








生物技术通报 ›› 2026, Vol. 42 ›› Issue (1): 170-183.doi: 10.13560/j.cnki.biotech.bull.1985.2025-1026
陈静欢( ), 房国楠, 朱文豪, 叶广继, 苏旺, 贺苗苗, 杨生龙(
), 房国楠, 朱文豪, 叶广继, 苏旺, 贺苗苗, 杨生龙( ), 周云(
), 周云( )
)
收稿日期:2025-09-26
出版日期:2026-01-26
发布日期:2026-02-04
通讯作者:
杨生龙,男,博士,助理研究员,研究方向 :马铃薯遗传育种;E-mail: ysl890224@163.com作者简介:陈静欢,女,硕士研究生,研究方向 :马铃薯遗传育种;E-mail: 18393899265@163.com
基金资助:
CHEN Jing-huan( ), FANG Guo-nan, ZHU Wen-hao, YE Guang-ji, SU Wang, HE Miao-miao, YANG Sheng-long(
), FANG Guo-nan, ZHU Wen-hao, YE Guang-ji, SU Wang, HE Miao-miao, YANG Sheng-long( ), ZHOU Yun(
), ZHOU Yun( )
)
Received:2025-09-26
Published:2026-01-26
Online:2026-02-04
摘要:
目的 明晰马铃薯种质资源的淀粉表征及相关基因表达差异,为品种改良选育提供优良种质资源,为揭示淀粉合成调控机制奠定研究基础。 方法 以100份马铃薯种质资源为材料,利用相关性分析、主成分分析及聚类分析等方法综合评价其淀粉含量、直链淀粉含量、支链淀粉含量、支/直比、还原糖含量和单株产量。 结果 相关性分析表明,直链淀粉含量与支/直比和支链淀粉含量呈极显著负相关,还原糖、淀粉含量与单株产量呈显著正相关。通过主成分分析共提取出3个主成分,分别为支链淀粉含量(支/直比)、还原糖含量、淀粉含量,累积贡献率为87.84%。聚类分析将马铃薯种质资源分为三类:cluster Ⅰ有32份,主要特征为晚熟高淀粉;cluster Ⅱ有40份,主要特征为早熟和淀粉含量中等;cluster Ⅲ有28份,主要特征为早熟低淀粉。为了揭示马铃薯品种间淀粉含量差异的分子机制,分析淀粉合成通路中8个结构基因(Susy4、AGPase、PTST1等)在高淀粉(青薯2号、大西洋、393034.7和深眼窝)和低淀粉(FBA-1、北薯1号、2017ch-1和720018)品种中的表达,结果显示,Susy4和PTST1表达量在决定淀粉含量方面起到关键作用。 结论 明确100份马铃薯种质资源的淀粉性状特征与3个类群划分,筛选出高淀粉含量的资源4份(青薯2号、大西洋、393034.7、深眼窝),证实Susy4和PTST1基因是调控淀粉含量的核心基因,为马铃薯品种选育与淀粉合成机制解析提供理论与材料支撑。
陈静欢, 房国楠, 朱文豪, 叶广继, 苏旺, 贺苗苗, 杨生龙, 周云. 马铃薯种质资源淀粉表征及相关基因表达分析[J]. 生物技术通报, 2026, 42(1): 170-183.
CHEN Jing-huan, FANG Guo-nan, ZHU Wen-hao, YE Guang-ji, SU Wang, HE Miao-miao, YANG Sheng-long, ZHOU Yun. Starch Characterization and Related Gene Expression Analysis of Potato Germplasm Resources[J]. Biotechnology Bulletin, 2026, 42(1): 170-183.
| 主要性状 Principal traits | 最大值 Max | 最小值 Min | 极差 Range | 平均值 Mean | 标准差 Deviation | 变异系数 CV |
|---|---|---|---|---|---|---|
| 淀粉含量 Starch content (%) | 18.77 | 8.29 | 10.48 | 13.70 | 2.36 | 17.20 |
| 直链淀粉含量 Amylose content (%) | 24.90 | 21.31 | 3.59 | 22.71 | 0.65 | 2.87 |
| 支链淀粉含量 Amylopectin content (%) | 78.69 | 75.10 | 3.59 | 77.29 | 0.65 | 0.84 |
| 支/直比 Ratio of amylopectin/amylose | 3.69 | 3.02 | 0.68 | 3.41 | 0.13 | 3.69% |
| 还原糖 Reducing sugar (%) | 1.15 | 0.02 | 1.13 | 0.60 | 0.24 | 40.25 |
| 单株产量 Yield per plant (kg) | 1.73 | 0.05 | 1.68 | 0.68 | 0.35 | 50.97% |
表1 100份马铃薯种质资源主要性状统计分析
Table 1 Statistical analysis of 100 potato germplasm resources for major traits
| 主要性状 Principal traits | 最大值 Max | 最小值 Min | 极差 Range | 平均值 Mean | 标准差 Deviation | 变异系数 CV |
|---|---|---|---|---|---|---|
| 淀粉含量 Starch content (%) | 18.77 | 8.29 | 10.48 | 13.70 | 2.36 | 17.20 |
| 直链淀粉含量 Amylose content (%) | 24.90 | 21.31 | 3.59 | 22.71 | 0.65 | 2.87 |
