








生物技术通报 ›› 2024, Vol. 40 ›› Issue (9): 113-122.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0514
• 薯类作物生物技术专题(专题主编:徐建飞,尚轶) • 上一篇 下一篇
王超1( ), 白如仟1, 管俊梅1, 罗稷林1, 何雪姣1, 迟绍轶2, 马玲1(
), 白如仟1, 管俊梅1, 罗稷林1, 何雪姣1, 迟绍轶2, 马玲1( )
)
收稿日期:2024-05-29
出版日期:2024-09-26
发布日期:2024-09-06
通讯作者:
马玲,女,博士,副研究员,研究方向:分子植物育种;E-mail: may_ynnu@ynnu.edu.cn作者简介:王超,男,硕士研究生,研究方向:马铃薯营养品质研究;E-mail: 1820392789@qq.com
基金资助:
WANG Chao1( ), BAI Ru-qian1, GUAN Jun-mei1, LUO Ji-lin1, HE Xue-jiao1, CHI Shao-yi2, MA Ling1(
), BAI Ru-qian1, GUAN Jun-mei1, LUO Ji-lin1, HE Xue-jiao1, CHI Shao-yi2, MA Ling1( )
)
Received:2024-05-29
Published:2024-09-26
Online:2024-09-06
摘要:
【目的】马铃薯因采后贮藏、运输及货架摆放等因素,容易造成块茎“绿变”,导致块茎中SGAs大量累积,而HY5是植物中光信号的重要传递因子,研究其在块茎变绿中的作用,为马铃薯采后环节因光照导致的龙葵素积累提供分子基础。【方法】通过基因表达分析、转录组分析、靶向代谢物分析、亚细胞定位、酵母单杂交、双荧光素酶等试验,阐明StHY5在光照后块茎变绿中的作用。【结果】StHY5在薯皮和薯肉中均有表达,但表达模式不同;绿变处理后,薯肉中StHY5的表达水平和SGAs含量的变化一致,与此同时,龙葵素合成基因StSGT1/GAME1和StGAME4表达显著上调;酵母单杂交试验和双荧光素酶试验证明,StHY5可以直接结合StSGT1/GAME1和StGAME4的启动子并激活其表达。【结论】在马铃薯绿变过程中,StHY5通过直接上调StSGT1/GAME1和StGAME4的表达,从而促进SGAs的积累。
王超, 白如仟, 管俊梅, 罗稷林, 何雪姣, 迟绍轶, 马玲. 马铃薯块茎变绿中StHY5对龙葵素合成的促进作用[J]. 生物技术通报, 2024, 40(9): 113-122.
WANG Chao, BAI Ru-qian, GUAN Jun-mei, LUO Ji-lin, HE Xue-jiao, CHI Shao-yi, MA Ling. Promotion of StHY5 in the Synthesis of SGAs during Tuber Turning-green of Potato[J]. Biotechnology Bulletin, 2024, 40(9): 113-122.
| 基因 Gene | 正向引物序列Forward primer sequence(5'-3') | 反向引物序列Reverse primer sequence(5'-3') |
|---|---|---|
| StActin | GGGATGGAGAAGTTTGGTGGTGG | CTTCGACCAAGGGATGGTGTAG |
| StHY5 | AGATCTGGAAGCAAGGGTGAAG | CACCTGCTGTTGTGTTCTTCAG |
表1 RT-qPCR所用引物
Table 1 Primers used in RT-qPCR
