








生物技术通报 ›› 2026, Vol. 42 ›› Issue (1): 230-240.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0546
王婷1( ), 孟义江2, 王晗1, 贾凯旋1, 乔晓宇1, 韩冰冰1, 刘晓清1, 葛淑俊1(
), 孟义江2, 王晗1, 贾凯旋1, 乔晓宇1, 韩冰冰1, 刘晓清1, 葛淑俊1( )
)
收稿日期:2025-05-27
出版日期:2026-01-26
发布日期:2026-02-04
通讯作者:
葛淑俊,女,教授,研究方向 :药用植物遗传育种;E-mail: gshj@hebau.edu.cn作者简介:王婷,女,硕士研究生,研究方向 :作物遗传资源研究与利用;E-mail: 13938172162@qq.com
基金资助:
WANG Ting1( ), MENG Yi-jiang2, WANG Han1, JIA Kai-xuan1, QIAO Xiao-yu1, HAN Bing-bing1, LIU Xiao-qing1, GE Shu-jun1(
), MENG Yi-jiang2, WANG Han1, JIA Kai-xuan1, QIAO Xiao-yu1, HAN Bing-bing1, LIU Xiao-qing1, GE Shu-jun1( )
)
Received:2025-05-27
Published:2026-01-26
Online:2026-02-04
摘要:
目的 紫菀(Aster tataricus L.)作为止咳润肺药材,黄酮类化合物是其主要活性成分,4-香豆酸辅酶A连接酶(4CL)为黄酮合成的关键限速酶,解析Ata4CL分子特征及在黄酮合成与抗旱中的双重功能,为紫菀药用成分优化及抗逆育种提供理论依据。 方法 硝酸铝比色法测定紫菀不同组织和生长阶段的总黄酮含量,克隆Ata4CL基因全长,结合生物信息学工具分析其结构特征,利用农杆菌介导转化烟草进行亚细胞定位,构建35S∶∶Ata4CL-6HA过表达载体并转化拟南芥,经干旱胁迫处理验证其功能。 结果 紫菀根部总黄酮呈双峰积累,为黄酮积累的主要部位,进入开花期的植株叶片和根茎中含量最高。克隆获得Ata4CL基因全长1 623 bp,编码540个氨基酸,分子量为59.04 kD,编码蛋白以无规则卷曲为主,含AAE保守结构域,定位于叶绿体;其氨基酸序列在菊科植物中高度保守,与小蓬草同源性高达96.48%;该基因在根部和叶片的表达量均呈“上升-下降-上升”的趋势,在开花植株中根部表达最强,盛花期的花中表达量最高。过表达Ata4CL的拟南芥总黄酮含量较野生型提高1.47-1.76倍,基因表达量上调5.41-12.23倍,同时在黄酮通路上下游合成基因表达量均上调;干旱胁迫下,转基因拟南芥比野生型存活率提高76.67%-86.67%,株高、根长及莲座直径均显著增加,SOD和POD活性增强,MDA含量降低。 结论 Ata4CL基因通过协同调控黄酮生物合成从而提高植物抗旱性,为紫菀药用成分优化及抗逆品种选育提供了关键分子靶点。
王婷, 孟义江, 王晗, 贾凯旋, 乔晓宇, 韩冰冰, 刘晓清, 葛淑俊. Ata4CL基因在紫菀黄酮合成与抗旱性功能研究[J]. 生物技术通报, 2026, 42(1): 230-240.
WANG Ting, MENG Yi-jiang, WANG Han, JIA Kai-xuan, QIAO Xiao-yu, HAN Bing-bing, LIU Xiao-qing, GE Shu-jun. Study on the Function of Ata4CL Gene in Flavonoid Synthesis and Drought Resistance in Aster tataricus[J]. Biotechnology Bulletin, 2026, 42(1): 230-240.