| 支链淀粉含量 Amylopectin content (%) | 78.69 | 75.10 | 3.59 | 77.29 | 0.65 | 0.84 |
| 支/直比 Ratio of amylopectin/amylose | 3.69 | 3.02 | 0.68 | 3.41 | 0.13 | 3.69% |
| 还原糖 Reducing sugar (%) | 1.15 | 0.02 | 1.13 | 0.60 | 0.24 | 40.25 |
| 单株产量 Yield per plant (kg) | 1.73 | 0.05 | 1.68 | 0.68 | 0.35 | 50.97% |
| 性状 Traits | 主成分1 Principal component 1 | 主成分2 Principal component 2 | 主成分3 Principal component 3 |
|---|---|---|---|
| 淀粉含量 Starch content | -0.04 | 0.87 | -0.07 |
| 直链淀粉含量 Amylose content | -0.99 | 0.02 | 0.07 |
| 支链淀粉含量 Amylopectin content | 0.99 | -0.02 | -0.07 |
| 支/直比 Ratio of amylopectin/amylose | 0.99 | -0.02 | -0.07 |
| 还原糖 Reducing sugar | -0.11 | 0.12 | 0.95 |
| 单株产量 Yield per plant | 0.01 | 0.69 | 0.31 |
| 特征值 Eigenvalue | 3.06 | 1.39 | 0.82 |
| 方差贡献率 Contribution rate (%) | 51.01 | 23.14 | 13.69 |
| 累计方差贡献率 Cumulative contribution rate (%) | 51.01 | 74.15 | 87.84 |
表2 100份马铃薯种质资源6个性状的主成分分析
Table 2 Principal component analysis of six traits in 100 potato germplasm resources
| 性状 Traits | 主成分1 Principal component 1 | 主成分2 Principal component 2 | 主成分3 Principal component 3 |
|---|---|---|---|
| 淀粉含量 Starch content | -0.04 | 0.87 | -0.07 |
| 直链淀粉含量 Amylose content | -0.99 | 0.02 | 0.07 |
| 支链淀粉含量 Amylopectin content | 0.99 | -0.02 | -0.07 |
| 支/直比 Ratio of amylopectin/amylose | 0.99 | -0.02 | -0.07 |
| 还原糖 Reducing sugar | -0.11 | 0.12 | 0.95 |
| 单株产量 Yield per plant | 0.01 | 0.69 | 0.31 |
| 特征值 Eigenvalue | 3.06 | 1.39 | 0.82 |
| 方差贡献率 Contribution rate (%) | 51.01 | 23.14 | 13.69 |
| 累计方差贡献率 Cumulative contribution rate (%) | 51.01 | 74.15 | 87.84 |
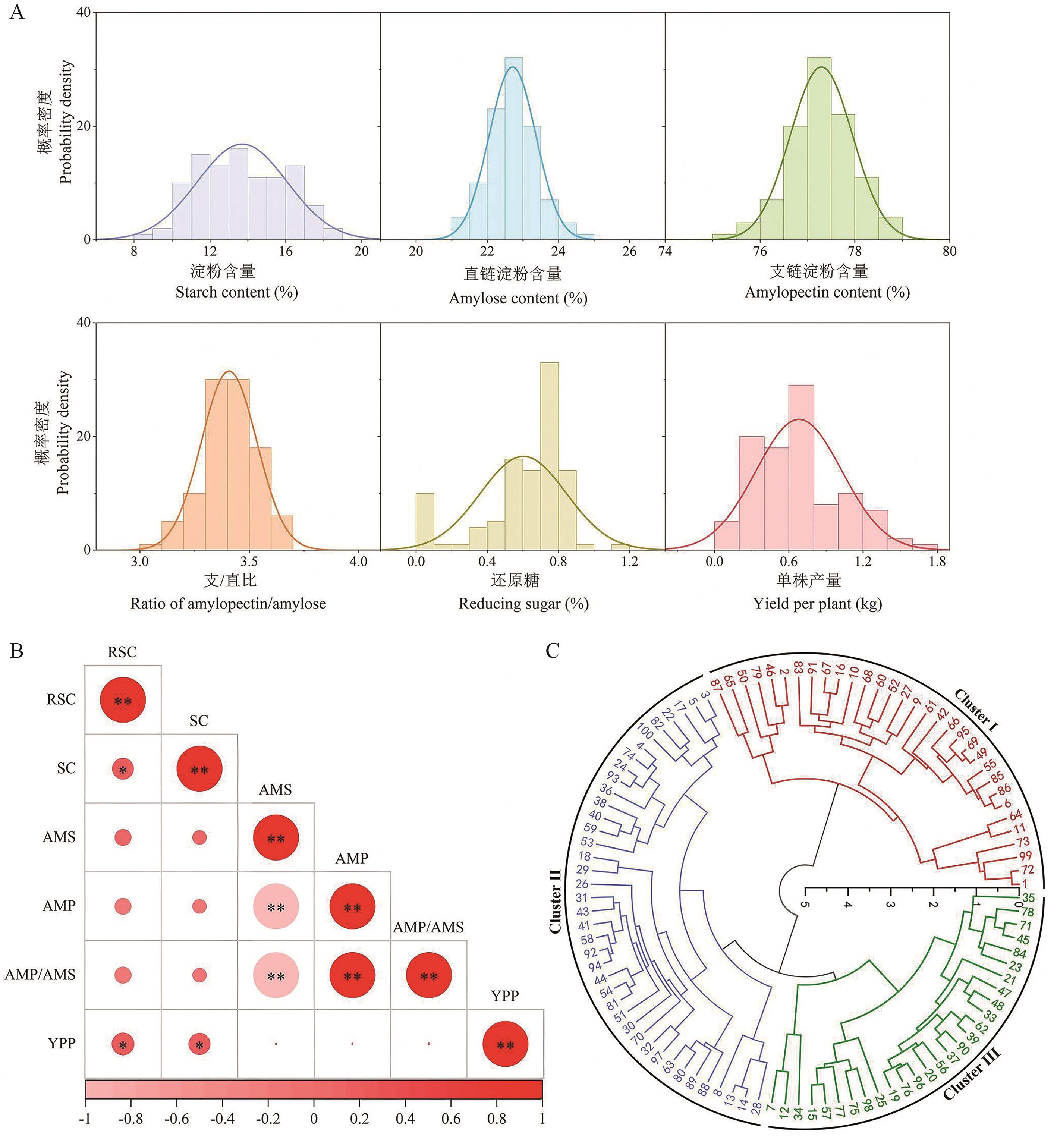
图 1 100 份马铃薯种质资源主要性状分布特征、指标相关性与聚类分析A:100份马铃薯种质资源主要性状的正态分布。B:100份马铃薯种质资源各单项指标的相关性分析。RSC:还原糖;SC:淀粉含量;AMS:直链淀粉含量;AMP:支链淀粉含量;AMP/AMS:支/直比;YPP:单株产量。*和**分别表示在0.05和0.01水平上显著相关。C:100份马铃薯种质资源的聚类分析。外圈数字对应于附表1中的材料序号
Fig. 1 Distribution characteristics, correlation analysis and clustering analysis of key traits in 100 potato germplasm resourcesA: Normal distribution of major traits in 100 potato germplasm resources. B: Correlation analysis of 100 potato germplasm resources for each individual index. RSC: Reducing sugar content. SC: Starch content. AMS: Amylose content. AMP: Amylopectin content. AMP/AMS: Ratio of amylopectin/amylose. YPP: Yield per plant. * and ** indicate significant correlation at the 0.05 and 0.01 levels, respectively. C: Cluster analysis of 100 potato germplasm resources. The numbers on the outer circle correspond to the material numbers in supplementary Table 1
