chromosome location | 亚细胞定位 Subcellular location |
|---|---|---|---|
| StG6PDH1 | Soltu.DM.01G040700 | chr.01 | 叶绿体Chloroplast |
| StG6PDH2 | Soltu.DM.02G033900 | chr.02 | 细胞质Cytoplasmic |
| StG6PDH3 | Soltu.DM.05G012290 | chr.05 | 叶绿体Chloroplast |
| StG6PDH4 | Soltu.DM.07G015360 | chr.07 | 叶绿体Chloroplast |
| 基因名称 Gene name | 理化性质Physicochemical property | 二级结构Secondary structure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 氨基酸长度 Amino acid length/aa | 相对分子量 Mw/kD | 等电点 pI | 不稳定系数Instability index | α-螺旋 α-helix/% | β-转角 β-turn/% | 无规则卷曲 Random coil/% | 延伸链 Extended strand/% | |||
| StG6PDH1 | 596 | 66.65 | 5.83 | 47.31 | 36.91 | 5.87 | 40.27 | 16.95 | ||
| StG6PDH2 | 511 | 58.48 | 5.97 | 47.53 | 40.51 | 5.28 | 39.33 | 16.87 | ||
| StG6PDH3 | 582 | 66.21 | 8.57 | 39.79 | 38.14 | 6.53 | 39.87 | 17.53 | ||
| StG6PDH4 | 577 | 65.72 | 6.88 | 39.84 | 37.78 | 6.59 | 39.17 | 16.46 | ||