| 基因 Gene | 正向引物序列Forward primer sequence(5'-3') | 反向引物序列Reverse primer sequence(5'-3') |
|---|---|---|
| StActin | GGGATGGAGAAGTTTGGTGGTGG | CTTCGACCAAGGGATGGTGTAG |
| StHY5 | AGATCTGGAAGCAAGGGTGAAG | CACCTGCTGTTGTGTTCTTCAG |
| 时间Time/min | A/% | B/% | 流速Flow/(mL·min-1) |
|---|---|---|---|
| 0.00 | 95.00 | 5.00 | 0.800 |
| 5.00 | 60.00 | 40.00 | 0.800 |
| 6.00 | 0.00 | 100.00 | 0.800 |
| 8.00 | 0.00 | 100.00 | 0.800 |
表2 流动相梯度
Table 2 Eluent gradient
| 时间Time/min | A/% | B/% | 流速Flow/(mL·min-1) |
|---|---|---|---|
| 0.00 | 95.00 | 5.00 | 0.800 |
| 5.00 | 60.00 | 40.00 | 0.800 |
| 6.00 | 0.00 | 100.00 | 0.800 |
| 8.00 | 0.00 | 100.00 | 0.800 |
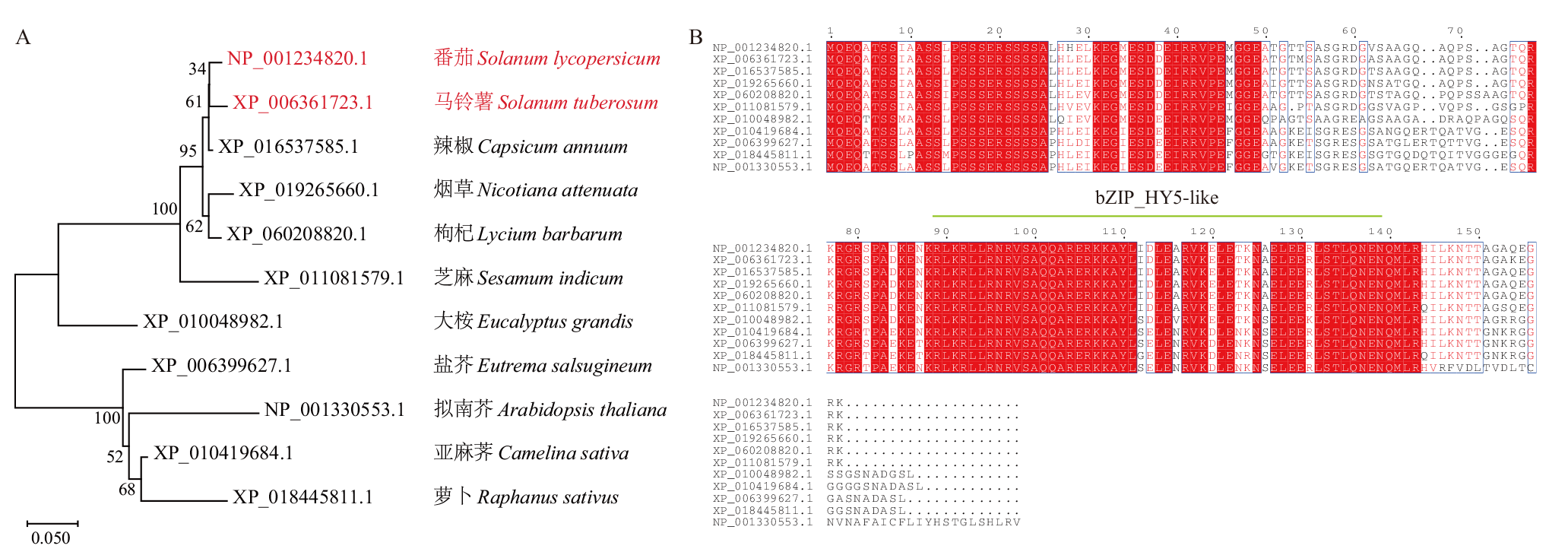
图1 茄科中StHY5的系统发育树分析及同源序列比对 A:StHY5蛋白的系统发育树分析,番茄和马铃薯用红色字体标出;B:StHY5的同源序列比对,绿色标出的氨基酸序列区间(89-140 aa)为bZIP-HY5-like结构域
Fig. 1 Phylogenetic tree and homologous sequence alignment of StHY5 in Solanaceae A: Phylogenetic tree analysis and homologous sequence alignment of StHY5, Solanum lycopersicum and Solanum tuberosum are marked in red; B: homologous sequence alignment of StHY5, the amino acid sequence interval(89-140 aa)marked in green is the bZIP-HY5-like domain

图2 马铃薯StHY5的时空表达分析(A)、原位杂交(B)和亚细胞定位分析(C) 不同小写字母表示在0.05水平差异显著。下同
Fig. 2 Temporal and spatial expression analysis (A), in-situ hybridization (B) and subcellular localization analysis (C) of StHY5 in potato Different lowercases indicate significant differences at the 0.05 level. The same below
| 名称 Name | 序列 Sequence | 数量 Account | 功能 Function |
|---|---|---|---|
| MBS | CAACTG | 1 | 参与干旱诱导的MYB结合位点MYB binding site involved in drought-inducibility |
| LTR | CCGAAA | 1 | 参与低温响应的顺式作用元件Cis-acting element involved in low-temperature responsiveness |