排序 Rank | 综合值得分 Comprehensive score | 品种名称 Variety | 排序 Rank | 综合值得分 Comprehensive score | 品种名称 Variety |
|---|---|---|---|---|---|
| 1 | 0.83 | 庄薯4号 | 51 | 0.55 | 晋薯4号 |
| 2 | 0.81 | UK3 | 52 | 0.55 | 中227 |
| 3 | 0.79 | CANCHAN-INIA | 53 | 0.55 | Andina |
| 4 | 0.79 | 139 | 54 | 0.54 | 388972.22 |
| 5 | 0.79 | 昆塔 | 55 | 0.54 | 06-26-11 |
| 6 | 0.78 | Peru08-1 | 56 | 0.53 | KY73 |
| 7 | 0.78 | Belmont | 57 | 0.53 | 大西洋 |
| 8 | 0.75 | VC75.3 | 58 | 0.53 | PHU1.22 |
| 9 | 0.73 | Peru08-3 | 59 | 0.53 | NX9 |
| 10 | 0.73 | Israel1 | 60 | 0.52 | KW-45 |
| 11 | 0.73 | 中薯5号 | 61 | 0.52 | I46 |
| 12 | 0.72 | 393140-3 | 62 | 0.52 | LB |
| 13 | 0.72 | 382171.1 | 63 | 0.52 | B18 |
| 14 | 0.70 | E23 | 64 | 0.51 | F8701 |
| 15 | 0.69 | C-50 | 65 | 0.51 | 中心02 |
| 16 | 0.68 | MEX-750.821 | 66 | 0.50 | Shepody |
| 17 | 0.67 | 393034.7 | 67 | 0.50 | J10828 |
| 18 | 0.66 | 青薯2号 | 68 | 0.50 | 早大白 |
| 19 | 0.66 | 呼自278 | 69 | 0.50 | 中心19 |
| 20 | 0.66 | 呼5号 | 70 | 0.49 | 中甸红 |
| 21 | 0.66 | 下寨65 | 71 | 0.49 | Calwhite |
| 22 | 0.66 | 福克212 | 72 | 0.48 | KB |
| 23 | 0.65 | R8 | 73 | 0.48 | Fundy |
| 24 | 0.64 | 中心06 | 74 | 0.47 | 720018 |
| 25 | 0.63 | DR-10 | 75 | 0.47 | S25 |
| 26 | 0.63 | CIP10-2 | 76 | 0.46 | GUS1 |
| 27 | 0.63 | RUSSIA2 | 77 | 0.46 | 加拿大红 |
| 28 | 0.63 | 兰旦 | 78 | 0.44 | Tylua |
| 29 | 0.63 | Vester | 79 | 0.44 | 高原4号 |
| 30 | 0.63 | 深眼窝 | 80 | 0.44 | NEGRA QJOSA |
| 31 | 0.62 | CHALLINA | 81 | 0.43 | NE303 |
| 32 | 0.62 | Peru08-2 | 82 | 0.43 | 陇薯8号 |
| 33 | 0.61 | 乌盟684 | 83 | 0.43 | 北薯1号 |
| 34 | 0.61 | KY28 | 84 | 0.41 | 闽薯1号 |
| 35 | 0.60 | 385499.1 | 85 | 0.41 | Colmo |
| 36 | 0.60 | 中联红 | 86 | 0.40 | 民薯2号 |
| 37 | 0.60 | 冀张薯12 | 87 | 0.40 | J10622 |
| 38 | 0.59 | 加拿大7637 | 88 | 0.39 | Jk5 |
| 39 | 0.59 | 2017ch-2 | 89 | 0.39 | 395015.6 |
| 40 | 0.59 | AC0338 | 90 | 0.38 | 388611.22 |
| 41 | 0.58 | 男爵薯 | 91 | 0.36 | 2017ch-1 |
| 42 | 0.58 | F70021-1 | 92 | 0.36 | NX162 |
| 43 | 0.58 | 甘谷红 | 93 | 0.36 | DANVA |
| 44 | 0.58 | 石延97-7 | 94 | 0.36 | FBA-1 |
| 45 | 0.57 | 2017ch-5 | 95 | 0.35 | 加大白 |
| 46 | 0.57 | 杂单4号 | 96 | 0.34 | cy-Ⅲ-29 |
| 47 | 0.56 | 青薯9号 | 97 | 0.30 | 抗疫白 |
| 48 | 0.56 | Wilia | 98 | 0.29 | 肯德 |
| 49 | 0.55 | BC37 | 99 | 0.28 | 费乌瑞它 |
| 50 | 0.55 | H2 | 100 | 0.23 | 克11 |
表3 100份马铃薯种质资源综合得分及排名
Table 3 Comprehensive scores and ranking of 100 potato germplasm resources
排序 Rank | 综合值得分 Comprehensive score | 品种名称 Variety | 排序 Rank | 综合值得分 Comprehensive score | 品种名称 Variety |
|---|---|---|---|---|---|
| 1 | 0.83 | 庄薯4号 | 51 | 0.55 | 晋薯4号 |
| 2 | 0.81 | UK3 | 52 | 0.55 | 中227 |
| 3 | 0.79 | CANCHAN-INIA | 53 | 0.55 | Andina |
