








生物技术通报 ›› 2025, Vol. 41 ›› Issue (10): 264-276.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0353
韩昱1( ), 袁青云1,2, 章青平1, 吴春来1, 贺巍1(
), 袁青云1,2, 章青平1, 吴春来1, 贺巍1( ), 张芬1(
), 张芬1( )
)
收稿日期:2025-04-02
出版日期:2025-10-26
发布日期:2025-10-28
通讯作者:
张芬,女,博士,讲师,研究方向 :茶树营养吸收代谢;E-mail: zhangfen2008.cool@163.com作者简介:韩昱,男,硕士,研究方向 :茶树营养吸收代谢;E-mail: 2311914325@qq.com
基金资助:
HAN Yu1( ), YUAN Qing-yun1,2, ZHANG Qing-ping1, WU Chun-lai1, HE Wei1(
), YUAN Qing-yun1,2, ZHANG Qing-ping1, WU Chun-lai1, HE Wei1( ), ZHANG Fen1(
), ZHANG Fen1( )
)
Received:2025-04-02
Published:2025-10-26
Online:2025-10-28
摘要:
目的 硝酸转运蛋白1/多肽转运蛋白家族(NRT1/PTR family, NPF)在植物氮素吸收、激素运输及逆境响应中发挥关键作用,解析茶树CsNPFs基因特性及表达模式,为茶树氮素高效利用和抗逆分子育种提供理论依据。 方法 通过基因克隆、生物信息学分析及RT-qPCR方法,鉴定CsNPFs基因家族成员,探究其组织表达特性及对激素(IAA、ABA、GA3)和硝酸盐(NO3-)处理的响应规律,并通过拟南芥异源表达验证CsNPF7.3的功能。 结果 共克隆得到6个CsNPFs基因全长CDS序列,其编码蛋白均为疏水性跨膜蛋白;系统进化分析显示,CsNPFs与狭叶油茶ClNPFs亲缘关系最近。基因组织表达特性分析显示,CsNPF5.5/7.3主要在根部表达,CsNPF2.13/2.7/3.1/7.1主要在叶片中积累。激素处理可显著诱导基因的表达,其中CsNPF7.3在根系中受激素诱导呈现明显的上调表达现象,推测其可能为根系中激素传递的关键基因,而CsNPF5.5则在激素的诱导下在叶片中呈现明显上调表达的现象,受到组织内部的相关因素调控。NO3-处理下,叶片中CsNPFs基因响应显著,且除CsNPF2.7和CsNPF3.1外,基本都呈下调表达模式;根系中CsNPF5.5和CsNPF7.3则呈显著上调的表达规律。拟南芥超表达验证显示,CsNPF7.3过表达可显著增加拟南芥的生物量积累,并增强植株对IAA、ABA和GA₃的响应,促进其侧根发育、根系伸长及叶柄长度的增加。 结论 CsNPFs在茶树的氮素吸收和激素响应过程中发挥着重要作用,其中CsNPF7.3为茶树根系中的关键功能基因,可参与调控茶树的生长及逆境适应。
韩昱, 袁青云, 章青平, 吴春来, 贺巍, 张芬. 茶树CsNPF家族6个基因的克隆与表达分析及CsNPF7.3功能验证[J]. 生物技术通报, 2025, 41(10): 264-276.
HAN Yu, YUAN Qing-yun, ZHANG Qing-ping, WU Chun-lai, HE Wei, ZHANG Fen. Cloning and Expression Analysis of Six Genes of the NPF Family in Tea Plants and Functional Verification of CsNPF7.3[J]. Biotechnology Bulletin, 2025, 41(10): 264-276.
目的 Purpose | 引物名称 Primer name | 前引物序列 Forward primer sequence (5′-3′) | 后引物序列 Reverse primer sequence (5′-3′) |
|---|---|---|---|
基因克隆 Gene cloneing | CsNPF2.13 | CCCAAGATAAGGAAAAATGGAGGTA | CATAGATTTGTCAAAGTGACTTCAT |
| CsNPF2.7 | AGACTCACACTCGGAGGCATAAACT | ACAAACAACACCACAACAACAGATG | |
| CsNPF3.1 | ATCCGACCGCCTCTACTCT | AGTTACAAACATATCGTTTAAGCTA | |
| CsNPF5.5 | GTGAGAGTGGAAAAGACAACGCATA | CCTAACAGCAGCAGCAACCCT | |
| CsNPF7.1 | TGGGGCTGTTTTATTAGTTGAG | AATATAGCCCGAAATAAGCCTGCA | |
| CsNPF7.3 | CCCAAACACCAACTTCTCTTCTCTC | CCTCACACAAATGCCTACTGTCCTT | |
表达分析 RT-qPCR analysis | CsGAPDH | TTGGCATCGTTGAGGGTCT | CAGTGGGAACACGGAAAGC |
| CsNPF2.13-q | AGGAAGGAAAGGAATCAACAGC | AACCTCGTCCCAACAAAGAAC | |
| CsNPF2.7-q | CGAACGCTGACACCTCTACA | GAGCCGCTTTAACTCCACCA | |
| CsNPF3.1-q | GCCACCTAATCCGAGGGATG | TGCCCAACTCAAGTGGACTG | |