| GC-motif | CCCCCG | 1 | 参与缺氧特异性诱导的增强剂Enhancer-like element involved in anoxic specific inducibility |
| P-box | CCTTTTG | 1 | 赤霉素反应元件Gibberellin-responsive element |
| GT1-motif | GGTTAA | 1 | 光敏元件Light-sensitive element |
| TCCC-motif | TCTCCCT | 1 | 光响应元件的一部分Part of a light responsive element |
| TCA-element | CCATCTTTTT/TCAGAAGAGG | 3 | 参与水杨酸反应的顺式作用元件Cis-acting element involved in salicylic acid responsiveness |
| ABRE | ACGTG/GCAACGTGTC | 3 | 参与脱落酸反应的顺式作用元件Cis-acting element involved in the abscisic acid responsiveness |
| ARE | AAACCA | 2 | 厌氧诱导所需的顺式作用元件Cis-acting regulatory element essential for the anaerobic induction |
| G-Box | CACGTT | 3 | 参与光反应的顺式作用元件Cis-acting regulatory element involved in light responsiveness |
| CGTCA-motif | CGTCA | 5 | 茉莉酸甲酯响应元件Cis-acting regulatory element involved in the MeJA-responsiveness |
| TGACG-motif | TGACG | 5 | 茉莉酸甲酯响应元件Cis-acting regulatory element involved in the MeJA-responsiveness |
表3 马铃薯StHY5启动子顺式作用元件分析
Table 3 Cis-acting elements of StHY5 promoter in potato
| 名称 Name | 序列 Sequence | 数量 Account | 功能 Function |
|---|---|---|---|
| MBS | CAACTG | 1 | 参与干旱诱导的MYB结合位点MYB binding site involved in drought-inducibility |
| LTR | CCGAAA | 1 | 参与低温响应的顺式作用元件Cis-acting element involved in low-temperature responsiveness |
| GC-motif | CCCCCG | 1 | 参与缺氧特异性诱导的增强剂Enhancer-like element involved in anoxic specific inducibility |
| P-box | CCTTTTG | 1 | 赤霉素反应元件Gibberellin-responsive element |
| GT1-motif | GGTTAA | 1 | 光敏元件Light-sensitive element |
| TCCC-motif | TCTCCCT | 1 | 光响应元件的一部分Part of a light responsive element |