| 4 | 0.79 | 139 | 54 | 0.54 | 388972.22 |
| 5 | 0.79 | 昆塔 | 55 | 0.54 | 06-26-11 |
| 6 | 0.78 | Peru08-1 | 56 | 0.53 | KY73 |
| 7 | 0.78 | Belmont | 57 | 0.53 | 大西洋 |
| 8 | 0.75 | VC75.3 | 58 | 0.53 | PHU1.22 |
| 9 | 0.73 | Peru08-3 | 59 | 0.53 | NX9 |
| 10 | 0.73 | Israel1 | 60 | 0.52 | KW-45 |
| 11 | 0.73 | 中薯5号 | 61 | 0.52 | I46 |
| 12 | 0.72 | 393140-3 | 62 | 0.52 | LB |
| 13 | 0.72 | 382171.1 | 63 | 0.52 | B18 |
| 14 | 0.70 | E23 | 64 | 0.51 | F8701 |
| 15 | 0.69 | C-50 | 65 | 0.51 | 中心02 |
| 16 | 0.68 | MEX-750.821 | 66 | 0.50 | Shepody |
| 17 | 0.67 | 393034.7 | 67 | 0.50 | J10828 |
| 18 | 0.66 | 青薯2号 | 68 | 0.50 | 早大白 |
| 19 | 0.66 | 呼自278 | 69 | 0.50 | 中心19 |
| 20 | 0.66 | 呼5号 | 70 | 0.49 | 中甸红 |
| 21 | 0.66 | 下寨65 | 71 | 0.49 | Calwhite |
| 22 | 0.66 | 福克212 | 72 | 0.48 | KB |
| 23 | 0.65 | R8 | 73 | 0.48 | Fundy |
| 24 | 0.64 | 中心06 | 74 | 0.47 | 720018 |
| 25 | 0.63 | DR-10 | 75 | 0.47 | S25 |
| 26 | 0.63 | CIP10-2 | 76 | 0.46 | GUS1 |
| 27 | 0.63 | RUSSIA2 | 77 | 0.46 | 加拿大红 |
| 28 | 0.63 | 兰旦 | 78 | 0.44 | Tylua |
| 29 | 0.63 | Vester | 79 | 0.44 | 高原4号 |
| 30 | 0.63 | 深眼窝 | 80 | 0.44 | NEGRA QJOSA |
| 31 | 0.62 | CHALLINA | 81 | 0.43 | NE303 |
| 32 | 0.62 | Peru08-2 | 82 | 0.43 | 陇薯8号 |
| 33 | 0.61 | 乌盟684 | 83 | 0.43 | 北薯1号 |
| 34 | 0.61 | KY28 | 84 | 0.41 | 闽薯1号 |
| 35 | 0.60 | 385499.1 | 85 | 0.41 | Colmo |
| 36 | 0.60 | 中联红 | 86 | 0.40 | 民薯2号 |
| 37 | 0.60 | 冀张薯12 | 87 | 0.40 | J10622 |
| 38 | 0.59 | 加拿大7637 | 88 | 0.39 | Jk5 |
| 39 | 0.59 | 2017ch-2 | 89 | 0.39 | 395015.6 |
| 40 | 0.59 | AC0338 | 90 | 0.38 | 388611.22 |
| 41 | 0.58 | 男爵薯 | 91 | 0.36 | 2017ch-1 |
| 42 | 0.58 | F70021-1 | 92 | 0.36 | NX162 |
| 43 | 0.58 | 甘谷红 | 93 | 0.36 | DANVA |
| 44 | 0.58 | 石延97-7 | 94 | 0.36 | FBA-1 |
| 45 | 0.57 | 2017ch-5 | 95 | 0.35 | 加大白 |
| 46 | 0.57 | 杂单4号 | 96 | 0.34 | cy-Ⅲ-29 |
| 47 | 0.56 | 青薯9号 | 97 | 0.30 | 抗疫白 |
| 48 | 0.56 | Wilia | 98 | 0.29 | 肯德 |
| 49 | 0.55 | BC37 | 99 | 0.28 | 费乌瑞它 |
| 50 | 0.55 | H2 | 100 | 0.23 | 克11 |
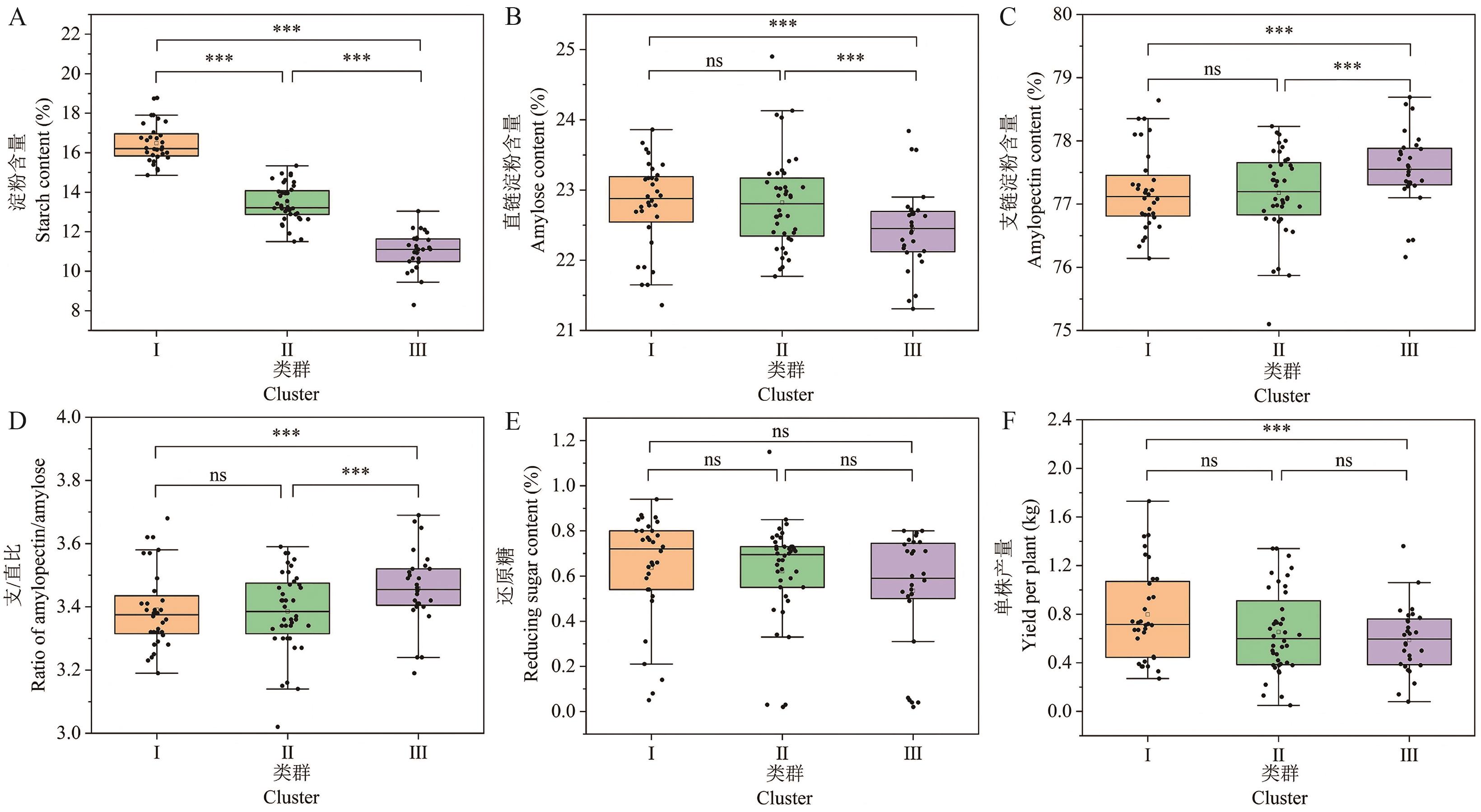
图2 100份马铃薯种质资源的相关性状差异显著性分析*、**和***分别表示0.05、0.01和0.001水平显著,ns表示不显著
Fig. 2 Significance analysis of differences in related traits among 100 potato germplasm resources*, ** and *** denote the significance at 0.05, 0.01 and 0.001 levels, respectively, and ns denotes non-significance
类群 Cluster | 淀粉 Starch content (%) | 直链淀粉 Amylose content (%) | 支链淀粉 Amylopectin content (%) | 支/直比 Ratio of amylopectin/amylose | 还原糖 Reducing sugar (%) | 单株产量 Yield per plant (kg) |
|---|---|---|---|---|---|---|