| 名称 Name | 上游引物 Forward primer sequence (5′‒3′) | 下游引物 Reverse primer sequence(5′‒3′ ) | 引物用途Application |
|---|---|---|---|
| Ata4CL | ATGGAATCACAAAAGGAAATCATTTTC | ATTTGGAACACCGGCTGCAA | 基因克隆 |
| Ata4CL-35s | GCCCAAGCTACGCGTC ATGGAATCACAAAAGGAAATCATTTTC | ATCGTATGGGTAACTAGAAC ATTTGGAACACCGGCTGCAA | 构建pGreen-Ata4CL-6HA载体 |
| Ata4CL-GFP | CGACTCTAGAGGATCC ATGGAATCACAAAAGGAAATCATTTTC | CTCACCATGGGCCCGGTACC ATTTGGAACACCGGCTGCAA | 构建pCameE-Ata4CL-GFP载体 |
| 35spro/Pgp2 | ACGAGGAGCATCGTGGAAAA | GCTCCGAGAAGTGCAAGCAG | 植株转基因阳性鉴定 |
| Ata4CL | GAAACCAACGAGGCGAGATT | TCGGAAATGTCGGGATGAGT | RT-qPCR |
| AtaActin | ACATCGCTCTTGACTATGAACAGG | ATGGCTGGAACAACACCTCTG | |
| Atactin2 | TCCATGAAACAACTTACAACTCCA | CGTACTCACTCTTTGAAATCCACA | |
| AtPAL1 | ACACTGTCTCTCAAGTGGCG | ACGTTGCGCTACAAGGATCA | |
| AtPAL2 | AGTCGTGAATCTTGGCGGAG | TCACACCGGCTCTTGAAGTC | |
| AtPAL4 | TACTTAGTCGCGCTTTGCCA | CTTGACGGATGTAGCTCCCC | |
| AtCHI | TCTTCGCTCTCTCCCCTACC | AGGTGACACACCGTTCTTCC |
表1 试验所用引物信息
Table 1 Information on the primers used in the experiment
| 名称 Name | 上游引物 Forward primer sequence (5′‒3′) | 下游引物 Reverse primer sequence(5′‒3′ ) | 引物用途Application |
|---|---|---|---|
| Ata4CL | ATGGAATCACAAAAGGAAATCATTTTC | ATTTGGAACACCGGCTGCAA | 基因克隆 |
| Ata4CL-35s | GCCCAAGCTACGCGTC ATGGAATCACAAAAGGAAATCATTTTC | ATCGTATGGGTAACTAGAAC ATTTGGAACACCGGCTGCAA | 构建pGreen-Ata4CL-6HA载体 |
| Ata4CL-GFP | CGACTCTAGAGGATCC ATGGAATCACAAAAGGAAATCATTTTC | CTCACCATGGGCCCGGTACC ATTTGGAACACCGGCTGCAA | 构建pCameE-Ata4CL-GFP载体 |
| 35spro/Pgp2 | ACGAGGAGCATCGTGGAAAA | GCTCCGAGAAGTGCAAGCAG | 植株转基因阳性鉴定 |
| Ata4CL | GAAACCAACGAGGCGAGATT | TCGGAAATGTCGGGATGAGT | RT-qPCR |
| AtaActin | ACATCGCTCTTGACTATGAACAGG | ATGGCTGGAACAACACCTCTG | |
| Atactin2 | TCCATGAAACAACTTACAACTCCA | CGTACTCACTCTTTGAAATCCACA | |
| AtPAL1 | ACACTGTCTCTCAAGTGGCG | ACGTTGCGCTACAAGGATCA | |
| AtPAL2 | AGTCGTGAATCTTGGCGGAG | TCACACCGGCTCTTGAAGTC | |
| AtPAL4 | TACTTAGTCGCGCTTTGCCA | CTTGACGGATGTAGCTCCCC | |
| AtCHI | TCTTCGCTCTCTCCCCTACC | AGGTGACACACCGTTCTTCC |