| TCA-element | CCATCTTTTT/TCAGAAGAGG | 3 | 参与水杨酸反应的顺式作用元件Cis-acting element involved in salicylic acid responsiveness |
| ABRE | ACGTG/GCAACGTGTC | 3 | 参与脱落酸反应的顺式作用元件Cis-acting element involved in the abscisic acid responsiveness |
| ARE | AAACCA | 2 | 厌氧诱导所需的顺式作用元件Cis-acting regulatory element essential for the anaerobic induction |
| G-Box | CACGTT | 3 | 参与光反应的顺式作用元件Cis-acting regulatory element involved in light responsiveness |
| CGTCA-motif | CGTCA | 5 | 茉莉酸甲酯响应元件Cis-acting regulatory element involved in the MeJA-responsiveness |
| TGACG-motif | TGACG | 5 | 茉莉酸甲酯响应元件Cis-acting regulatory element involved in the MeJA-responsiveness |
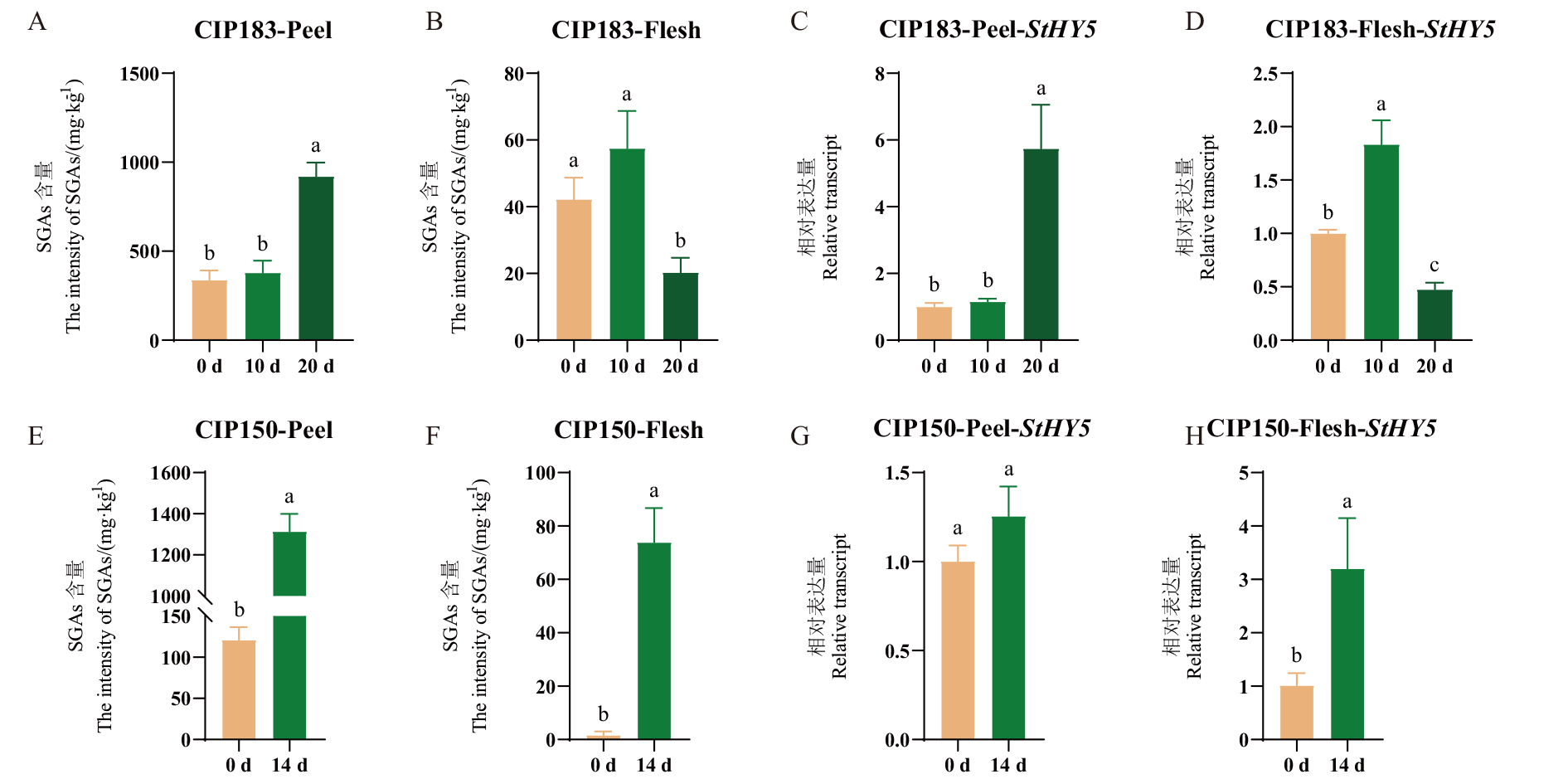
图3 绿变处理中SGAs含量及StHY5表达量 A-B:CIP183薯皮、薯肉的SGAs含量;C-D:CIP183薯皮、薯肉的StHY5表达量;E-F:CIP150薯皮、薯肉的SGAs含量;G-H:CIP150薯皮、薯肉的StHY5表达量
Fig. 3 SGAs content and StHY5 expression in green turning treatment A-B: CIP183 SGAs intensity of potato peel and flesh. C-D: StHY5 expression of CIP183 potato peel and flesh. E-F: CIP150 SGAs intensity of potato peel and flesh.G-H: StHY5 expression in CIP150 potato peel and flesh
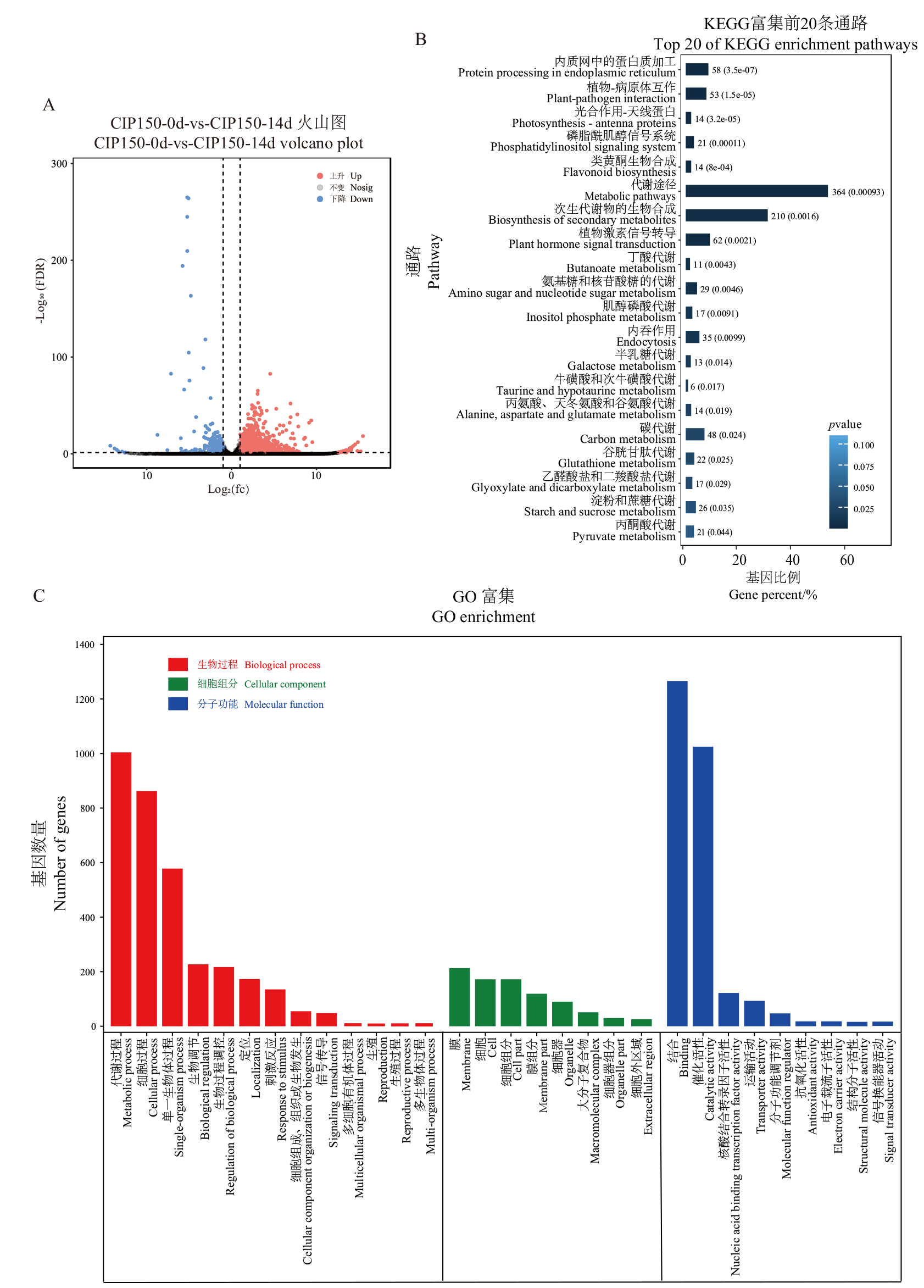
图4 马铃薯块茎CIP150薯肉绿变前后的转录组分析 A:火山图分析;B:KEGG富集分析;C:GO富集分析
Fig. 4 Transcriptome analysis of potato tuber CIP150 before and after flesh turning green A: Volcanic map analysis. B: KEGG enrichment analysis. C: GO enrichment analysis
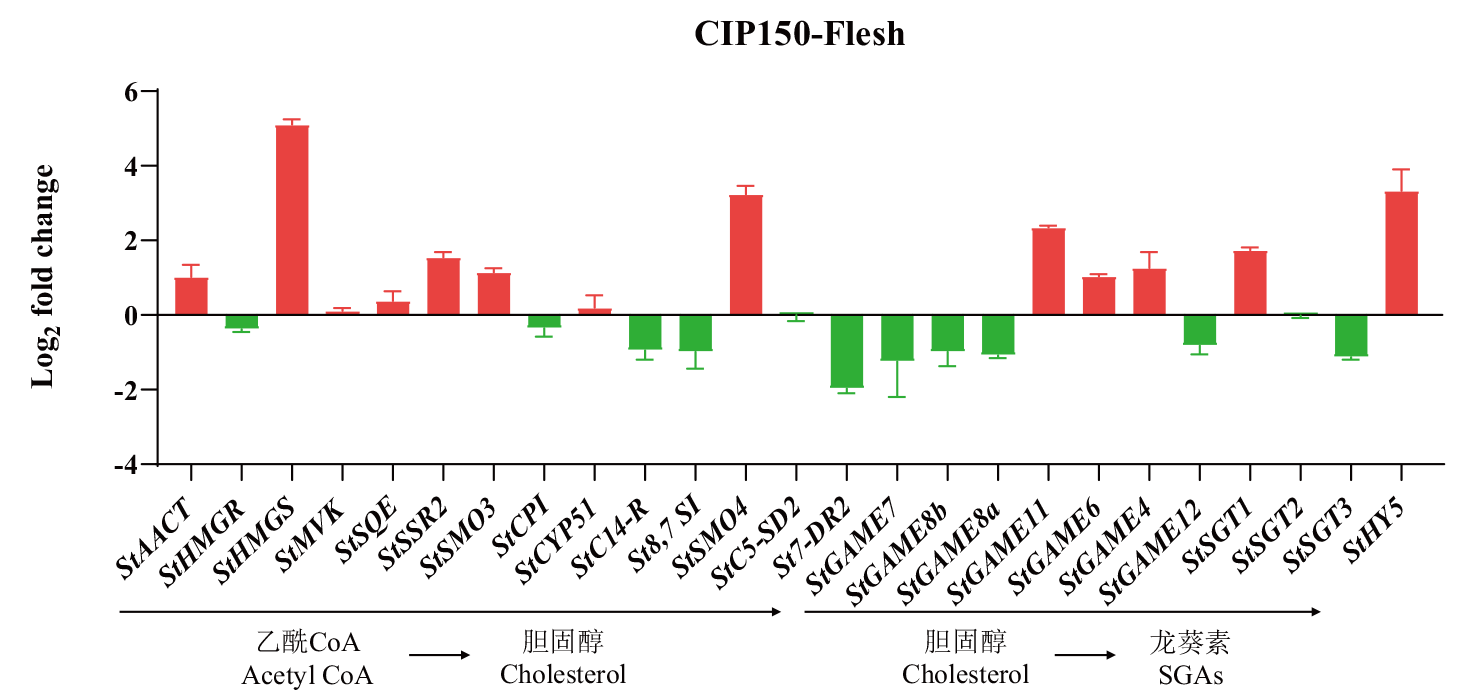
图5 马铃薯块茎CIP150薯肉绿变前后SGAs合成基因的差异表达 StAACT至St7-DR2为胆固醇合成途径,StGAME7至StSGT3为龙葵素合成途径,箭头指示的基因顺序即为龙葵素合成通路基因顺序
Fig. 5 Differential expression of SGAs synthetic genes in potato tuber CIP150 before and after flesh turning green StAACT to St7-DR2 is the synthesis pathway of cholesterol, StGAME7 to StSGT3 is the synthesis pathway of SGAs, the arrow indicates the sequence of genes involved in the SGAs synthesis pathway