| Ⅰ | 16.48±0.98a | 22.79±0.62a | 77.21±0.62b | 3.39±0.12b | 0.64±0.24a | 0.80±0.38a |
| Ⅱ | 13.43±0.93b | 22.82±0.67a | 77.18±0.67b | 3.39±0.13b | 0.62±0.22a | 0.65±0.34ab |
| Ⅲ | 11.02±0.95c | 22.45±0.58b | 77.55±0.58a | 3.46±0.12ba | 0.53±0.26a | 0.59±0.27b |
表4 三种类群中7个性状的平均值比较
Table 4 Comparison of mean values of seven traits in three clusters
类群 Cluster | 淀粉 Starch content (%) | 直链淀粉 Amylose content (%) | 支链淀粉 Amylopectin content (%) | 支/直比 Ratio of amylopectin/amylose | 还原糖 Reducing sugar (%) | 单株产量 Yield per plant (kg) |
|---|---|---|---|---|---|---|
| Ⅰ | 16.48±0.98a | 22.79±0.62a | 77.21±0.62b | 3.39±0.12b | 0.64±0.24a | 0.80±0.38a |
| Ⅱ | 13.43±0.93b | 22.82±0.67a | 77.18±0.67b | 3.39±0.13b | 0.62±0.22a | 0.65±0.34ab |
| Ⅲ | 11.02±0.95c | 22.45±0.58b | 77.55±0.58a | 3.46±0.12ba | 0.53±0.26a | 0.59±0.27b |
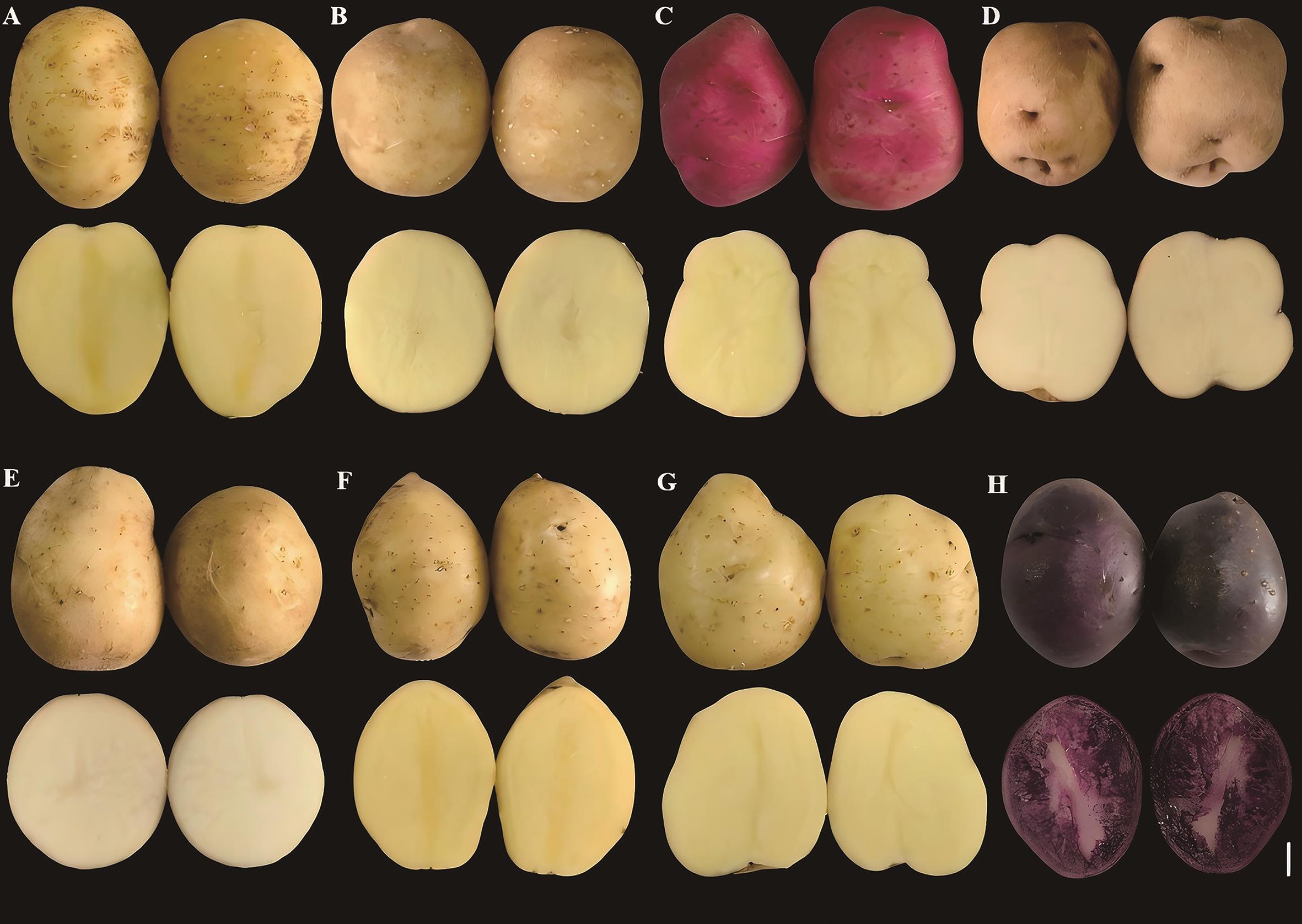
图3 高淀粉与低淀粉品种的块茎表型A:青薯2号;B:大西洋;C:393034.7;D:深眼窝;E:720018;F:2017ch-1;G:北薯1号;H:FBA-1;比例尺为2 cm(右下角白色线段)
Fig. 3 Tuber phenotypes of high-starch and low-starch varietiesA: Qingshu 2. B: Atlantic. C: 393034.7. D: Shenyanwo. E: 720018. F: 2017ch-1. G: Beishu 1. H: FBA-1. The scale is 2 cm (White line segment in the lower right corner)

图4 淀粉合成通路图及相关基因表达分析A:淀粉合成通路图。B:淀粉合成相关基因在高、低淀粉品种及高、低淀粉组中的表达差异显著性分析。不同字母表示不同品种在0.01水平上存在显著性差异,***表示在0.001水平上存在极显著差异
Fig. 4 Starch synthesis pathway diagram and related gene expression analysisA: Starch synthesis pathway diagram. B: Significance analysis of expression differences in starch synthesis-related genes among high-starch and low-starch varieties and high-starch and low-starch groups. Different letters indicate significant differences between varieties at the 0.01 level, and *** indicates an extremely significant difference at the 0.001 level
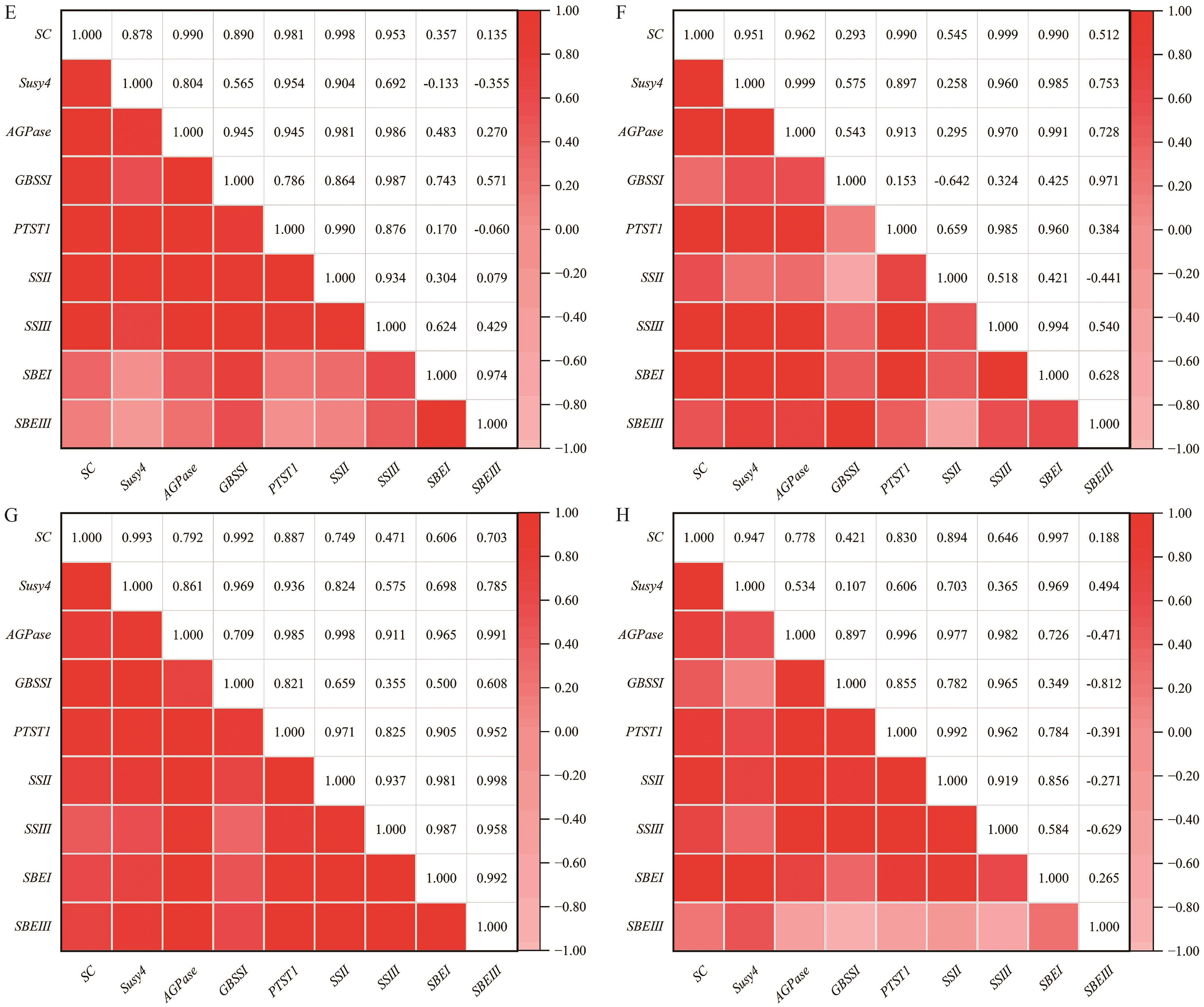
图5 淀粉含量与淀粉相关基因表达量的相关性分析SC:淀粉含量;A:青薯2号淀粉含量与淀粉相关基因表达量的相关性热图;B:大西洋;C:393034.7;D:深眼窝;E:720018;F:2017ch-1;G:北薯1号;H:FBA-1
Fig. 5 Correlation analysis between starch content and expressions of starch-related genesSC: Starch content. A: Heatmap showing the correlation between starch content and starch-related gene expression levels in Qingshu 2. B: Atlantic. C: 393034.7. D: Shenyanwo. E: 720018. F: 2017ch-1. G: Beishu 1. H: FBA-1
| [1] | Xaba S, Bello Z, Rapiya M, et al. Sustainable nutrient management strategies for enhancing potato production: the role of cover crops—a systematic review [J]. Horticulturae, 2025, 11(9): 1051. |
| [2] | Narváez-Cuenca CE, Peña C, Restrepo-Sánchez LP, et al. Macronutrient contents of potato genotype collections in the Solanum tuberosum Group Phureja [J]. J Food Compos Anal, 2018, 66: 179-184. |