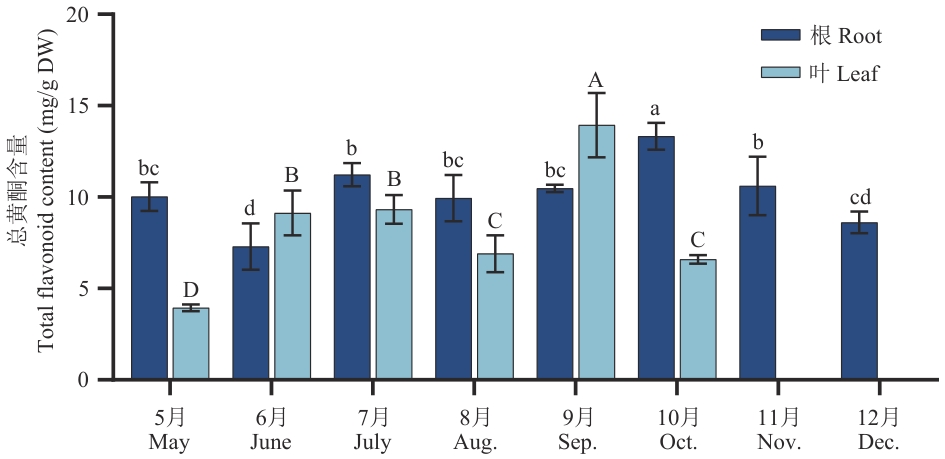
图1 紫菀不同发育阶段总黄酮含量大写字母和小写字母分别表示根部和叶片间差异显著。误差线为3次重复的平均数±标准差,Duncan检验差异显著水平小于0.05,下同
Fig. 1 Total flavonoid contents at different developmental stages of Aster tataricusCapital letters and lowercase letters respectively indicate significant differences between roots and leaves. Error bars indicate the average values of 3 repetitions ± standard deviation. Duncan test indicates a significant difference level less than 0.05, the same below
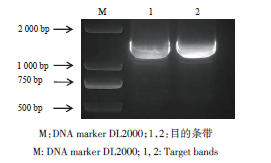
图3 紫菀Ata4CL基因克隆M:DNA marker DL2000;1,2:目的条带
Fig. 3 Cloning and characterization of the Ata4CL gene in A. tataricusM: DNA marker DL2000; 1, 2: Target bands
图7 Ata4CL编码蛋白的亚细胞定位A:过表达35S::Ata4CL-6HA载体构建;B:过表达拟南芥除草剂筛选;C:PCR验证过表达株系(M:2 000 bp标记;水:空白对照;WT:野生型;编号1-8:过表达植株)
Fig. 7 Subcellular localization of protein encoded by Ata4CLA: Constructing vector for overexpressing 35S::Ata4CL-6HA. B: Screening of transgenic Arabidopsis herbicides. C: PCR was used to validate transgenic lines (M: 2 000 bp label. Water: Blank control; WT: Wild type; Number 1-8: overexpressed plants)

图8 不同发育阶段和不同组织中Ata4CL的表达水平A:不同月份Ata4CL的表达量;B:花期开花植株不同组织Ata4CL的表达量;C:不同花时期Ata4CL的表达量
Fig. 8 Ata4CL expression patterns across tissues and developmental stagesA: The expressions of Ata4CL in different months; B: Expressions of Ata4CL in different tissues of bolting plants during flowering; C: Expressions of Ata4CL in different flower stages
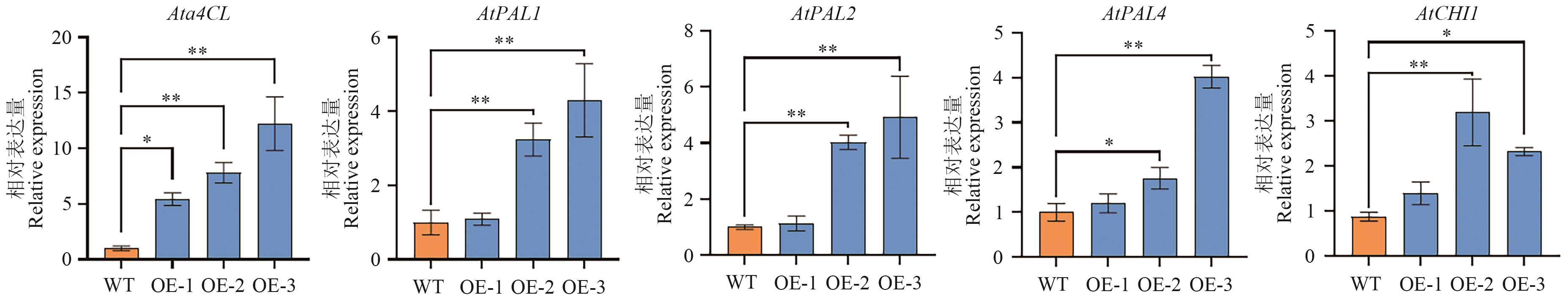
图11 过表达Ata4CL拟南芥中Ata4CL、AtPAL和AtCHI的相对表达量误差线表示3次重复平均值±标准差,采用t检验进行统计学分析(*P<0.05,**P<0.01)
Fig. 11 Relative expressions of Ata4CL, AtPAL and AtCHI in Ata4CL overexpressing A. thalianaThe error line indicates the mean ± standard deviation of three repetitions, and statistical analysis was performed using the t-test (*P<0.05, **P<0.01)
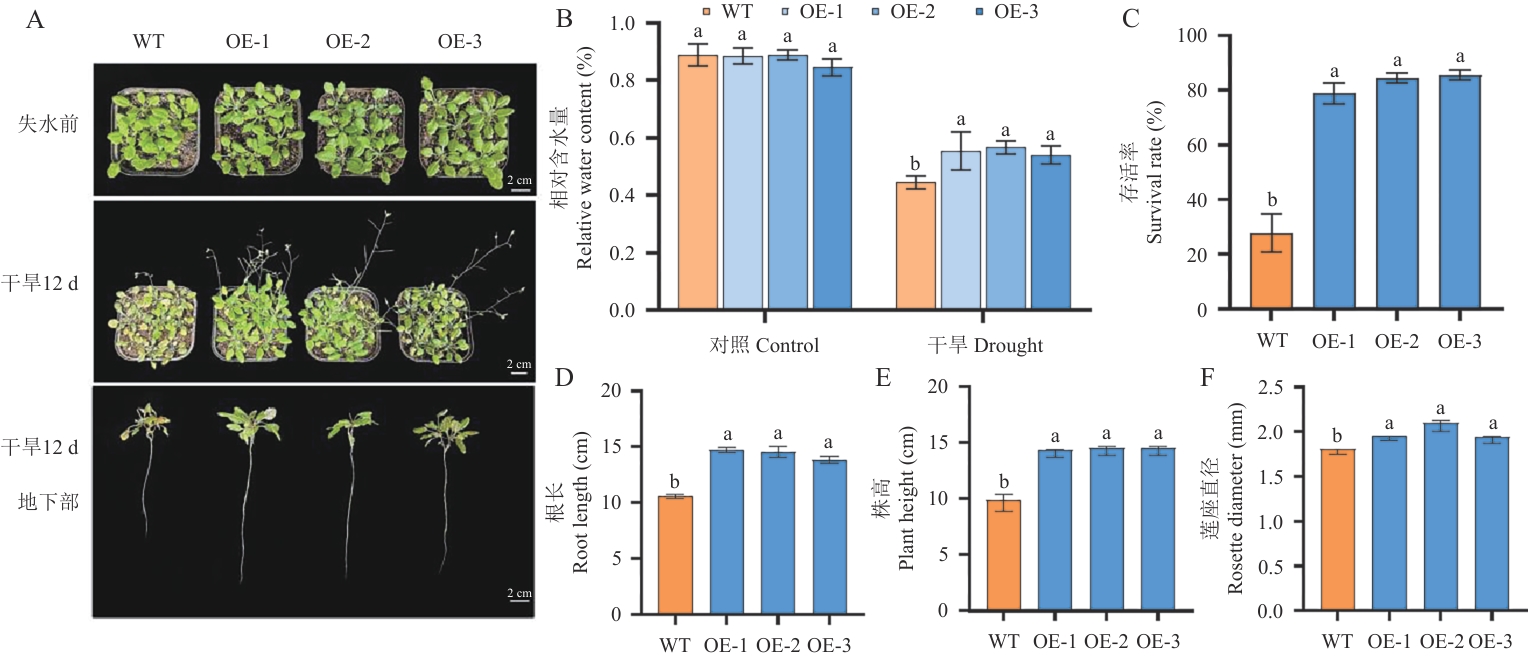
图12 Ata4CL过表达拟南芥的耐旱性分析A:成株期拟南芥干旱胁迫12 d前后生长表型及根系形态;B:干旱胁迫12 d前后WT和Ata4CL相对含水量;C-F:干旱12 d后WT和Ata4CL株高、存活率、根长及莲座直径
