








Biotechnology Bulletin ›› 2023, Vol. 39 ›› Issue (7): 277-287.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1517
Previous Articles Next Articles
MEI Huan( ), LI Yue, LIU Ke-meng, LIU Ji-hua(
), LI Yue, LIU Ke-meng, LIU Ji-hua( )
)
Received:2022-12-16
Online:2023-07-26
Published:2023-08-17
Contact:
LIU Ji-hua
E-mail:3220020340@stu.cpu.edu.cn;liujihua@cpu.edu.cn
MEI Huan, LI Yue, LIU Ke-meng, LIU Ji-hua. Study on the Biosynthesis of l-SLR by Efficient Prokaryotic Expression of Berberine Bridge Enzyme[J]. Biotechnology Bulletin, 2023, 39(7): 277-287.
| 项目 Item | 菌株和质粒Strains and plasmids | 详细信息Detailed information | 来源Source |
|---|---|---|---|
| 菌株Strains | BL21(DE3)-SMR-MEcBO | pMAL-c4x, Tac P-EcBO-Tac T, EcoR I/Sal I; pBR322 ori, Smr+ | Lab stock |
| BL21(DE3)-pCold-II- ECBO | pCold-II, CSPA P-MBP-EcBO - CSPA T, Nde I/Xho I; pBR322 ori, Amp+ | This work | |
| BL21(DE3)-pTrc99a-ECBO | pTrc99a, Trc P-EcBO - Trc T, EcoR I/Hind III; pBR322 ori, Amp+ | This work | |
| BL21(DE3)-pBad24- ECBO | pBad24, araBAD P-EcBO - araBAD T, EcoR I/ Hind III; pBR322 ori, Amp+ | This work | |
| BL21(DE3)-pET28a-sumo-EcBO | pET28a-sumo, T7 P-EcBO - T7 T, BamH I/Sac I ; pBR322 ori, Kan+ | This work | |
| E. coli DH5α | F-φ80 lacZΔM15Δ(lacZYA-argF)U169 endA1 recA1 hsdR17(rk-, mk-)supE44λ- thi-1 gyrA96 relA1 phoA | General Biol | |
| E. coli BL21(DE3) | F-ompT hsdS(rB-mB-)gal dcm(DE3) | General Biol | |
| Strain A | BL21(DE3)with plasmid SMR-MEcBO and pGro7 | This work | |
| Strain B | BL21(DE3)with plasmid SMR-MEcBO and pTf16 | This work | |
| Strain C | BL21(DE3)with plasmid SMR-MEcBO and pKJE7 | This work | |
| Strain D | BL21(DE3)with plasmid SMR-MEcBO and pG-KJE8 | This work | |
| Strain E | BL21(DE3)with plasmid SMR-MEcBO and pG-Tf2 | This work | |
| 质粒Plasmids | pCold II | CSPA Promoter,Amp+ | Lab stock |
| pET-28a-sumo | T7 Promoter,Kan+ | Lab stock | |
| pTrc99a | Trc Promoter,Amp+ | Lab stock | |
| pBad24 | araBAD Promoter,Amp+ | Lab stock |
Table 1 Strain and plasmid information
| 项目 Item | 菌株和质粒Strains and plasmids | 详细信息Detailed information | 来源Source |
|---|---|---|---|
| 菌株Strains | BL21(DE3)-SMR-MEcBO | pMAL-c4x, Tac P-EcBO-Tac T, EcoR I/Sal I; pBR322 ori, Smr+ | Lab stock |
| BL21(DE3)-pCold-II- ECBO | pCold-II, CSPA P-MBP-EcBO - CSPA T, Nde I/Xho I; pBR322 ori, Amp+ | This work | |
| BL21(DE3)-pTrc99a-ECBO | pTrc99a, Trc P-EcBO - Trc T, EcoR I/Hind III; pBR322 ori, Amp+ | This work | |
| BL21(DE3)-pBad24- ECBO | pBad24, araBAD P-EcBO - araBAD T, EcoR I/ Hind III; pBR322 ori, Amp+ | This work | |
| BL21(DE3)-pET28a-sumo-EcBO | pET28a-sumo, T7 P-EcBO - T7 T, BamH I/Sac I ; pBR322 ori, Kan+ | This work | |
| E. coli DH5α | F-φ80 lacZΔM15Δ(lacZYA-argF)U169 endA1 recA1 hsdR17(rk-, mk-)supE44λ- thi-1 gyrA96 relA1 phoA | General Biol | |