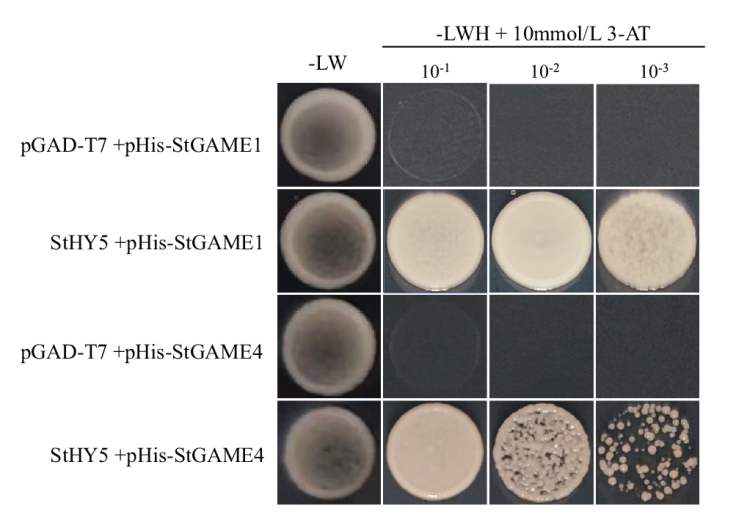
图6 StHY5与StSGT1/GAME1和StGAME4启动子间的酵母单杂交分析 L、W、H分别代表亮氨酸、色氨酸和组氨酸,10-1、10-2、10-3分别代表酵母菌OD600分别调至0.1、0.01、0.001时的点板浓度
Fig. 6 Yeast single hybridization analysis between StHY5 and StSGT1/GAME1 and StGAME4 promoters L, W and H indicate Leu, Trp and His respectively, and 10-1, 10-2 and 10-3 indicate the point plate concentration of yeast OD600 when it is adjusted to 0.1, 0.01 and 0.001, respectively
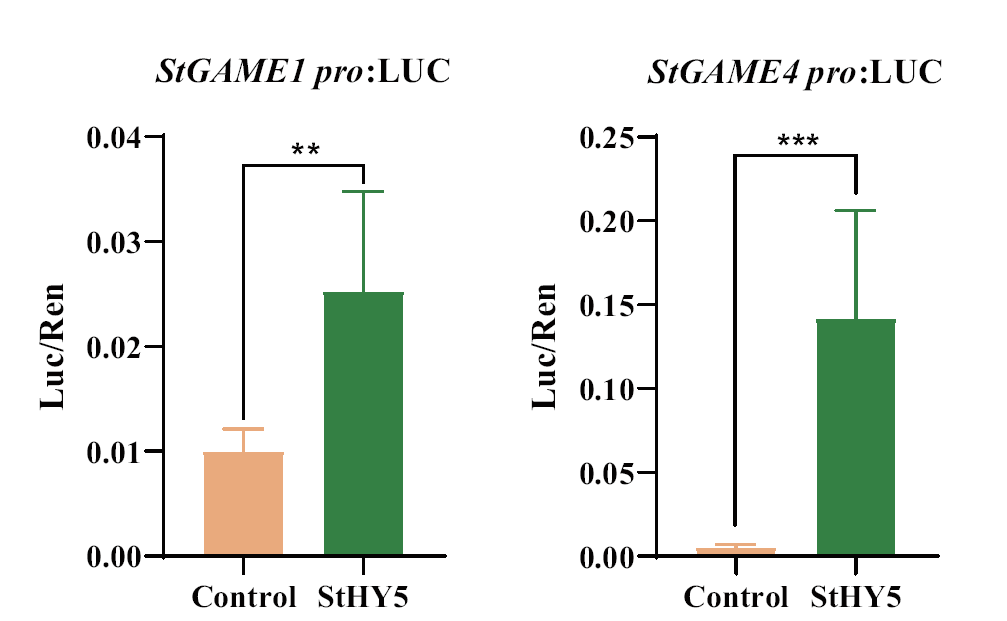
图7 StHY5与StSGT1/GAME1和StGAME4启动子间的双荧光素酶分析
Fig. 7 Double luciferase analysis between StHY5 and StS-GT1/GAME1 and StGAME4 promoters **P<0.01, ***P<0.001
| [1] | Hardigan MA, Laimbeer FPE, Newton L, et al. Genome diversity of tuber-bearing Solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato[J]. Proc Natl Acad Sci USA, 2017, 114(46): E9999-E10008. |
| [2] | Menoble C. Research and comparative analysis about potato production situation between China and continents in the world[C]. Agricultural Engineering, 2011. |
| [3] |
Begum SS, Das D, Gour NK, et al. Computational modelling of nanotube delivery of anti-cancer drug into glutathione reductase enzyme[J]. Sci Rep, 2021, 11(1): 4950.
doi: 10.1038/s41598-021-84006-1 pmid: 33654109 |
| [4] |
何虎翼, 唐洲萍, 杨鑫, 等. 马铃薯淀粉合成与降解研究进展[J]. 生物技术通报, 2019, 35(4): 101-107.
doi: 10.13560/j.cnki.biotech.bull.1985.2018-0829 |
| He HY, Tang ZP, Yang X, et al. Research progress on potato starch synthesis and degradation[J]. Biotechnol Bull, 2019, 35(4): 101-107. | |
| [5] | 聂碧华, 谢从华, 聂先舟. 马铃薯抗病毒机制研究进展[J]. 园艺学报, 2012, 39(9): 1703-1714. |