Fig. 12 Drought tolerance of Ata4CL-overexpressing A. thalianaA: Growth phenotype and root morphology of adult A. thaliana after 12 d of drought stress. B: Relative water content of WT and Ata4CL before and after 12 d of drought stress. C-F: Plant length, root length, rosette diameter and survival rate of WT and Ata4CL after 12 d of drought stress
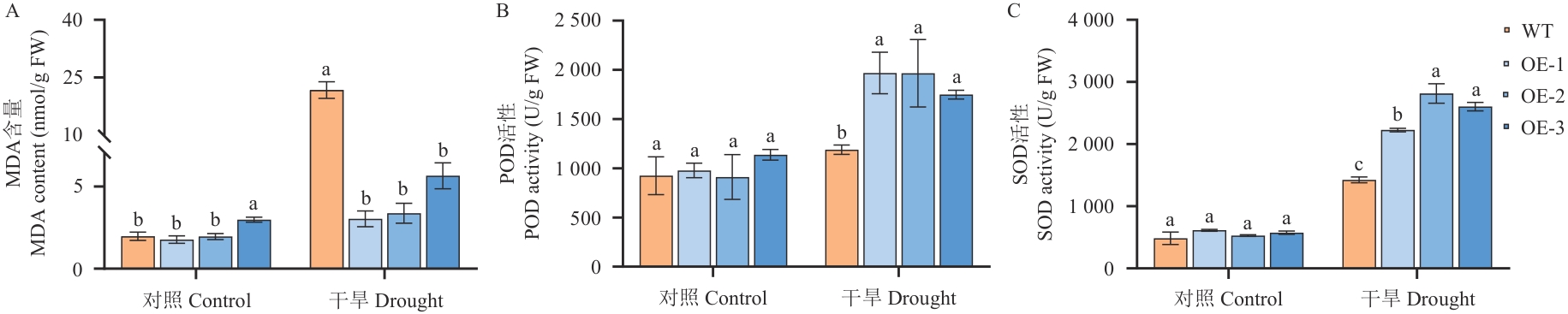
图13 干旱胁迫前后成株期拟南芥POD/SOD活性及MDA含量变化
Fig. 13 Changes in POD/SOD activities and MDA contents of A. thaliana at the mature plant stage before and after drought stress
| [1] | 国家药典委员会. 中华人民共和国药典-一部2020年版 [M]. 北京: 中国医药科技出版社, 2020. |
| Chinese Pharmacopoeia Commission. People’s republic of China (PRC) pharmacopoeia-No.1 department [M]. Beijing: China Medical Science Press, 2020. | |
| [2] | 蔡一杰, 史学礼, 刘红云, 等. 中药紫菀有效成分及药理作用 [J]. 中兽医医药杂志, 2023, 42(2): 39-42. |
| Cai YJ, Shi XL, Liu HY, et al. Effective components and pharmacological effects of Asteris Radix [J]. J Tradit Chin Vet Med, 2023, 42(2): 39-42. | |
| [3] | 陈志威, 张华敏, 王乐, 等. 新型冠状病毒肺炎验案3则 [J]. 中医杂志, 2020, 61(9): 745-748. |
| Chen ZW, Zhang HM, Wang L, et al. Three cases of coronavirus disease 2019 (COVID-19) report [J]. J Tradit Chin Med, 2020, 61(9): 745-748. | |
| [4] | 李园园, 李洪娟, 侯桂革, 等. 大孔吸附树脂纯化紫菀总黄酮工艺 [J]. 中成药, 2019, 41(3): 501-505. |
| Li YY, Li HJ, Hou GG, et al. Purification process for total flavonoids in Asteris Radix et Rhizoma with macroporous absorption resin [J]. Chin Tradit Pat Med, 2019, 41(3): 501-505. | |
| [5] | 赵彤, 邵瑾, 杨颖, 等. 藏紫菀不同溶剂提取物的体外抗氧化活性 [J]. 中成药, 2021, 43(4): 1103-1107. |
| Zhao T, Shao J, Yang Y, et al. Antioxidant activity of different solvent extracts from Aster Tibetan in vitro [J]. Chin Tradit Pat Med, 2021, 43(4): 1103-1107. | |
| [6] | Chen ZW, Tong L, Li SM, et al. Identification of major parent compounds and metabolites in bile, plasma and urine of rats after oral administration of Radix Scutellariae extract by UFLC-IT-TOF/MS[J]. J. Chin. Pharm. Sci, 2013, 22: 319-328. |