| CsNPF5.5-q | GGCTAACTTTCATCGCCTGC | CAAGCTTGCGGTTCATGTGG | |
| CsNPF6.1-q | CGATAGCAGCGTGTTTGTGG | GGCTGCCTTGTCCAAACATC | |
| CsNPF6.3-q | GCCTCACTGACTGTTTTCTTCG | AACCGCTTGATTTCGCCTA | |
| CsNPF7.1-q | TAGCCACATTCGGAGCAGAC | CCGACGTTGAGTGCAGAGTA | |
| CsNPF7.3-q | GCCAACCGGCAATACGAGAA | GACATTATTAGCTGCGTCGGC | |
| Atβ-tubulin-q | ACCACTCCTAGCTTTGGTGATCTG | AGGTTCACTGCGAGCTTCCTCA |
表 1 基因克隆及荧光定量分析引物序列
Table 1 Primers’ sequences for gene cloning and RT-qPCR analysis
目的 Purpose | 引物名称 Primer name | 前引物序列 Forward primer sequence (5′-3′) | 后引物序列 Reverse primer sequence (5′-3′) |
|---|---|---|---|
基因克隆 Gene cloneing | CsNPF2.13 | CCCAAGATAAGGAAAAATGGAGGTA | CATAGATTTGTCAAAGTGACTTCAT |
| CsNPF2.7 | AGACTCACACTCGGAGGCATAAACT | ACAAACAACACCACAACAACAGATG | |
| CsNPF3.1 | ATCCGACCGCCTCTACTCT | AGTTACAAACATATCGTTTAAGCTA | |
| CsNPF5.5 | GTGAGAGTGGAAAAGACAACGCATA | CCTAACAGCAGCAGCAACCCT | |
| CsNPF7.1 | TGGGGCTGTTTTATTAGTTGAG | AATATAGCCCGAAATAAGCCTGCA | |
| CsNPF7.3 | CCCAAACACCAACTTCTCTTCTCTC | CCTCACACAAATGCCTACTGTCCTT | |
表达分析 RT-qPCR analysis | CsGAPDH | TTGGCATCGTTGAGGGTCT | CAGTGGGAACACGGAAAGC |
| CsNPF2.13-q | AGGAAGGAAAGGAATCAACAGC | AACCTCGTCCCAACAAAGAAC | |
| CsNPF2.7-q | CGAACGCTGACACCTCTACA | GAGCCGCTTTAACTCCACCA | |
| CsNPF3.1-q | GCCACCTAATCCGAGGGATG | TGCCCAACTCAAGTGGACTG | |
| CsNPF5.5-q | GGCTAACTTTCATCGCCTGC | CAAGCTTGCGGTTCATGTGG | |
| CsNPF6.1-q | CGATAGCAGCGTGTTTGTGG | GGCTGCCTTGTCCAAACATC | |
| CsNPF6.3-q | GCCTCACTGACTGTTTTCTTCG | AACCGCTTGATTTCGCCTA | |
| CsNPF7.1-q | TAGCCACATTCGGAGCAGAC | CCGACGTTGAGTGCAGAGTA | |
| CsNPF7.3-q | GCCAACCGGCAATACGAGAA | GACATTATTAGCTGCGTCGGC | |
| Atβ-tubulin-q | ACCACTCCTAGCTTTGGTGATCTG | AGGTTCACTGCGAGCTTCCTCA |
基因名称 Gene name | 开放阅读框长度 Open reading frame (ORF) (bp) | 编码氨基酸数目 Number of amino acids |
|---|---|---|
| CsNPF2.13 | 1 923 | 640 |
| CsNPF2.7 | 1 470 | 489 |
| CsNPF3.1 | 1 815 | 604 |
| CsNPF5.5 | 1 659 | 552 |
| CsNPF7.1 | 1 734 | 577 |
| CsNPF7.3 | 1 800 | 599 |
表2 茶树CsNPFs序列信息
Table 2 Sequence information of CsNPFs in tea plants
基因名称 Gene name | 开放阅读框长度 Open reading frame (ORF) (bp) | 编码氨基酸数目 Number of amino acids |
|---|---|---|
| CsNPF2.13 | 1 923 | 640 |
| CsNPF2.7 | 1 470 | 489 |
| CsNPF3.1 | 1 815 | 604 |
| CsNPF5.5 | 1 659 | 552 |
| CsNPF7.1 | 1 734 | 577 |
| CsNPF7.3 | 1 800 | 599 |