| [3] | Ierna A, Mauromicale G. How physicochemical and nutritional traits of potatoes may vary under field conditions over long periods [J]. J Sci Food Agric, 2024, 104(7): 3842-3852. |
| [4] | Hofvander P, Andersson M, Larsson CT, et al. Field performance and starch characteristics of high-amylose potatoes obtained by antisense gene targeting of two branching enzymes [J]. Plant Biotechnol J, 2004, 2(4): 311-320. |
| [5] | Seefeldt HF, Tønning E, Thybo AK. Exploratory sensory profiling of three culinary preparations of potatoes (Solanum tuberosum L.) [J]. J Sci Food Agric, 2011, 91(1): 104-112. |
| [6] | Shirani-Bidabadi M, Nazarian-Firouzabadi F, Sorkheh K, et al. Transcriptomic analysis of potato (Solanum tuberosum L.) tuber development reveals new insights into starch biosynthesis [J]. PLoS One, 2024, 19(4): e0297334. |
| [7] | Geigenberger P, Stitt M, Fernie AR. Metabolic control analysis and regulation of the conversion of sucrose to starch in growing potato tubers [J]. Plant Cell Environ, 2004, 27(6): 655-673. |
| [8] | Wang W, Wei XJ, Jiao GA, et al. GBSS-BINDING PROTEIN, encoding a CBM48 domain-containing protein, affects rice quality and yield [J]. J Integr Plant Biol, 2020, 62(7): 948-966. |
| [9] | Zhang XL, Szydlowski N, Delvallé D, et al. Overlapping functions of the starch synthases SSII and SSIII in amylopectin biosynthesis in Arabidopsis [J]. BMC Plant Biol, 2008, 8: 96. |
| [10] | Kang GZ, Li SY, Zhang MQ, et al. Molecular cloning and expression analysis of the starch-branching enzyme III gene from common wheat (Triticum aestivum) [J]. Biochem Genet, 2013, 51(5/6): 377-386. |
| [11] | Lindqvist-Kreuze H, Bonierbale M, Grüneberg WJ, et al. Potato and sweetpotato breeding at the international potato center: approaches, outcomes and the way forward [J]. Theor Appl Genet, 2023, 137(1): 12. |
| [12] | Hu J, Mei M, Jin F, et al. Phenotypic variability and genetic diversity analysis of cultivated potatoes in China [J]. Front Plant Sci, 2022, 13: 954162. |
| [13] | Hu TY, Yang HK, Zhang KQ, et al. Effects of different altitudes on the structure and properties of potato starch [J]. Front Plant Sci, 2023, 14: 1111843. |
| [14] | Dang B, Zhang WG, Zhang J, et al. Evaluation of nutritional components, phenolic composition, and antioxidant capacity of highland barley with different grain colors on the Qinghai Tibet Plateau [J]. Foods, 2022, 11(14): 2025. |
| [15] | Bordoloi A, Kaur L, Singh J. Parenchyma cell microstructure and textural characteristics of raw and cooked potatoes [J]. Food Chem, 2012, 133(4): 1092-1100. |