| [7] | Sun Y, Li L, Liao M, et al. A systematic data acquisition and mining strategy for chemical profiling of Aster tataricus rhizoma (Ziwan) by UHPLC-Q-TOF-MS and the corresponding anti-depressive activity screening[J]. Journal of Pharmaceutical and Biomedical Analysis, 2018, 154: 216-226. |
| [8] | Magalingam KB, Radhakrishnan AK, Haleagrahara N. Protective mechanisms of flavonoids in Parkinson’s disease[J]. Oxidative medicine and cellular longevity, 2015, 2015(1): 314560. |
| [9] | Marín L, Miguélez EM, Villar CJ, et al. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties[J]. BioMed research international, 2015, 2015(1): 905215. |
| [10] | Chen M, Zhai J, Zhang J, et al. Transcriptomic and physiological analyses of pigment accumulation in eucommia ulmoides Hongye[J]. Phyton (0031-9457), 2022, 91(5): 1027-1044. |
| [11] | Alkahtani M, Omer SA, El-Naggar MA, et al. Pathogenesis-related protein and phytoalexin induction against cucumber powdery mildew by elicitors[J]. International journal of plant pathology, 2011, 2(2): 63-71. |
| [12] | Li B, Fan R, Sun G, et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species[J]. Plant and Soil, 2021, 461: 389-405. |
| [13] | Quiroz A, Mendez L, Mutis A, et al. Antifeedant activity of red clover root isoflavonoids on Hylastinus obscurus[J]. Journal of soil science and plant nutrition, 2017, 17(1): 231-239. |
| [14] | 杨晓云, 杨智敏, 罗小娇, 等. 青稞4-香豆酸辅酶A连接酶基因4CL的克隆及表达分析[J]. 麦类作物学报, 2014, 34(12): 1603-1610. |
| Yang XY, Yang ZM, Luo XJ, et al. Cloning and expression analysis of 4-coumarate-CoA ligase gene 4CL in highland barley [J]. Journal of Triticeae Crops, 2014, 34(12): 1603-1610. | |
| [15] | Dixon RA, Steele CL. Flavonoids and isoflavonoids-a gold mine for metabolic engineering [J]. Trends Plant Sci, 1999, 4(10): 394-400. |
| [16] | Raes J, Rohde A, Christensen JH, et al. Genome-wide characterization of the lignification toolbox in Arabidopsis[J]. Plant Physiol, 2003, 133(3): 1051-1071. |