蛋白 Protein | 蛋白分子量 Molecular weight (kD) | 蛋白等电点 Theoretical pI | 总原子数 Total number of atom | 不稳定系数 Instability index | 脂肪系数 Aliphatic index | 亲疏水性平均系数 Grand average of hydropathicity (GRAVY) | 信号肽 Signal peptide | 亚细胞定位 Sub-cellular localization | 跨膜螺旋预测 Transmembrane helices prediction |
|---|---|---|---|---|---|---|---|---|---|
| CsNPF2.13 | 70.26 | 8.82 | 9 950 | 46.14 | 97.66 | 0.228 | 0.001 3 | 质膜(8.48) | 12 |
| CsNPF2.7 | 53.62 | 8.81 | 7 633 | 42.13 | 108.4 | 0.332 | 0.287 5 | 质膜(9.4) | 12 |
| CsNPF3.1 | 67.10 | 6.11 | 9 523 | 32.75 | 104.59 | 0.183 | 0.000 5 | 质膜(8.3) | 11 |
| CsNPF5.5 | 61.38 | 8.70 | 8 713 | 32.29 | 102.46 | 0.375 | 0.526 5 | 质膜(8.5) | 9 |
| CsNPF7.1 | 62.92 | 6.05 | 8 849 | 25.63 | 97.14 | 0.337 | 0.004 6 | 质膜(8.4) | 12 |
| CsNPF7.3 | 66.51 | 8.53 | 9 378 | 29.64 | 96.51 | 0.263 | 0.002 0 | 质膜(9.1) | 12 |
表3 茶树CsNPFs蛋白基本特性
Table 3 Basic characteristics of CsNPFs in tea plants
蛋白 Protein | 蛋白分子量 Molecular weight (kD) | 蛋白等电点 Theoretical pI | 总原子数 Total number of atom | 不稳定系数 Instability index | 脂肪系数 Aliphatic index | 亲疏水性平均系数 Grand average of hydropathicity (GRAVY) | 信号肽 Signal peptide | 亚细胞定位 Sub-cellular localization | 跨膜螺旋预测 Transmembrane helices prediction |
|---|---|---|---|---|---|---|---|---|---|
| CsNPF2.13 | 70.26 | 8.82 | 9 950 | 46.14 | 97.66 | 0.228 | 0.001 3 | 质膜(8.48) | 12 |
| CsNPF2.7 | 53.62 | 8.81 | 7 633 | 42.13 | 108.4 | 0.332 | 0.287 5 | 质膜(9.4) | 12 |
| CsNPF3.1 | 67.10 | 6.11 | 9 523 | 32.75 | 104.59 | 0.183 | 0.000 5 | 质膜(8.3) | 11 |
| CsNPF5.5 | 61.38 | 8.70 | 8 713 | 32.29 | 102.46 | 0.375 | 0.526 5 | 质膜(8.5) | 9 |
| CsNPF7.1 | 62.92 | 6.05 | 8 849 | 25.63 | 97.14 | 0.337 | 0.004 6 | 质膜(8.4) | 12 |
| CsNPF7.3 | 66.51 | 8.53 | 9 378 | 29.64 | 96.51 | 0.263 | 0.002 0 | 质膜(9.1) | 12 |
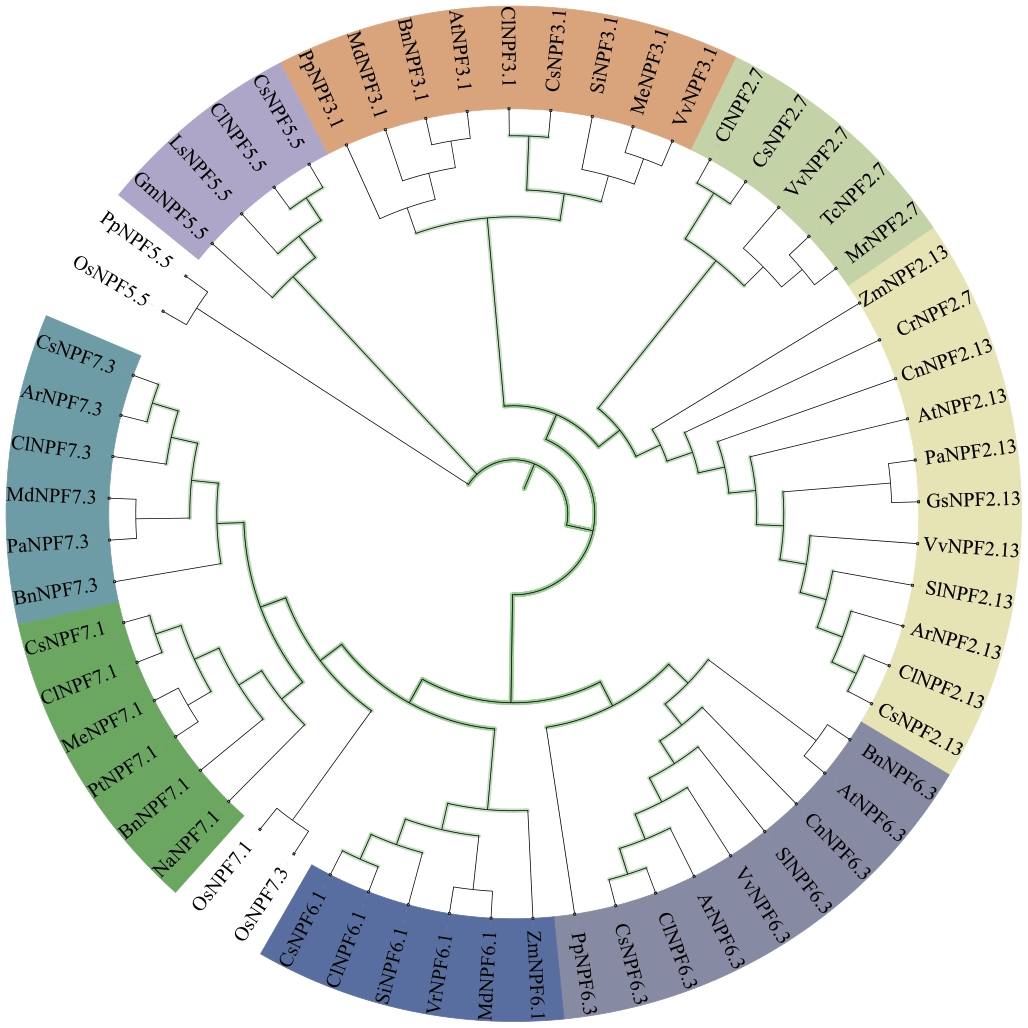
图2 CsNPFs蛋白的系统发育树At:拟南芥;Os:水稻;Zm:玉米;Mt:苜蓿;Md:苹果;Cl:狭叶油茶;Vv:葡萄;Si:芝麻;Pa:白杨树;Cn:椰子;Cr:长春花;Tc:可可树;Pp:海松;Bn:甘蓝型油菜;Gm:大豆;Na:渐狭叶烟草;Pt:毛果杨;Ls:莴苣;Me:木薯;Mr:杨梅;Ar:山黎猕猴桃;Vr:河岸葡萄;Sl:番茄;Gs:野生大豆
Fig. 2 Phylogenetic tree ofCsNPFs proteinsAt: Arabidopsis thaliana; Os: Oryza sativa; Zm: Zea mays; Mt: Medicago truncatula; Md: Malus domestica; Cl: Camellia lanceoleosa; Vv: Vitis vinifera; Si: Sesamum indicum; Pa: Populus alba; Cn: Cocos nucifera; Cr: Catharanthus roseus; Tc: Theobroma cacao; Pp: Pinus pinaster; Bn: Brassica napus; Gm: Glycine max; Na: Nicotiana attenuata;Pt: Populus trichocarpa; Ls: Lactuca sativais; Me: Manihot esculenta; Mr: Morella rubra; Ar: Actinidia rufa; Vr: Vitis riparis; Sl: Solanum lycopersicum; Gs: Glycine soja
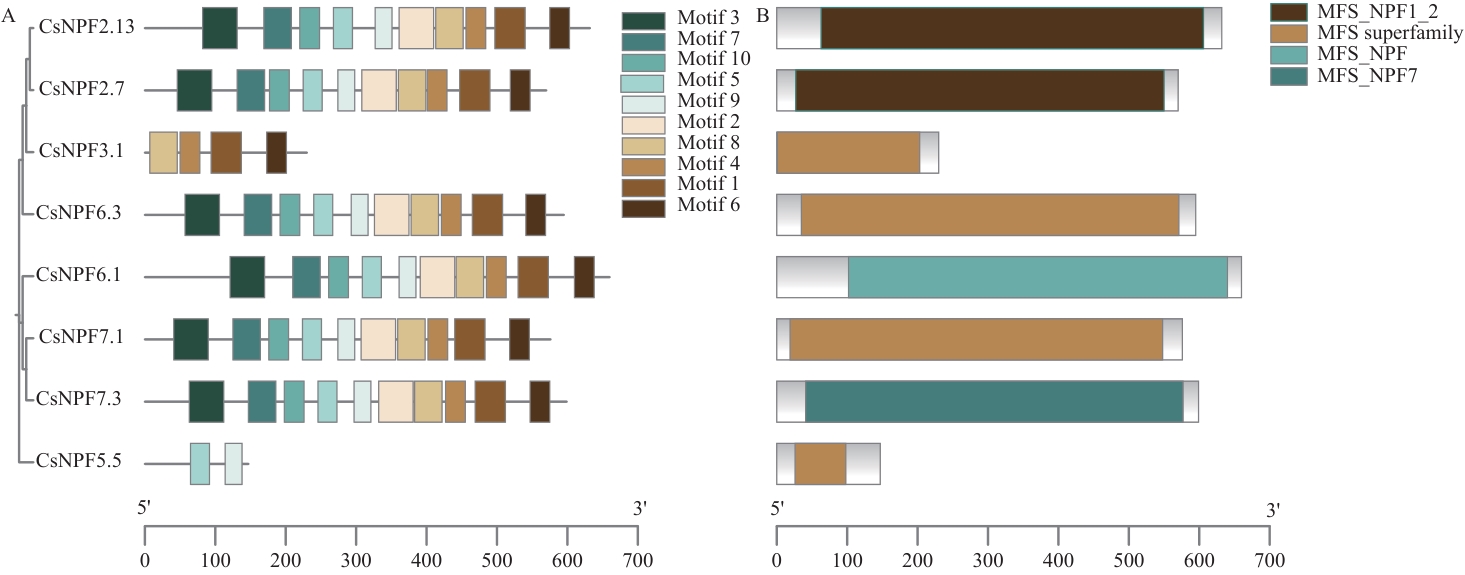