表3 马铃薯G6PDH基因家族蛋白理化性质和二级结构分析
Table 3 Protein physicochemical properties and secondary structures of potato G6PDH gene family
| 基因名称 Gene name | 理化性质Physicochemical property | 二级结构Secondary structure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 氨基酸长度 Amino acid length/aa | 相对分子量 Mw/kD | 等电点 pI | 不稳定系数Instability index | α-螺旋 α-helix/% | β-转角 β-turn/% | 无规则卷曲 Random coil/% | 延伸链 Extended strand/% | |||
| StG6PDH1 | 596 | 66.65 | 5.83 | 47.31 | 36.91 | 5.87 | 40.27 | 16.95 | ||
| StG6PDH2 | 511 | 58.48 | 5.97 | 47.53 | 40.51 | 5.28 | 39.33 | 16.87 | ||
| StG6PDH3 | 582 | 66.21 | 8.57 | 39.79 | 38.14 | 6.53 | 39.87 | 17.53 | ||
| StG6PDH4 | 577 | 65.72 | 6.88 | 39.84 | 37.78 | 6.59 | 39.17 | 16.46 | ||
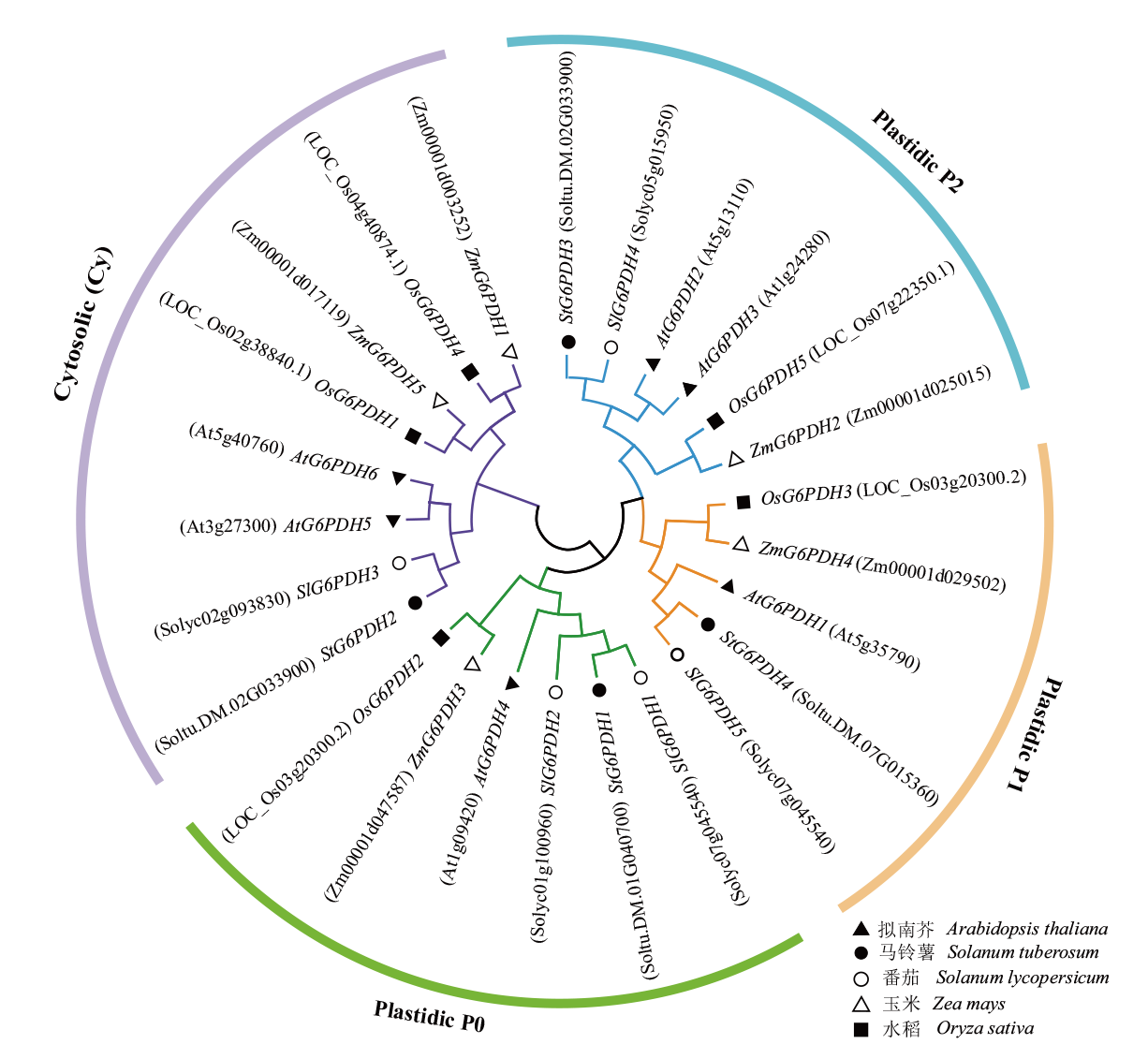
图2 马铃薯、拟南芥、番茄、玉米和水稻G6PDH蛋白的系统进化树
Fig. 2 Phylogenetic tree of G6PDH proteins in Solanum tuberosum, Arabidopsis thaliana, Solanum lycopersicum, Zea mays and Oryza sativa