| [17] | Gui JS, Shen JH, Li LG. Functional characterization of evolutionarily divergent 4-coumarate: coenzyme a ligases in rice [J]. Plant Physiol, 2011, 157(2): 574-586. |
| [18] | Carocha V, Soler M, Hefer C, et al. Genome-wide analysis of the lignin toolbox of Eucalyptus grandis [J]. New Phytol, 2015, 206(4): 1297-1313. |
| [19] | Cao YP, Li XX, Jiang L. Integrative analysis of the core fruit lignification toolbox in pear reveals targets for fruit quality bioengineering [J]. Biomolecules, 2019, 9(9): 504. |
| [20] | Schneider K, Hövel K, Witzel K, et al. The substrate specificity-determining amino acid code of 4-coumarate: CoA ligase [J]. Proc Natl Acad Sci U S A, 2003, 100(14): 8601-8606. |
| [21] | Ehlting J, Büttner D, Wang Q, et al. Three 4-coumarate: coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms [J]. Plant J, 1999, 19(1): 9-20. |
| [22] | Lindermayr C, Möllers B, Fliegmann J, et al. Divergent members of a soybean (Glycine max L.) 4-coumarate: coenzyme A ligase gene family: Primary structures, catalytic properties, and differential expression [J]. Eur J Biochem, 2002, 269(4): 1304-1315. |
| [23] | Sun SC, Xiong XP, Zhang XL, et al. Characterization of the Gh4CL gene family reveals a role of Gh4CL7 in drought tolerance [J]. BMC Plant Biol, 2020, 20(1): 125. |
| [24] | Liu MX, Liu YT, Hu W, et al. Transcriptome and metabolome analyses reveal the regulatory role of MdPYL9 in drought resistance in apple [J]. BMC Plant Biol, 2024, 24(1): 452. |
| [25] | Hossain MA, Mizanur Rahman SM. Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple [J]. Food Res Int, 2011, 44(3): 672-676. |
| [26] | Wu WH, Xu RY, Liu N, et al. The physiological responses of maize seedlings with different amylose content to drought stress [J]. J Plant Growth Regul, 2023, 42(5): 3291-3301. |
| [27] | Xiao N, Ma HZ, Wang WX, et al. Overexpression of ZmSUS1 increased drought resistance of maize (Zea mays L.) by regulating sucrose metabolism and soluble sugar content [J]. Planta, 2024, 259(2): 43. |