图3 CsNPFs蛋白的基序(A)及保守结构域(B)分析MFS_NPF1_2、MFS superfamily 和MFS_NPF7表示硝酸盐传输相关结构域
Fig. 3 Conserved motif (A) and domain (B) analysis of CsNPFs proteinsMFS_NPF1_2, MFS superfamily, and MFS_NPF7 denote nitrate transport-related domains
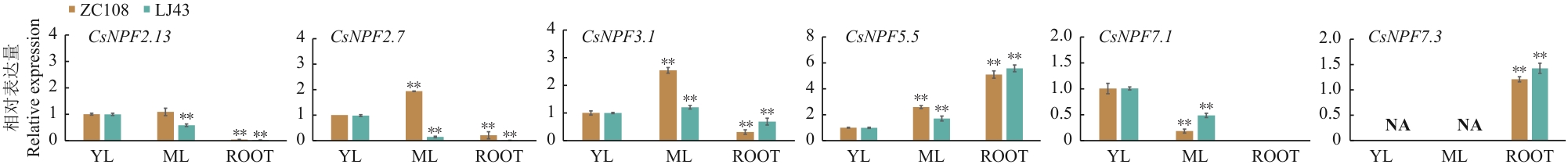
图5 CsNPFs基因组织特性分析不同茶树品种‘中茶108’(ZC108)、‘龙井43’(LJ43),不同组织部位嫩叶(YL)、成熟叶(ML)、根(ROOT)。NA代表未检测到数据。误差线代表3个重复的标准方差,图中数据为平均数±标准差,* P <0.05,** P <0.01。下同
Fig. 5 Organizational characteristics analysis of CsNPFs genesDifferent tea varieties ‘Zhongcha 108’ (ZC108), ‘Longjing 43’ (LJ43), different tissue parts of the young leaf (YL), mature leaf (ML), root (ROOT). NA indicates that data is not detected. The error bars indicate the standard deviation among the three replicates. The data are three biological replicates ± SD. * P < 0.05, ** P < 0.01. The same below
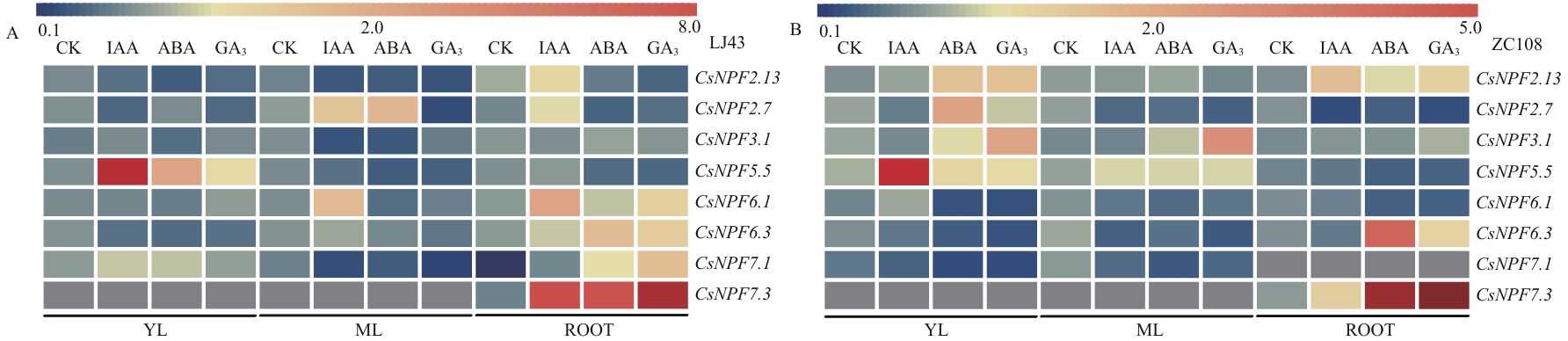
图6 激素处理下CsNPFs基因在不同组织部位的表达水平使用MeV软件构建基因表达量热图,表达量数据为3个生物学重复的平均值,蓝色代表低表达,红色代表高表达,灰色代表未检测到数据
Fig. 6 Expressions of CsNPFs in different tissues under hormone treatmentThe average values of three biological replicates were used to generate a heat map using MeV software. The intensity value bars are shown above the heat map. Blue denotes low expression, and red denotes high expression. The grey color indicates that no data has been detected

图7 硝酸盐处理下叶片和根系中CsNPFs基因的表达水平氮饥饿处理10 d(-N)、加氮1 mol/L(低氮,+LN)、4 mol/L(高氮,+HN),分别处理2 h和2 d
Fig. 7 Expressions of CsNPFs genes in the leaves and roots under nitrate treatmentNitrogen starvation treatment for 10 d (-N), nitrogen addition 1 mol/L (low nitrogen, +LN), 4 mol/L (high nitrogen, +HN), respectively, for 2 h and 2 d
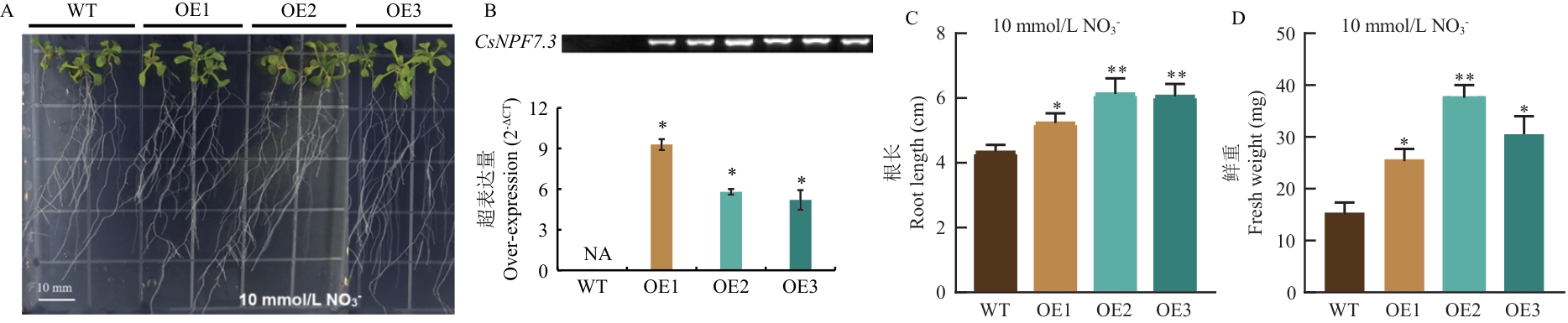
图8 CsNPF7.3超表达株系(OE)与野生型(WT)的表型差异分析A:1/4 MS(10 mmol/L NO3-)条件下拟南芥生长表型图;B:超表达株系的阳性鉴定;C:10 mmol/L NO3-条件下不同株系根长;D:10 mmol/L NO3-条件下不同株系鲜重。 WT为Col-0野生型拟南芥,OE为CsNPF7.3转基因超表达拟南芥(OE1、OE2、OE3分别为3个超表达系列)。下同
Fig. 8 Phenotypic difference analysis of CsNPF7.3 overexpressing lines (OE) compared with WT (Col-0) under different treatment conditionsA: Phenotype of Arabidopsis thaliana growth under 1/4 MS (10 mmol/L NO3-) conditions. B: Positive identification of overexpressing lines. C: Root lengths of different lines under 10 mmol/L NO3- conditions. D: Fresh weights of different lines under 10 mmol/L NO3- conditions. WT refers to the wild-type A. thaliana Col-0, and OE refers to the CsNPF7.3 transgenic overexpressing A. thaliana (OE1, OE2, and OE3 are 3 overexpressing lines). The same below