| Nie BH, Xie CH, Nie XZ. Progress in research on the resistance mechanism against viruses in potatoes[J]. Acta Hortic Sin, 2012, 39(9): 1703-1714. | |
| [6] | 邓仁菊, 邓宽平, 何天久, 等. 马铃薯主要病害抗性育种研究进展[J]. 西南农业学报, 2014, 27(3):1337-1342. |
| Deng RJ, Deng KP, He TJ, et al. Progresses of main diseases resistance breeding in potato[J]. Southwest China J Agric Sci, 2014, 27(3): 1337-1342. | |
| [7] | Wang T, He WS, Jiang HG, et al. The effects of nitrogen, phosphorus and potassium application on yield and starch content of potato plants[J]. Soil Fertil Sci China, 2016(3): 80-86. |
| [8] | Dhalsamant K, Singh CB, Lankapalli R. A review on greening and glycoalkaloids in potato tubers: potential solutions[J]. J Agric Food Chem, 2022, 70(43): 13819-13831. |
| [9] | Percival G, Dixon GR. Glycoalkaloid concentrations in aerial tubers of potato(Solanum tuberosum L.)[J]. J Sci Food Agric, 1996, 70(4): 439-448. |
| [10] | Bamberg J, Moehninsi, Navarre R, et al. Variation for tuber greening in the diploid wild potato Solanum microdontum[J]. Am J Potato Res, 2015, 92(3): 435-443. |
| [11] | Baur S, Bellé N, Hausladen H, et al. Quantitation of toxic steroidal glycoalkaloids and newly identified saponins in post-harvest light-stressed potato(Solanum tuberosum L.) varieties[J]. J Agric Food Chem, 2022, 70(27): 8300-8308. |
| [12] | Ostreikova TO, Kalinkina OV, Bogomolov NG, et al. Glycoalkaloids of plants in the family solanaceae(Nightshade)as potential drugs[J]. Pharm Chem J, 2022, 56(7): 948-957. |
| [13] |
Itkin M, Heinig U, Tzfadia O, et al. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes[J]. Science, 2013, 341(6142): 175-179.