| [16] | Castanha N, Villar J, da Matta MD Jr, et al. Structure and properties of starches from Arracacha (Arracacia xanthorrhiza) roots [J]. Int J Biol Macromol, 2018, 117: 1029-1038. |
| [17] | Tian JM, Qin LK, Zeng XF, et al. The role of amylose in gel forming of rice flour [J]. Foods, 2023, 12(6): 1210. |
| [18] | Ma PP, Yuan Y, Shen QC, et al. Evolution and expression analysis of starch synthase gene families in Saccharum spontaneum [J]. Trop Plant Biol, 2019, 12(3): 158-173. |
| [19] | Nakamura Y. Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue [J]. Plant Cell Physiol, 2002, 43(7): 718-725. |
| [20] | Seung D, Soyk S, Coiro M, et al. Protein targeting to starch is required for localising granule-bound starch synthase to starch granules and for normal amylose synthesis in Arabidopsis [J]. PLoS Biol, 2015, 13(2): e1002080. |
| [21] | Guo YX, Wang GY, Guo X, et al. Genetic dissection of protein and starch during wheat grain development using QTL mapping and GWAS [J]. Front Plant Sci, 2023, 14: 1189887. |
| [22] | Jama-Rodzenska A, Janik G, Walczak A, et al. Tuber yield and water efficiency of early potato varieties (Solanum tuberosum L.) cultivated under various irrigation levels [J]. Sci Rep, 2021, 11: 19121. |
| [23] | Yan W, Wu XY, Li YN, et al. Cell wall invertase 3 affects cassava productivity via regulating sugar allocation from source to sink [J]. Front Plant Sci, 2019, 10: 541. |
| [24] | Okamoto Y, Kajimura T, Ikeda TM, et al. Evidence from principal component analysis for improvement of grain shape- and spikelet morphology-related traits after hexaploid wheat speciation [J]. Genes Genet Syst, 2012, 87(5): 299-310. |
| [25] | Guo Z, Han JC, Zhang Y, et al. The impact of high-temperature treatments on maize growth parameters and soil nutrients: a comprehensive evaluation through principal component analysis [J]. PLoS One, 2024, 19(8): e0309070. |
| [26] | Stutte GW, Yorio NC, Wheeler RM. Interacting effects of photoperiod and photosynthetic photon flux on net carbon assimilation and starch accumulation in potato leaves [J]. J Am Soc Hortic Sci, 1996, 121(2): 264-268. |
| [27] | Adly WMRM, Niedbała G, El-Denary ME, et al. Somaclonal variation for genetic improvement of starch accumulation in potato (Solanum tuberosum) tubers [J]. Plants, 2023, 12(2): 232. |
| [28] | Li T, Heuvelink E, Marcelis LFM. Quantifying the source-sink balance and carbohydrate content in three tomato cultivars [J]. Front Plant Sci, 2015, 6: 416. |