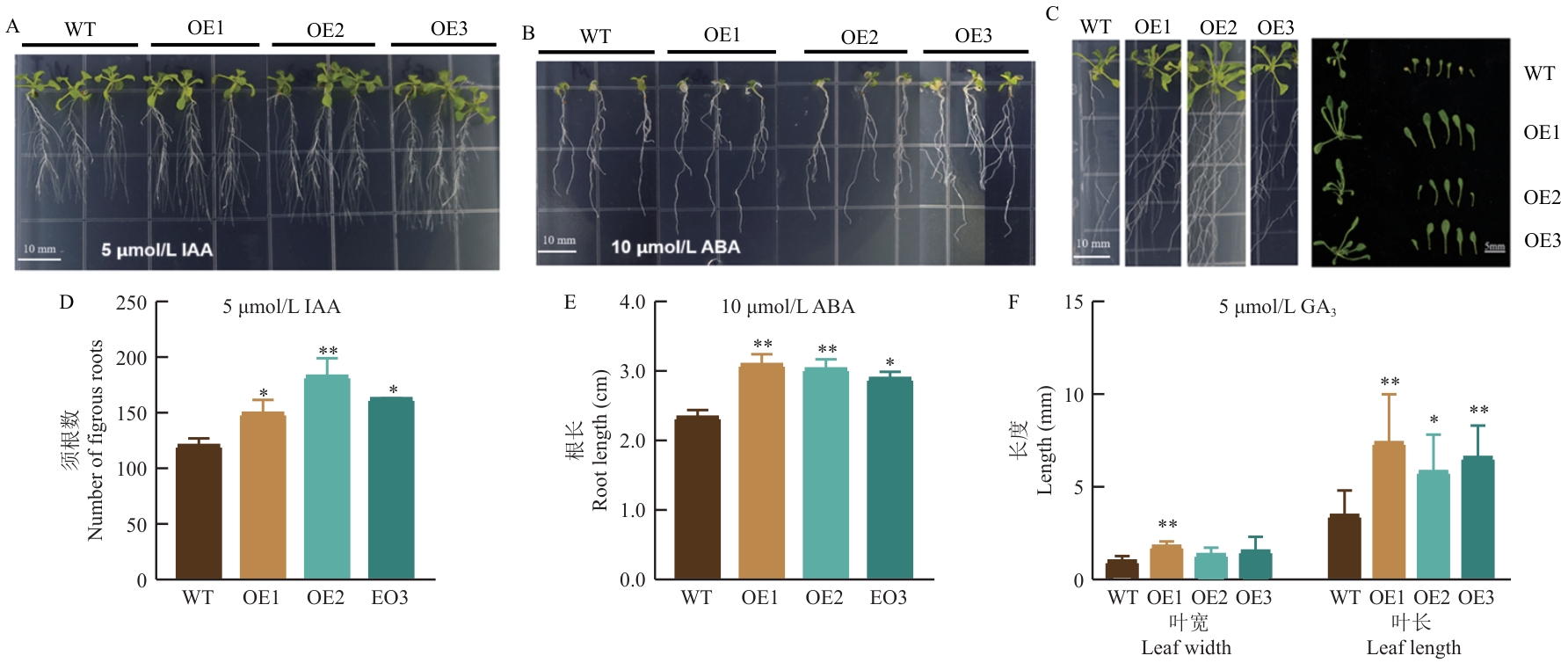
图9 不同激素处理下CsNPF7.3超表达株系(OE)与野生型(WT)的表型差异分析A:5 μmol/L IAA条件下植株生长表型图;B:10 μmol/L ABA条件下植株生长表型图;C:5 μmol/L GA3条件下植株生长表型图;D:5 μmol/L IAA条件下不同株系须根数差异分析;E:10 μmol/L ABA条件下植株根长差异分析;F:5 μmol/L GA3条件下植株叶宽和叶长差异分析
Fig. 9 Phenotypic difference analysis of CsNPF7.3 overexpressing lines (OE) compared with WT (Col-0) under different hormone treatment conditionsA: Phenotype of plant growth under 5 μmol/L IAA conditions. B: Phenotype of plant growth under 10 μmol/L ABA conditions. C: Phenotype of plant growth under 5 μmol/L GA3 conditions. D: The number of fibrous roots analysis among different lines under 5 μmol/L IAA conditions. E: Analysis of the difference in root length among plants under 10 μmol/L ABA conditions. F: Analysis of the difference in leaf width and leaf length among plants under 5 μmol/L GA3 conditions
| [1] | 袁青云, 贺巍, 苏会, 等. 植物硝酸根/多肽转运蛋白NPF家族功能研究进展 [J]. 植物生理学报, 2022, 58(10): 1840-1850. |
| Yuan QY, He W, Su H, et al. Research progress in nitrate/peptide transporter NPF family in plants [J]. Plant Physiol J, 2022, 58(10): 1840-1850. | |
| [2] | 黄慧梅, 高永康, 台玉莹, 等. 硝酸盐转运蛋白NRT2在植物中的功能及分子机制研究进展 [J]. 植物学报, 2023, 58(5): 783-798. |
| Huang HM, Gao YK, Tai YY, et al. Research advances in elucidating the function and molecular mechanism of the nitrate transporter 2 (NRT2) proteins in plants [J]. Chin Bull Bot, 2023, 58(5): 783-798. | |
| [3] | Alboresi A, Gestin C, Leydecker MT, et al. Nitrate, a signal relieving seed dormancy in Arabidopsis [J]. Plant Cell Environ, 2005, 28(4): 500-512. |
| [4] | 钱雨轩, 武占会, 刘宁, 等. 生菜NRT2/3的生物信息及功能的初步验证 [J]. 植物营养与肥料学报, 2024, 30(11): 2161-2180. |
| Qian YX, Wu ZH, Liu N, et al. Biological information of lettuce NRT2/3 and preliminary functional validation [J]. J Plant Nutr Fertil, 2024, 30(11): 2161-2180. | |
| [5] | 胡书婷, 王青. 植物氮代谢与氮素利用遗传基础研究进展 [J]. 山西农业科学, 2024, 52(6): 1-11. |
| Hu ST, Wang Q. Research progress on nitrogen metabolism and genetic basis of nitrogen utilization in plant [J]. J Shanxi Agric Sci, 2024, 52(6): 1-11. | |
| [6] | 尹卓然, 王驰, 轩栋栋, 等. 烟草硝酸盐转运蛋白NtNRT1.7基因的克隆、亚细胞定位及表达模式分析 [J]. 烟草科技, 2022, 55(5): 1-8. |
| Yin ZR, Wang C, Xuan DD, et al. Cloning, subcellular localization and expression pattern analysis of tobacco nitrate transporter NtNRT1.7 gene [J]. Tob Sci Technol, 2022, 55(5): 1-8. | |
| [7] | Fan SC, Lin CS, Hsu PK, et al. The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate [J]. Plant Cell, 2009, 21(9): 2750-2761. |