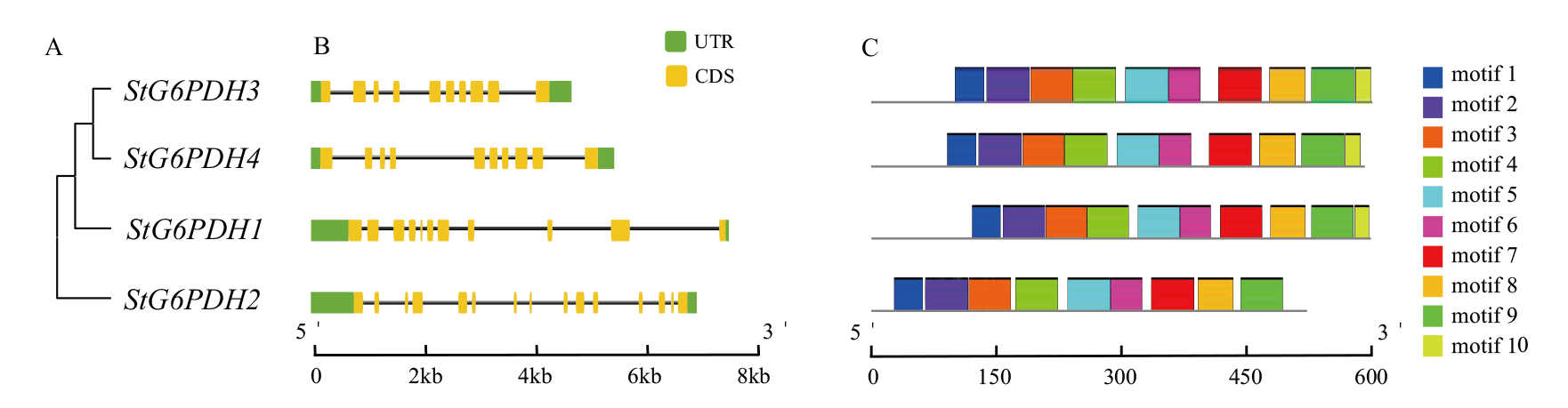
图3 马铃薯G6PDH基因家族成员的进化关系(A)、基因结构(B)和保守基序(C)分析
Fig. 3 Evolutionary relationships(A), gene structure(B)and conserved motifs(C)of the potato G6PDH gene family
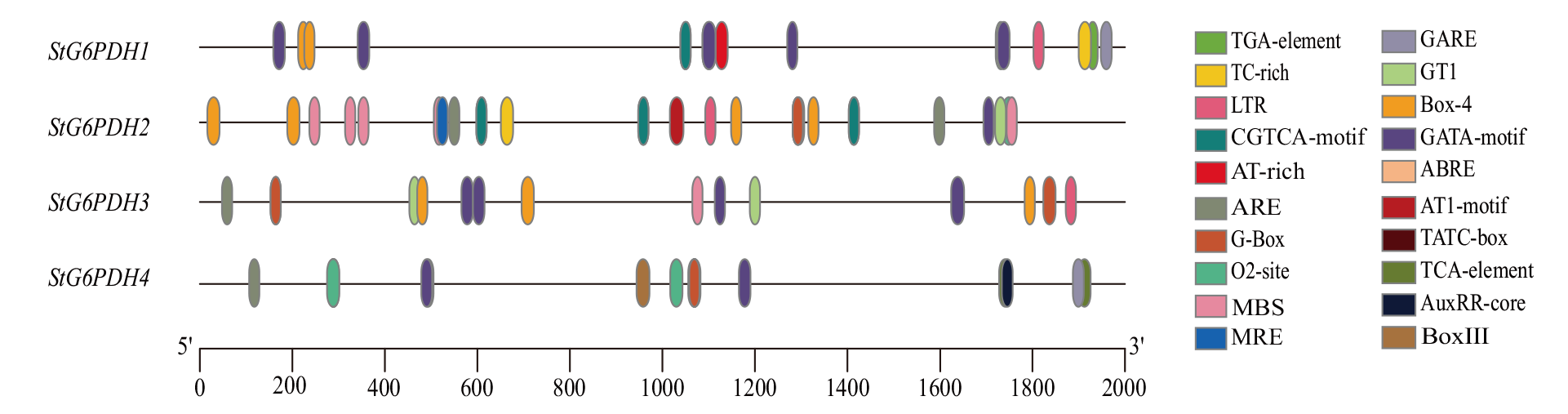
图4 马铃薯G6PDH基因家族顺式作用元件分析 光响应元件:GATA-motif、GT1、Box-4、MRE、G-Box、AT1-motif;低温响应元件:LTR;厌氧诱导:ARE;赤霉素响应元件:GARE、TATC-box;生长素响应元件:AuxRE-core、TGA-element;脱落酸响应元件:ABRE;茉莉酸响应元件:CGTCA-motif;水杨酸响应元件:TCA-element;干旱响应元件:MBS;玉米醇溶蛋白代谢调节元件:O2-site;胁迫和防御响应元件:TC-rich;其他:BoxIII、AT-rich
Fig. 4 Cis-acting elements of potato G6PDH gene family Light responsiveness: GATA-motif, GT1, Box-4, MRE, G-Box, AT1-motif. Low-temperature responsiveness: LTR. Anaerobic induction: ARE. Gibberellin responsiveness: GARE, TATC-box. Auxin responsiveness: AuxRE-core, TGA-element. Abscisic acid responsiveness: ABRE. Me-JA responsiveness: CGTCA-motif. Salicylic acid responsiveness: TCA-element. Drought-induced response element: MBS. Zein metabolism regulation: O2-site. Defense and stress responsiveness: TC-rich. Other: Box III, AT-rich