| [29] | Payyavula RS, Singh RK, Navarre DA. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism [J]. J Exp Bot, 2013, 64(16): 5115-5131. |
| [30] | Lai YC, Wang SY, Gao HY, et al. Physicochemical properties of starches and expression and activity of starch biosynthesis-related genes in sweet potatoes [J]. Food Chem, 2016, 199: 556-564. |
| [31] | Gann PJ, Esguerra M, Counce PA, et al. Genotype-dependent and heat-induced grain chalkiness in rice correlates with the expression patterns of starch biosynthesis genes [J]. Plant Environ Interact, 2021, 2(4): 165-176. |
| [32] | Zhong YX, Blennow A, Kofoed-Enevoldsen O, et al. Protein Targeting to Starch 1 is essential for starchy endosperm development in barley [J]. J Exp Bot, 2019, 70(2): 485-496. |
| [33] | Crofts N, Domon A, Miura S, et al. Starch synthases SSIIa and GBSSI control starch structure but do not determine starch granule morphology in the absence of SSIIIa and SSIVb [J]. Plant Mol Biol, 2022, 108(4/5): 379-398. |
| [1] | 巩慧玲, 邢玉洁, 马俊贤, 蔡霞, 冯再平. 马铃薯LAC基因家族的鉴定及盐胁迫下表达分析[J]. 生物技术通报, 2025, 41(9): 82-93. |
| [2] | 卢瑶, 袁平平, 金鑫, 毛向红, 范向斌, 白小东. 基于SSR标记的马铃薯野生种和地方种遗传多样性分析和指纹图谱构建[J]. 生物技术通报, 2025, 41(9): 94-104. |
| [3] | 任睿斌, 司二静, 万广有, 汪军成, 姚立蓉, 张宏, 马小乐, 李葆春, 王化俊, 孟亚雄. 大麦条纹病菌GH17基因家族的鉴定及表达分析[J]. 生物技术通报, 2025, 41(8): 146-154. |
| [4] | 曾丹, 黄园, 王健, 张艳, 刘庆霞, 谷荣辉, 孙庆文, 陈宏宇. 铁皮石斛bZIP转录因子家族全基因组鉴定及表达分析[J]. 生物技术通报, 2025, 41(8): 197-210. |
| [5] | 韩燚, 侯昌林, 唐露, 孙璐, 谢晓东, 梁晨, 陈小强. 大麦HvERECTA基因的克隆及功能分析[J]. 生物技术通报, 2025, 41(7): 106-116. |
| [6] | 黄丹丹, 吴云翼, 邹建华, 俞婷, 朱炎辉, 杨梅宏, 董文丽, 高冬丽. 马铃薯StPTST2a基因的克隆及互作分析[J]. 生物技术通报, 2025, 41(7): 172-180. |
| [7] | 李霞, 张泽伟, 刘泽军, 王楠, 郭江波, 辛翠花, 张彤, 简磊. 马铃薯转录因子StMYB96的克隆及功能研究[J]. 生物技术通报, 2025, 41(7): 181-192. |
| [8] | 罗稷林, 栗锦烨, 贾玉鑫. 马铃薯中重力响应调节基因鉴定及功能分析[J]. 生物技术通报, 2025, 41(6): 109-118. |
| [9] | 许慧珍, SHANTWANA Ghimire, RAJU Kharel, 岳云, 司怀军, 唐勋. 马铃薯SUMO E3连接酶基因家族分析及StSIZ1基因的克隆与表达模式分析[J]. 生物技术通报, 2025, 41(6): 119-129. |
| [10] | 段永红, 杨欣, 于冠群, 夏俊俊, 宋陆帅, 白小东, 彭锁堂. 125份马铃薯种质资源遗传多样性及主成分分析[J]. 生物技术通报, 2025, 41(6): 130-143. |
| [11] | 宋慧洋, 苏宝杰, 李京昊, 梅超, 宋倩娜, 崔福柱, 冯瑞云. 马铃薯StAS2-15基因的克隆及盐胁迫下功能分析[J]. 生物技术通报, 2025, 41(5): 119-128. |
| [12] | 牛若宇, 高瞻, 熊显鹏, 祝德, 罗皓天, 马学远, 胡冠菁. 棉花野生种质资源的育种应用研究与前景[J]. 生物技术通报, 2025, 41(4): 21-32. |
| [13] | 文博霖, 万敏, 胡建军, 王克秀, 景晟林, 王心悦, 朱博, 唐铭霞, 李兵, 何卫, 曾子贤. 马铃薯川芋50遗传转化及基因编辑体系的建立[J]. 生物技术通报, 2025, 41(4): 88-97. |
| [14] | 刘涛, 王志淇, 吴文博, 石文婷, 王超楠, 杜崇, 杨中敏. 马铃薯GRAM基因家族鉴定与表达分析[J]. 生物技术通报, 2025, 41(4): 145-155. |
| [15] | 张益瑄, 马宇, 王童童, 盛苏奥, 宋家凤, 吕钊彦, 朱晓彪, 侯华兰. 马铃薯DIR家族全基因组鉴定及表达模式分析[J]. 生物技术通报, 2025, 41(3): 123-136. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||