| [28] | Fu F, Qin HT, Xin YJ, et al. Characterization of 4-coumarate CoA ligase (4CL) gene family and functional study of Sm4CL2/3/7/9 in Salvia miltiorrhiza [J]. Funct Integr Genom, 2025, 25(1): 35. |
| [29] | 申晚霞, 王志彬, 薛杨, 等. 柑橘4CL基因家族的结构及其功能分析 [J]. 园艺学报, 2019, 46(6): 1068-1078. |
| Shen WX, Wang ZB, Xue Y, et al. Characterization of 4-coumarate: CoA ligase(4CL)gene family in Citrus [J]. Acta Hortic Sin, 2019, 46(6): 1068-1078. | |
| [30] | Yan C, Li CL, Jiang MC, et al. Systematic characterization of gene families and functional analysis of PvRAS3 and PvRAS4 involved in rosmarinic acid biosynthesis in Prunella vulgaris [J]. Front Plant Sci, 2024, 15: 1374912. |
| [31] | 范丙友, 陆海, 蒋湘宁. 维管植物4-香豆酸: 辅酶A连接酶(4CL)研究进展 [J]. 林业科学, 2007, 43(2): 96-103. |
| Fan BY, Lu H, Jiang XN. Review on 4-coumarate: coenzyme a ligase (4CL) of vascular plants [J]. Sci Silvae Sin, 2007, 43(2): 96-103. | |
| [32] | 谢海娟, 范希德, 叶广继, 等. 马铃薯St4CL的克隆及表达分析 [J]. 生物技术通报, 2019, 35(11): 1-8. |
| Xie HJ, Fan XD, Ye GJ, et al. Cloning and expression analysis of St4CL gene in Solanum tuberosum [J]. Biotechnol Bull, 2019, 35(11): 1-8. | |
| [33] | Ma ZH, Nan XT, Li WF, et al. Comprehensive genomic identification and expression analysis 4CL gene family in apple [J]. Gene, 2023, 858: 147197. |
| [34] | Kao YY, Harding SA, Tsai CJ. Differential expression of two distinct phenylalanine ammonia-lyase genes in condensed tannin-accumulating and lignifying cells of quaking aspen [J]. Plant Physiol, 2002, 130(2): 796-807. |
| [35] | Wang CH, Yu J, Cai YX, et al. Characterization and functional analysis of 4-coumarate: CoA ligase genes in mulberry [J]. PLoS One, 2016, 11(5): e0155814. |
| [36] | Ma JY, Zuo DJ, Zhang XD, et al. Genome-wide identification analysis of the 4-Coumarate: CoA ligase (4CL) gene family expression profiles in Juglans regia and its wild relatives J. Mandshurica resistance and salt stress [J]. BMC Plant Biol, 2024, 24(1): 211. |