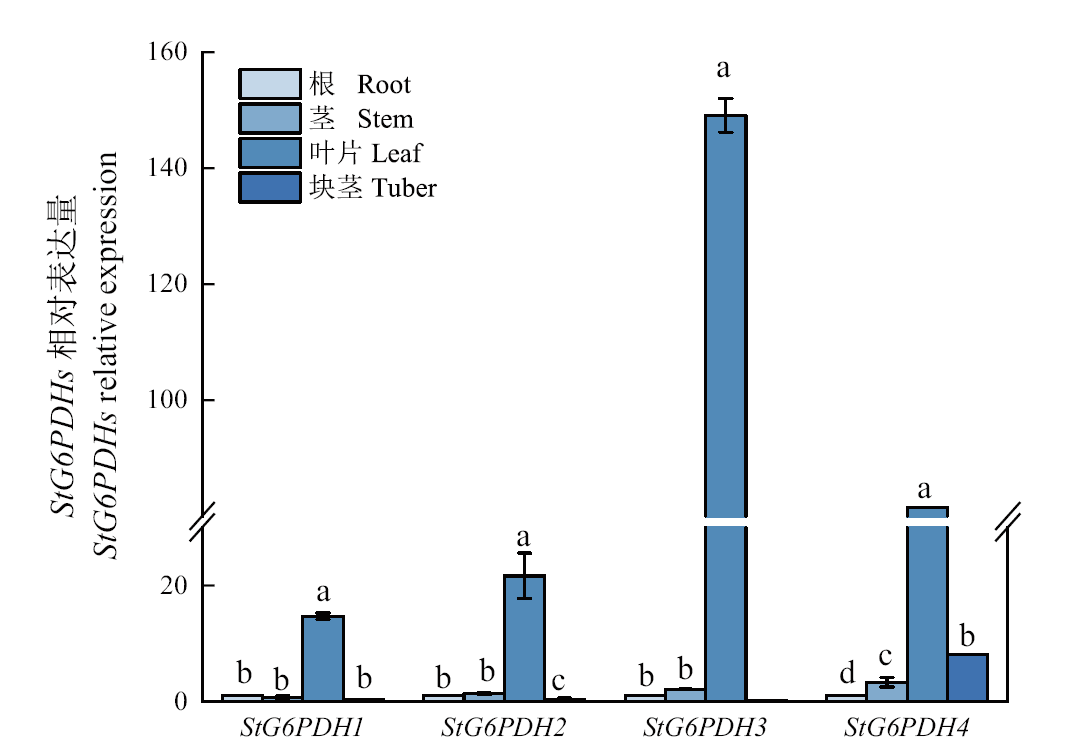
图5 马铃薯不同器官中G6PDH的相对表达量 同一基因中的不同小写字母表示差异显著(P<0.05)
Fig. 5 Relative expressions of G6PDH in different organs of potato Different lowercase letters in the same gene indicate significant difference(P<0.05)
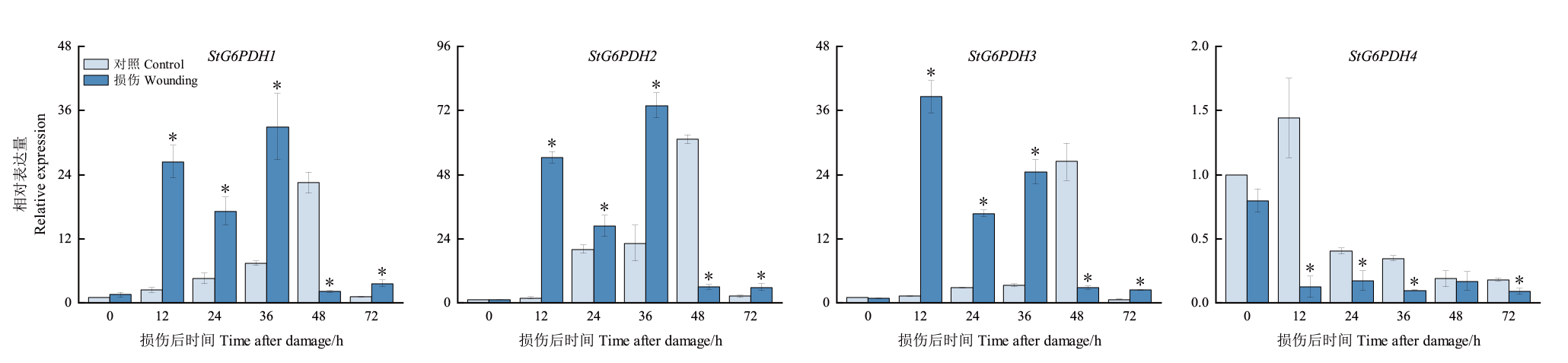
图6 损伤马铃薯块茎中StG6PDHs的相对表达量 *代表同一时间的对照组和处理组之间差异显著(P<0.05)
Fig. 6 Relative expression of StG6PDH1s in damaged potato tubers * indicates a significant difference between the control group and the treatment group(P<0.05)
| [1] |
Beumer K, Stemerding D. A breeding consortium to realize the potential of hybrid diploid potato for food security[J]. Nat Plants, 2021, 7(12): 1530-1532.
doi: 10.1038/s41477-021-01035-4 pmid: 34815537 |