| [8] | Corratgé-Faillie C, Lacombe B. Substrate (un) specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins [J]. J Exp Bot, 2017, 68(12): 3107-3113. |
| [9] | 冯凯月, 赵鑫焱, 李子妍, 等. 植物响应盐碱胁迫的分子机制研究进展 [J]. 生物技术通报, 2024, 40(10): 122-138. |
| Feng KY, Zhao XY, Li ZY, et al. Research advances in the molecular mechanisms of plant response to saline-alkali stress [J]. Biotechnol Bull, 2024, 40(10): 122-138. | |
| [10] | Segonzac C, Boyer JC, Ipotesi E, et al. Nitrate efflux at the root plasma membrane: identification of an Arabidopsis excretion transporter [J]. Plant Cell, 2007, 19(11): 3760-3777. |
| [11] | Chiu CC, Lin CS, Hsia AP, et al. Mutation of a nitrate transporter, AtNRT1: 4, results in a reduced petiole nitrate content and altered leaf development [J]. Plant Cell Physiol, 2004, 45(9): 1139-1148. |
| [12] | Almagro A, Lin SH, Tsay YF. Characterization of the Arabidopsis nitrate transporter NRT1.6 reveals a role of nitrate in early embryo development [J]. Plant Cell, 2008, 20(12): 3289-3299. |
| [13] | Fang ZM, Bai GX, Huang WT, et al. The rice peptide transporter OsNPF7.3 is induced by organic nitrogen, and contributes to nitrogen allocation and grain yield [J]. Front Plant Sci, 2017, 8: 1338. |
| [14] | Wang J, Lu K, Nie HP, et al. Rice nitrate transporter OsNPF7.2 positively regulates tiller number and grain yield [J]. Rice, 2018, 11(1): 12. |
| [15] | Krapp A. Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces [J]. Curr Opin Plant Biol, 2015, 25: 115-122. |
| [16] | Krouk G, Crawford NM, Coruzzi GM, et al. Nitrate signaling: adaptation to fluctuating environments [J]. Curr Opin Plant Biol, 2010, 13(3): 265-272. |
| [17] | Tal I, Zhang Y, Jørgensen ME, et al. The Arabidopsis NPF3 protein is a GA transporter [J]. Nat Commun, 2016, 7: 11486. |
| [18] | Shimizu T, Kanno Y, Suzuki H, et al. Arabidopsis NPF4.6 and NPF5.1 control leaf stomatal aperture by regulating abscisic acid transport [J]. Genes, 2021, 12(6): 885. |
| [19] | Tang Z, Chen Y, Chen F, et al. OsPTR7 (OsNPF8.1), a putative peptide transporter in rice, is involved in dimethylarsenate accumulation in rice grain [J]. Plant Cell Physiol, 2017, 58(5): 904-913. |
| [20] | Niu HL, Wang JJ, Liao ZW, et al. Root-specific expression of CsNPF2.3 is involved in modulating fluoride accumulation in tea plant (Camellia sinensis) [J]. Hortic Res, 2025, 12(6): uhaf072. |