doi: 10.1126/science.1240230 pmid: 23788733 |
| [14] |
Akiyama R, Umemoto N, Mizutani M. Recent advances in steroidal glycoalkaloid biosynthesis in the genus Solanum[J]. Plant Biotechnol, 2023, 40(3): 185-191.
doi: 10.5511/plantbiotechnology.23.0717b pmid: 38293253 |
| [15] | Yang LW, Jiang ZM, Jing YJ, et al. PIF1 and RVE1 form a transcriptional feedback loop to control light-mediated seed germination in Arabidopsis[J]. J Integr Plant Biol, 2020, 62(9): 1372-1384. |
| [16] | Zhou P, Song MF, Yang QH, et al. Both PHYTOCHROME RAPIDLY REGULATED1(PAR1)and PAR2 promote seedling photomorphogenesis in multiple light signaling pathways[J]. Plant Physiol, 2014, 164(2): 841-852. |
| [17] | Yu JW, Rubio V, Lee NY, et al. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability[J]. Mol Cell, 2008, 32(5): 617-630. |
| [18] |
Jiao YL, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants[J]. Nat Rev Genet, 2007, 8(3): 217-230.
doi: 10.1038/nrg2049 pmid: 17304247 |
| [19] |
Gangappa SN, Botto JF. The BBX family of plant transcription factors[J]. Trends Plant Sci, 2014, 19(7): 460-470.
doi: 10.1016/j.tplants.2014.01.010 pmid: 24582145 |
| [20] | Koornneef M, Rolff E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana(L.) heynh[J]. Z Für Pflanzenphysiol, 1980, 100(2): 147-160. |
| [21] | Oyama T, Shimura Y, Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl[J]. Genes Dev, 1997, 11(22): 2983-2995. |
| [22] | Jing YJ, Zhang D, Wang X, et al. Arabidopsis chromatin remodeling factor PICKLE interacts with transcription factor HY5 to regulate hypocotyl cell elongation[J]. Plant Cell, 2013, 25(1): 242-256. |
| [23] |
Gangappa SN, Botto JF. The multifaceted roles of HY5 in plant growth and development[J]. Mol Plant, 2016, 9(10): 1353-1365.
doi: S1674-2052(16)30134-4 pmid: 27435853 |
| [24] | Wang WH, Wang PW, Li XJ, et al. The transcription factor SlHY5 regulates the ripening of tomato fruit at both the transcriptional and translational levels[J]. Hortic Res, 2021, 8(1): 83. |
| [25] | Wang CC, Meng LH, Gao Y, et al. Manipulation of light signal transduction factors as a means of modifying steroidal glycoalkaloids accumulation in tomato leaves[J]. Front Plant Sci, 2018, 9: 437. |
| [26] | Itkin M, Rogachev I, Alkan N, et al. GLYCOALKALOID METABOLISM1 is required for steroidal alkaloid glycosylation and prevention of phytotoxicity in tomato[J]. Plant Cell, 2011, 23(12): 4507-4525. |
| [27] | Wan LB, Gao HD, Gao HL, et al. Selective extraction and determination of steroidal glycoalkaloids in potato tissues by electromembrane extraction combined with LC-MS/MS[J]. Food Chem, 2022, 367: 130724. |
| [28] | Zhu GT, Wang SC, Huang ZJ, et al. Rewiring of the fruit metabolome in tomato breeding[J]. Cell, 2018, 172(1/2): 249-261.e12. |
| [29] |
Jakoby M, Weisshaar B, Dröge-Laser W, et al. bZIP transcription factors in Arabidopsis[J]. Trends Plant Sci, 2002, 7(3): 106-111.
doi: 10.1016/s1360-1385(01)02223-3 pmid: 11906833 |
| [30] | Kurihara Y, Makita Y, et al. Time-course transcriptome study reveals mode of bZIP transcription factors on light exposure in Arabidopsis[J]. Int J Mol Sci, 2020, 21(6): 1993. |
| [31] |
Agarwal P, Baranwal VK, Khurana P. Genome-wide analysis of bZIP transcription factors in wheat and functional characterization of a TabZIP under abiotic stress[J]. Sci Rep, 2019, 9(1): 4608.
doi: 10.1038/s41598-019-40659-7 pmid: 30872683 |
| [32] |
Chattopadhyay S, Ang LH, Puente P, et al. Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression[J]. Plant Cell, 1998, 10(5): 673-683.
doi: 10.1105/tpc.10.5.673 pmid: 9596629 |
| [33] | Sa QL, Li WB, Sun YR. Transcriptional regulation of the G-box and G-box-binding proteins in plant gene expression[J]. Plant Physiol Commun, 2003, 39(1): 89-92. |
| [34] |
McCue KF, Allen PV, Shepherd LVT, et al. Potato glycosterol rhamnosyltransferase, the terminal step in triose side-chain biosynthesis[J]. Phytochemistry, 2007, 68(3): 327-334.
doi: 10.1016/j.phytochem.2006.10.025 pmid: 17157337 |
| [35] |
McCue KF, Allen PV, Shepherd LVT, et al. The primary in vivo steroidal alkaloid glucosyltransferase from potato[J]. Phytochemistry, 2006, 67(15): 1590-1597.