| [2] | Hu WZ, Guan YG, Ji YR, et al. Effect of cutting styles on quality, antioxidant activity, membrane lipid peroxidation, and browning in fresh-cut potatoes[J]. Food Biosci, 2021, 44: 101435. |
| [3] | París R, Lamattina L, Casalongué CA. Nitric oxide promotes the wound-healing response of potato leaflets[J]. Plant Physiol Biochem, 2007, 45(1): 80-86. |
| [4] |
姜红, 王毅, 毕阳. 马铃薯块茎的愈伤过程、机制和影响因素[J]. 园艺学报, 2019, 46(9): 1842-1852.
doi: 10.16420/j.issn.0513-353x.2019-0358 |
| Jiang H, Wang Y, Bi Y. Healing processes, mechanisms and factors affecting potato tubers[J]. Journal of Horticulture, 2019, 46(9): 1842-1852. | |
| [5] | Bussell JD, Keech O, Fenske R, et al. Requirement for the plastidial oxidative pentose phosphate pathway for nitrate assimilation in Arabidopsis[J]. Plant J, 2013, 75(4): 578-591. |
| [6] |
Corpas FJ, González-Gordo S, Palma JM. Nitric oxide and hydrogen sulfide modulate the NADPH-generating enzymatic system in higher plants[J]. J Exp Bot, 2021, 72(3): 830-847.