| [21] | Zhang F, Wang LY, Bai PX, et al. Identification of regulatory networks and hub genes controlling nitrogen uptake in tea plants [Camellia sinensis (L.) O. kuntze [J]. J Agric Food Chem, 2020, 68(8): 2445-2456. |
| [22] | 袁青云, 韩昱, 贺巍, 等. 茶树CsNPF6.1/6.3基因克隆及ABA转运功能验证 [J]. 园艺学报, 2024, 51(12): 2817-2828. |
| Yuan QY, Han Y, He W, et al. Cloning and ABA transport function analysis of CsNPF6.1/6.3 in tea plants [J]. Acta Hortic Sin, 2024, 51(12): 2817-2828. | |
| [23] | Muoki RC, Paul A, Kumari A, et al. An improved protocol for the isolation of RNA from roots of tea (Camellia sinensis (L.) O. Kuntze) [J]. Mol Biotechnol, 2012, 52(1): 82-88. |
| [24] | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method [J]. Methods, 2001, 25(4): 402-408. |
| [25] | Bent A. Arabidopsis thaliana floral dip transformation method [J]. Methods Mol Biol, 2006, 343: 87-103. |
| [26] | Zhang XR, Henriques R, Lin SS, et al. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method [J]. Nat Protoc, 2006, 1(2): 641-646. |
| [27] | 李婧, 左欣欣, 赵培伶, 等. 茶树高亲和硝酸盐转运蛋白家族基因NRT2的鉴定与表达 [J]. 应用与环境生物学报, 2022, 28(1): 50-56. |
| Li J, Zuo XX, Zhao PL, et al. Identification and expression analysis of high-affinity nitrate transporter family genes NRT2 in Camellia sinensis [J]. Chin J Appl Environ Biol, 2022, 28(1): 50-56. | |
| [28] | 马嘉俊, 吴英华, 李璿, 等. 白菜NPF基因家族成员的鉴定及其生物信息学分析 [J]. 河南农业科学, 2021, 50(9): 117-127. |
| Ma JJ, Wu YH, Li X, et al. Identification and bioinformatics analysis of NPF gene family members in Chinese Cabbage (Brassica rapa subsp. pekinensis) [J]. J Henan Agric Sci, 2021, 50(9): 117-127. | |
| [29] | Wen J, Li PF, Ran F, et al. Genome-wide characterization, expression analyses, and functional prediction of the NPF family in Brassica napus [J]. BMC Genomics, 2020, 21(1): 871. |
| [30] | Tsay YF, Chiu CC, Tsai CB, et al. Nitrate transporters and peptide transporters [J]. FEBS Lett, 2007, 581(12): 2290-2300. |
| [31] | Parker JL, Newstead S. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1 [J]. Nature, 2014, 507(7490): 68-72. |
| [32] | 汪芳珍. 荒漠植物沙芥NAXT家族基因的克隆及其成员PcNPF2.7的功能分析 [D]. 兰州: 兰州大学, 2021. |
| Wang FZ. Cloning of NAXT family genes from desert plant Pugionium cornutum and functional analysis of its member PcNPF2.7 [D]. Lanzhou: Lanzhou University, 2021. | |
| [33] | Léran S, Garg B, Boursiac Y, et al. AtNPF5.5, a nitrate transporter affecting nitrogen accumulation in Arabidopsis embryo [J]. Sci Rep, 2015, 5: 7962. |