| [37] | Li SS, Chang Y, Li B, et al. Functional analysis of 4-coumarate: CoA ligase from Dryopteris fragrans in transgenic tobacco enhances lignin and flavonoids [J]. Genet Mol Biol, 2020, 43(2): e20180355. |
| [38] | Wang YB, Liu W, Li W, et al. Integrative analysis of metabolome and transcriptome reveals regulatory mechanisms of flavonoid biosynthesis in soybean under salt stress [J]. Front Plant Sci, 2024, 15: 1415867. |
| [39] | Fan JY, Luo ZP, Wang YK, et al. Maize 4-coumarate coenzyme A ligase Zm4CL-like9 gene positively regulates drought stress response in Arabidopsis thaliana [J]. GM Crops Food, 2025, 16(1): 199-215. |
| [1] | 刘佳丽, 宋经荣, 赵文宇, 张馨元, 赵子洋, 曹一博, 张凌云. 蓝莓R2R3-MYB基因鉴定及类黄酮调控基因表达分析[J]. 生物技术通报, 2025, 41(9): 124-138. |
| [2] | 翟莹, 计俊杰, 陈炯辛, 于海伟, 李珊珊, 赵艳, 马天意. 异源过表达大豆GmNF-YB24提高转基因烟草抗旱性[J]. 生物技术通报, 2025, 41(8): 137-145. |
| [3] | 田琴, 刘奎, 吴翔纬, 纪媛媛, 曹一博, 张凌云. 转录因子VcMYB17调控蓝莓抗旱性的功能研究[J]. 生物技术通报, 2025, 41(4): 198-210. |
| [4] | 韩凯, 周永顺, 张凯月, 王路, 高剑峰, 陈福龙. 三株小球藻抗旱性能评价[J]. 生物技术通报, 2024, 40(8): 244-254. |
| [5] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [6] | 翟莹, 李铭杨, 张军, 赵旭, 于海伟, 李珊珊, 赵艳, 张梅娟, 孙天国. 异源表达大豆转录因子GmNF-YA19提高转基因烟草抗旱性[J]. 生物技术通报, 2023, 39(5): 224-232. |
| [7] | 罗艳菊, 谢林艳, 邹清林, 李四杰, 刘涵, 刘鲁峰, 何丽莲, 李富生. 内生菌对干旱胁迫下甘蔗的生理响应及抗旱性评价[J]. 生物技术通报, 2023, 39(12): 219-228. |
| [8] | 陈光, 李佳, 杜瑞英, 王旭. pOsHAK1:OsFLN2提高水稻的糖代谢水平和抗旱性[J]. 生物技术通报, 2022, 38(8): 92-100. |
| [9] | 马旭辉, 陈茹梅, 柳小庆, 赵军, 张霞. 褪黑素对玉米幼苗根系发育和抗旱性的影响[J]. 生物技术通报, 2021, 37(2): 1-14. |
| [10] | 张云川, 林熠轩, 曹新文, 王海楠, 闫洁. 橡胶草TkDREB2基因的克隆以及在烟草中的抗旱功能分析[J]. 生物技术通报, 2021, 37(11): 212-224. |
| [11] | 袁闯, 许兴, 唐三元, 毛桂莲, 朱林. 孕穗期甜高粱耐旱性鉴定[J]. 生物技术通报, 2019, 35(12): 1-9. |
| [12] | 李瑞雪,孙任洁,汪泰初,陈丹丹,李荣芳,李龙,赵卫国,. 植物抗旱性鉴定评价方法及抗旱机制研究进展[J]. 生物技术通报, 2017, 33(7): 40-48. |
| [13] | 刘延娟, 杨玉梅, 刘娴, 高艳秀, 袁航, 龚明, 邹竹荣. 甲基戊糖梭菌膜整合焦磷酸酶提高转基因烟草的抗旱性[J]. 生物技术通报, 2017, 33(6): 81-88. |
| [14] | 李志亮, 吴忠义, 杨清, 张希太, 叶嘉, 邢浩春, 陈建中, 黄丛林. 基因枪转化法对抗旱基因导入玉米的研究[J]. 生物技术通报, 2016, 32(5): 61-68. |
| [15] | 黎玉顺, 刘逸泠, 刘步仓, 穆建强, 祝建波. 新疆雪莲水孔蛋白sikPIP1基因的克隆与功能分析[J]. 生物技术通报, 2015, 31(9): 97-105. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||