doi: 10.1093/jxb/eraa440 pmid: 32945878 |
| [7] | Chen PH, Tjong WY, Yang HC, et al. Glucose-6-phosphate dehydrogenase, redox homeostasis and embryogenesis[J]. Int J Mol Sci, 2022, 23(4): 2017. |
| [8] | Landi S, Nurcato R, De Lillo A, et al. Glucose-6-phosphate dehydrogenase plays a central role in the response of tomato(Solanum lycopersicum)plants to short and long-term drought[J]. Plant Physiol Biochem, 2016, 105: 79-89. |
| [9] | Li X, Cai Q, Yu T, et al. ZmG6PDH1 in glucose-6-phosphate dehydrogenase family enhances cold stress tolerance in maize[J]. Front Plant Sci, 2023, 14: 1116237. |
| [10] | Zhao Y, Cui YF, Huang SY, et al. Genome-wide analysis of the glucose-6-phosphate dehydrogenase family in soybean and functional identification of GmG6PDH2 involvement in salt stress[J]. Front Plant Sci, 2020, 11: 214. |
| [11] | Li XA, Li BR, Min DD, et al. Transcriptomic analysis reveals key genes associated with the biosynthesis regulation of phenolics in fresh-cut pitaya fruit(Hylocereus undatus)[J]. Postharvest Biol Technol, 2021, 181: 111684. |
| [12] | Chintha P, Sarkar D, Ramakrishna R, et al. Biological elicitors to enhance wound healing responses in cut potato tubers[J]. Sci Hortic, 2023, 319: 112152. |
| [13] | Wakao S, Benning C. Genome-wide analysis of glucose-6-phosphate dehydrogenases in Arabidopsis[J]. Plant J, 2005, 41(2): 243-256. |
| [14] |
Chen CJ, Chen H, Zhang Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data[J]. Mol Plant, 2020, 13(8): 1194-1202.
doi: S1674-2052(20)30187-8 pmid: 32585190 |
| [15] | 韩占红, 王斌, 杨瑞瑞, 等. 一氧化氮处理对马铃薯采后块茎愈伤的促进及机制[J]. 食品科学, 2020, 41(21): 222-229. |
| Han ZH, Wang B, Yang RR, et al. Effect and mechanism of postharvest nitric oxide treatment on promoting wound healing in potato tubers[J]. Food Sci, 2020, 41(21): 222-229. | |
| [16] | Esposito S. Nitrogen assimilation, abiotic stress and glucose 6-phosphate dehydrogenase: the full circle of reductants[J]. Plants, 2016, 5(2): 24. |
| [17] | Yang YT, Fu ZW, Su YC, et al. A cytosolic glucose-6-phosphate dehydrogenase gene, ScG6PDH, plays a positive role in response to various abiotic stresses in sugarcane[J]. Sci Rep, 2014, 4: 7090. |
| [18] | Hou FY, Huang J, Yu SL, et al. The 6-phosphogluconate dehydrogenase genes are responsive to abiotic stresses in rice[J]. J Integr Plant Biol, 2007, 49(5): 655-663. |
| [19] | 田宇, 彭瞰看, 宋春华, 等. 小麦G6PDH基因的生物信息学分析及其低温胁迫下苗期的表达特征[J]. 麦类作物学报, 2019, 39(6): 631-638. |
| Tian Y, Peng KK, Song CH, et al. Bioinformatics analysis of wheat G6PDH genes and their patterns in tillering node and leaf under cold stress[J]. J Triticeae Crops, 2019, 39(6): 631-638. | |
| [20] | Zhang YT, Luo MW, Cheng LJ, et al. Identification of the cytosolic glucose-6-phosphate dehydrogenase gene from strawberry involved in cold stress response[J]. Int J Mol Sci, 2020, 21(19): 7322. |
| [21] | Jiang ZR, Wang M, Nicolas M, et al. Glucose-6-phosphate dehydrogenases: the hidden players of plant physiology[J]. Int J Mol Sci, 2022, 23(24): 16128. |
| [22] | Landi S, Capasso G, Esposito S. Different G6PDH isoforms show specific roles in acclimation to cold stress at various growth stages of barley(Hordeum vulgare)and Arabidopsis thaliana[J]. Plant Physiol Biochem, 2021, 169: 190-202. |
| [23] | Whisstock JC, Lesk M. 预测的蛋白质的功能的蛋白质的顺序和结构[J]. 季度审查的生物物理学, 2003, 36(3): 307-340. |
| Whisstock JC, Lesk M. Order and structure of proteins for predicted protein function[J]. Quarterly Review of Biophysics, 2003, 36(3): 307-340. | |
| [24] | 车卓, 张沛沛, 陈涛, 等. 小麦G6PDH基因家族的鉴定与表达分析[J]. 麦类作物学报, 2023, 43(8): 947-957. |
| Che Z, Zhang PP, Chen T, et al. Identification and expression analysis of the G6PDH gene family in wheat[J]. J Triticeae Crops, 2023, 43(8): 947-957. | |
| [25] |
Preiser AL, Fisher N, Banerjee A, et al. Plastidic glucose-6-phosphate dehydrogenases are regulated to maintain activity in the light[J]. Biochem J, 2019, 476(10): 1539-1551.