pmid: 16298403 |
| [36] | McCue KF, Shepherd LVT, Allen PV, et al. Metabolic compensation of steroidal glycoalkaloid biosynthesis in transgenic potato tubers: using reverse genetics to confirm the in vivo enzyme function of a steroidal alkaloid galactosyltransferase[J]. Plant Sci, 2005, 168(1): 267-273. |
| [37] | Shakya R, Navarre DA. LC-MS analysis of solanidane glycoalkaloid diversity among tubers of four wild potato species and three cultivars(Solanum tuberosum)[J]. J Agric Food Chem, 2008, 56(16): 6949-6958. |
| [38] |
Iijima Y, Watanabe B, Sasaki R, et al. Steroidal glycoalkaloid profiling and structures of glycoalkaloids in wild tomato fruit[J]. Phytochemistry, 2013, 95: 145-157.
doi: 10.1016/j.phytochem.2013.07.016 pmid: 23941899 |
| [39] |
Cárdenas PD, Sonawane PD, Heinig U, et al. The bitter side of the nightshades: Genomics drives discovery in Solanaceae steroidal alkaloid metabolism[J]. Phytochemistry, 2015, 113: 24-32.
doi: 10.1016/j.phytochem.2014.12.010 pmid: 25556315 |
| [40] | Sawai S, Ohyama K, Yasumoto S, et al. Sterol side chain reductase 2 is a key enzyme in the biosynthesis of cholesterol, the common precursor of toxic steroidal glycoalkaloids in potato[J]. Plant Cell, 2014, 26(9): 3763-3774. |
| [41] |
Cárdenas PD, Sonawane PD, Pollier J, et al. GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway[J]. Nat Commun, 2016, 7: 10654.
doi: 10.1038/ncomms10654 pmid: 26876023 |
| [1] | 申鹏, 高雅彬, 丁红. 马铃薯SAT基因家族的鉴定和表达分析[J]. 生物技术通报, 2024, 40(9): 64-73. |
| [2] | 宋兵芳, 柳宁, 程新艳, 徐晓斌, 田文茂, 高悦, 毕阳, 王毅. 马铃薯G6PDH基因家族鉴定及其在损伤块茎的表达分析[J]. 生物技术通报, 2024, 40(9): 104-112. |
| [3] | 夏士轩, 耿泽栋, 祝光涛, 张春芝, 李大伟. 基于深度学习的马铃薯花粉活力快速检测[J]. 生物技术通报, 2024, 40(9): 123-130. |
| [4] | 毛向红, 卢瑶, 范向斌, 杜培兵, 白小东. 基于SSR荧光标记毛细管电泳的马铃薯品种遗传多样性分析及分子身份证构建[J]. 生物技术通报, 2024, 40(9): 131-140. |
| [5] | 袁兰, 黄娅楠, 张贝妮, 熊雨萌, 王洪洋. 基于流式细胞仪鉴定马铃薯倍性的高通量样品制备方法[J]. 生物技术通报, 2024, 40(9): 141-147. |
| [6] | 宋倩娜, 段永红, 冯瑞云. CRISPR/Cas9介导的高效四倍体马铃薯试管薯基因编辑体系的建立[J]. 生物技术通报, 2024, 40(9): 33-41. |
| [7] | 王柯然, 闫俊杰, 刘建凤, 高玉林. RNAi技术在马铃薯害虫防控中的应用和风险[J]. 生物技术通报, 2024, 40(9): 4-10. |
| [8] | 张小妹, 周南伶, 张赛行, 王超, 沈玉龙, 管俊梅, 马玲. 马铃薯StDREBs基因的克隆及其表达分析[J]. 生物技术通报, 2024, 40(9): 42-50. |
| [9] | 满全财, 孟姿诺, 李伟, 蔡心汝, 苏润东, 付长青, 高顺娟, 崔江慧. 马铃薯AQP基因家族鉴定及表达分析[J]. 生物技术通报, 2024, 40(9): 51-63. |
| [10] | 吴娟, 武小娟, 王沛捷, 谢锐, 聂虎帅, 李楠, 马艳红. 彩色马铃薯花青素合成相关ERF基因筛选及表达分析[J]. 生物技术通报, 2024, 40(9): 82-91. |
| [11] | 乔岩, 杨芳, 任盼荣, 祁伟亮, 安沛沛, 李茜, 李丹, 肖俊飞. 马铃薯野生种烯酰水合酶超家族基因ScDHNS的克隆与功能分析[J]. 生物技术通报, 2024, 40(9): 92-103. |
| [12] | 张震, 李清, 徐菁, 陈凯园, 张春芝, 祝光涛. 马铃薯线粒体靶向表达载体的构建与应用[J]. 生物技术通报, 2024, 40(5): 66-73. |
| [13] | 张玉, 石磊, 巩檑, 聂峰杰, 杨江伟, 刘璇, 杨文静, 张国辉, 颉瑞霞, 张丽. 马铃薯WOX基因家族的鉴定及在离体再生和非生物胁迫中的表达分析[J]. 生物技术通报, 2024, 40(3): 170-180. |
| [14] | 梅显军, 宋慧洋, 李京昊, 梅超, 宋倩娜, 冯瑞云, 陈喜明. 马铃薯StDof5的克隆及表达分析[J]. 生物技术通报, 2024, 40(3): 181-192. |
| [15] | 张春芝, 周倩, 吴瑶瑶, 尚轶, 黄三文. 基因组学研究助力马铃薯育种方式的变革[J]. 生物技术通报, 2024, 40(10): 11-18. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||