| [34] | Cui YN, Li XT, Yuan JZ, et al. Nitrate transporter NPF7.3/NRT1.5 plays an essential role in regulating phosphate deficiency responses in Arabidopsis [J]. Biochem Biophys Res Commun, 2019, 508(1): 314-319. |
| [35] | Lin SH, Kuo HF, Canivenc G, et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport [J]. Plant Cell, 2008, 20(9): 2514-2528. |
| [36] | 郑冬超, 夏新莉, 尹伟伦. 生长素促进拟南芥AtNRT1.1基因表达增强硝酸盐吸收 [J]. 北京林业大学学报, 2013, 35(2): 80-85. |
| Zheng DC, Xia XL, Yin WL. Auxin promotes nitrate uptake by up-regulating AtNRT1.1gene transcript level in Arobidopsis thaliana [J]. J Beijing For Univ, 2013, 35(2): 80-85. | |
| [37] | Chen HF, Zhang Q, Wang XR, et al. Nitrogen form-mediated ethylene signal regulates root-to-shoot K+ translocation via NRT1.5 [J]. Plant Cell Environ, 2021, 44(12): 3806-3818. |
| [38] | Guo FQ, Wang RC, Crawford NM. The Arabidopsis dual‐affinity nitrate transporter gene AtNRT1.1 (CHL1) is regulated by auxin in both shoots and roots [J]. J Exp Bot, 2002, 53(370): 835-844. |
| [1] | 徐小萍, 杨成龙, 和兴, 郭文杰, 吴健, 方少忠. 百合LoAPS1克隆及其在休眠解除过程的功能分析[J]. 生物技术通报, 2025, 41(9): 195-206. |
| [2] | 董向向, 缪百灵, 许贺娟, 陈娟娟, 李亮杰, 龚守富, 朱庆松. 森林草莓FveBBX20基因的生物信息学分析及开花调控功能[J]. 生物技术通报, 2025, 41(9): 115-123. |
| [3] | 李珊, 马登辉, 马红义, 姚文孔, 尹晓. 葡萄SKP1基因家族鉴定与表达分析[J]. 生物技术通报, 2025, 41(9): 147-158. |
| [4] | 巩慧玲, 邢玉洁, 马俊贤, 蔡霞, 冯再平. 马铃薯LAC基因家族的鉴定及盐胁迫下表达分析[J]. 生物技术通报, 2025, 41(9): 82-93. |
| [5] | 张永艳, 郭思健, 李晶, 郝思怡, 李瑞得, 刘嘉鹏, 程春振. 蓝莓花青素相关VcGSTF19基因的克隆及功能研究[J]. 生物技术通报, 2025, 41(9): 139-146. |
| [6] | 化文平, 刘菲, 浩佳欣, 陈尘. 丹参ADH基因家族的鉴定与表达模式分析[J]. 生物技术通报, 2025, 41(8): 211-219. |
| [7] | 白雨果, 李婉迪, 梁建萍, 石志勇, 卢庚龙, 刘红军, 牛景萍. 哈茨木霉T9131对黄芪幼苗的促生机理[J]. 生物技术通报, 2025, 41(8): 175-185. |
| [8] | 李开杰, 吴瑶, 李丹丹. 红花CtbHLH128基因克隆及调控干旱胁迫应答功能研究[J]. 生物技术通报, 2025, 41(8): 234-241. |
| [9] | 任睿斌, 司二静, 万广有, 汪军成, 姚立蓉, 张宏, 马小乐, 李葆春, 王化俊, 孟亚雄. 大麦条纹病菌GH17基因家族的鉴定及表达分析[J]. 生物技术通报, 2025, 41(8): 146-154. |
| [10] | 康琴, 汪霞, 谌明洋, 徐静天, 陈诗兰, 廖平杨, 许文志, 吴卫, 徐东北. 薄荷UV-B受体基因McUVR8的克隆与表达分析[J]. 生物技术通报, 2025, 41(8): 255-266. |
| [11] | 曾丹, 黄园, 王健, 张艳, 刘庆霞, 谷荣辉, 孙庆文, 陈宏宇. 铁皮石斛bZIP转录因子家族全基因组鉴定及表达分析[J]. 生物技术通报, 2025, 41(8): 197-210. |
| [12] | 李凯月, 邓晓霞, 殷缘, 杜亚彤, 徐元静, 王竞红, 于耸, 蔺吉祥. 蓖麻LEA基因家族的鉴定和铝胁迫响应分析[J]. 生物技术通报, 2025, 41(7): 128-138. |
| [13] | 牛景萍, 赵婧, 郭茜, 王书宏, 赵晋忠, 杜维俊, 殷丛丛, 岳爱琴. 基于WGCNA鉴定大豆抗大豆花叶病毒NAC转录因子及其诱导表达分析[J]. 生物技术通报, 2025, 41(7): 95-105. |
| [14] | 韩燚, 侯昌林, 唐露, 孙璐, 谢晓东, 梁晨, 陈小强. 大麦HvERECTA基因的克隆及功能分析[J]. 生物技术通报, 2025, 41(7): 106-116. |
| [15] | 郭秀娟, 冯宇, 吴瑞香, 王利琴, 杨建春. Ca2+处理对胡麻种子萌发影响的转录组分析[J]. 生物技术通报, 2025, 41(7): 139-149. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||