doi: 10.1042/BCJ20190234 pmid: 31092702 |
| [26] | Lei DY, Lin YX, Luo MW, et al. Genome-wide investigation of G6PDH gene in strawberry: evolution and expression analysis during development and stress[J]. Int J Mol Sci, 2022, 23(9): 4728-4728. |
| [27] | Cardi M, Chibani K, Cafasso D, et al. Abscisic acid effects on activity and expression of barley(Hordeum vulgare)plastidial glucose-6-phosphate dehydrogenase[J]. J Exp Bot, 2011, 62(11): 4013-4023. |
| [28] | Tian Y, Peng KK, Bao YZ, et al. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase genes of winter wheat enhance the cold tolerance of transgenic Arabidopsis[J]. Plant Physiol Biochem, 2021, 161: 86-97. |
| [29] | Wang HH, Yang LD, Li Y, et al. Involvement of ABA- and H2O2-dependent cytosolic glucose-6-phosphate dehydrogenase in maintaining redox homeostasis in soybean roots under drought stress[J]. Plant Physiol Biochem, 2016, 107: 126-136. |
| [30] | Feng RJ, Wang XM, He L, et al. Identification, characterization, and stress responsiveness of glucose-6-phosphate dehydrogenase genes in highland barley[J]. Plants, 2020, 9(12): 1800. |
| [31] |
Knight JS, Emes MJ, Debnam PM. Isolation and characterisation of a full-length genomic clone encoding a plastidic glucose 6-phosphate dehydrogenase from Nicotiana tabacum[J]. Planta, 2001, 212(4): 499-507.
doi: 10.1007/s004250000419 pmid: 11525506 |
| [32] |
Weise SE, Liu T, Childs KL, et al. Transcriptional regulation of the glucose-6-phosphate/ phosphate translocator 2 is related to carbon exchange across the chloroplast envelope[J]. Front Plant Sci, 2019, 10: 827.
doi: 10.3389/fpls.2019.00827 pmid: 31316533 |
| [33] | Gao S, Zheng ZB, Huan L, et al. G6PDH activity highlights the operation of the cyclic electron flow around PSI in Physcomitrella patens during salt stress[J]. Sci Rep, 2016, 6: 21245. |
| [34] | Wei XB, Huang XL, Yang WL, et al. A chloroplast-localized glucose-6-phosphate dehydrogenase positively regulates stripe rust resistance in wheat[J]. Int J Mol Sci, 2022, 24(1): 459. |
| [35] |
Lulai EC, Suttle JC. Signals involved in tuber wound-healing[J]. Plant Signal Behav, 2009, 4(7): 620-622.
doi: 8922 pmid: 19820323 |
| [36] | Wang L, Wang WX, Shan JW, et al. A genome-wide view of the transcriptome dynamics of fresh-cut potato tubers[J]. Genes, 2023, 14(1): 181. |
| [1] | 申鹏, 高雅彬, 丁红. 马铃薯SAT基因家族的鉴定和表达分析[J]. 生物技术通报, 2024, 40(9): 64-73. |
| [2] | 王超, 白如仟, 管俊梅, 罗稷林, 何雪姣, 迟绍轶, 马玲. 马铃薯块茎变绿中StHY5对龙葵素合成的促进作用[J]. 生物技术通报, 2024, 40(9): 113-122. |
| [3] | 夏士轩, 耿泽栋, 祝光涛, 张春芝, 李大伟. 基于深度学习的马铃薯花粉活力快速检测[J]. 生物技术通报, 2024, 40(9): 123-130. |
| [4] | 毛向红, 卢瑶, 范向斌, 杜培兵, 白小东. 基于SSR荧光标记毛细管电泳的马铃薯品种遗传多样性分析及分子身份证构建[J]. 生物技术通报, 2024, 40(9): 131-140. |
| [5] | 袁兰, 黄娅楠, 张贝妮, 熊雨萌, 王洪洋. 基于流式细胞仪鉴定马铃薯倍性的高通量样品制备方法[J]. 生物技术通报, 2024, 40(9): 141-147. |
| [6] | 吴慧琴, 王延宏, 刘涵, 司政, 刘雪晴, 王静, 阳宜, 成妍. 辣椒UGT基因家族的鉴定及表达分析[J]. 生物技术通报, 2024, 40(9): 198-211. |
| [7] | 宋倩娜, 段永红, 冯瑞云. CRISPR/Cas9介导的高效四倍体马铃薯试管薯基因编辑体系的建立[J]. 生物技术通报, 2024, 40(9): 33-41. |
| [8] | 王柯然, 闫俊杰, 刘建凤, 高玉林. RNAi技术在马铃薯害虫防控中的应用和风险[J]. 生物技术通报, 2024, 40(9): 4-10. |
| [9] | 张小妹, 周南伶, 张赛行, 王超, 沈玉龙, 管俊梅, 马玲. 马铃薯StDREBs基因的克隆及其表达分析[J]. 生物技术通报, 2024, 40(9): 42-50. |
| [10] | 满全财, 孟姿诺, 李伟, 蔡心汝, 苏润东, 付长青, 高顺娟, 崔江慧. 马铃薯AQP基因家族鉴定及表达分析[J]. 生物技术通报, 2024, 40(9): 51-63. |
| [11] | 吴娟, 武小娟, 王沛捷, 谢锐, 聂虎帅, 李楠, 马艳红. 彩色马铃薯花青素合成相关ERF基因筛选及表达分析[J]. 生物技术通报, 2024, 40(9): 82-91. |
| [12] | 乔岩, 杨芳, 任盼荣, 祁伟亮, 安沛沛, 李茜, 李丹, 肖俊飞. 马铃薯野生种烯酰水合酶超家族基因ScDHNS的克隆与功能分析[J]. 生物技术通报, 2024, 40(9): 92-103. |
| [13] | 李雨晴, 吴楠, 罗建让. 卵叶牡丹花色苷合成相关基因bHLH的克隆与功能分析[J]. 生物技术通报, 2024, 40(8): 174-185. |
| [14] | 李亦君, 杨小贝, 夏琳, 罗朝鹏, 徐馨, 杨军, 宁黔冀, 武明珠. 烟草NtPRR37基因克隆及功能分析[J]. 生物技术通报, 2024, 40(8): 221-231. |
| [15] | 崔原瑗, 王昭懿, 白双宇, 任毓昭, 豆飞飞, 刘彩霞, 刘凤楼, 王掌军, 李清峰. 大麦非特异性磷脂酶C基因家族全基因组鉴定及苗期胁迫表达分析[J]. 生物技术通报, 2024, 40(8): 74-82. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||