| E. coli BL21(DE3) | F-ompT hsdS(rB-mB-)gal dcm(DE3) | General Biol | |
| Strain A | BL21(DE3)with plasmid SMR-MEcBO and pGro7 | This work | |
| Strain B | BL21(DE3)with plasmid SMR-MEcBO and pTf16 | This work | |
| Strain C | BL21(DE3)with plasmid SMR-MEcBO and pKJE7 | This work | |
| Strain D | BL21(DE3)with plasmid SMR-MEcBO and pG-KJE8 | This work | |
| Strain E | BL21(DE3)with plasmid SMR-MEcBO and pG-Tf2 | This work | |
| 质粒Plasmids | pCold II | CSPA Promoter,Amp+ | Lab stock |
| pET-28a-sumo | T7 Promoter,Kan+ | Lab stock | |
| pTrc99a | Trc Promoter,Amp+ | Lab stock | |
| pBad24 | araBAD Promoter,Amp+ | Lab stock |
| 名称Name | 引物序列Primer sequence(5'-3') |
|---|---|
| pCold-II-Nde I-F | catcatcatcatcatcatatgAAAATCGAAGAAGGTAAACTGGTAATC |
| pCold-II-Xho I-R | cttgaattcggatccctcgagAATAACCACTTCACCACCATCACTG |
| pTrc99a-EcoR I-F | aggaaacagaccatggaattcATGGGTAATGATCTGCTGAGCTGTCTG |
| pTrc99a-Hind III-R | tccgccaaaacagccaagcttAATAACCACTTCACCACCATCACTG |
| pBad24-EcoR I-F | ttgggctagcaggaggaattcATGGGTAATGATCTGCTGAGCTGTCTG |
| pBad24-Hind III-R | TccgccaaaacagccaagcttAATAACCACTTCACCACCATCACTG |
| pET28a-sumo- BamH I-F | cttgaattcggatccctcgagGGTAATGATCTGCTGAGCTGTCTG |
| pET28a-sumo- Sac I -R | catcatcatcatcatcatatgAATAACCACTTCACCACCATCACTG |
| T7 | TAATACGACTCACTATAGGG |
| T7 ter | TGCTAGTTATTGCTCAGCGG |
| pCold-II-F | ACGCCATATCGCCGAAAGG |
| pCold-II-R | GGCAGGGATCTTAGATTCTG |
| pBad-F | ATGCCATAGCATTTTTATCC |
| pBad-R | GATTTAATCTGTATCAGG |
| pTrc99a-F | GAGCGGATAACAATTTCACACAGG |
| pTrc99a-R | GATTTAATCTGTATCAGG |
Table 2 List of primers
| 名称Name | 引物序列Primer sequence(5'-3') |
|---|---|
| pCold-II-Nde I-F | catcatcatcatcatcatatgAAAATCGAAGAAGGTAAACTGGTAATC |
| pCold-II-Xho I-R | cttgaattcggatccctcgagAATAACCACTTCACCACCATCACTG |
| pTrc99a-EcoR I-F | aggaaacagaccatggaattcATGGGTAATGATCTGCTGAGCTGTCTG |
| pTrc99a-Hind III-R | tccgccaaaacagccaagcttAATAACCACTTCACCACCATCACTG |
| pBad24-EcoR I-F | ttgggctagcaggaggaattcATGGGTAATGATCTGCTGAGCTGTCTG |
| pBad24-Hind III-R | TccgccaaaacagccaagcttAATAACCACTTCACCACCATCACTG |
| pET28a-sumo- BamH I-F | cttgaattcggatccctcgagGGTAATGATCTGCTGAGCTGTCTG |
| pET28a-sumo- Sac I -R | catcatcatcatcatcatatgAATAACCACTTCACCACCATCACTG |
| T7 | TAATACGACTCACTATAGGG |
| T7 ter | TGCTAGTTATTGCTCAGCGG |
| pCold-II-F | ACGCCATATCGCCGAAAGG |
| pCold-II-R | GGCAGGGATCTTAGATTCTG |
| pBad-F | ATGCCATAGCATTTTTATCC |
| pBad-R | GATTTAATCTGTATCAGG |
| pTrc99a-F | GAGCGGATAACAATTTCACACAGG |
| pTrc99a-R | GATTTAATCTGTATCAGG |
| 质粒Plasmid | 分子伴侣组合Chaperone | 启动子Promoter | 抗性标签Resistant marker | 诱导物Inducer |
|---|---|---|---|---|
| pG-KJE8 | dnaK-dnaJ-grpE groES-groEL | araB Pzt-1 | Chl+ | L-阿拉伯糖 四环素 |
| pGro7 | groES-groEL | araB | Chl+ | L-阿拉伯糖 |
| pKJE7 | dnaK-dnaJ-grpE | araB | Chl+ | L-阿拉伯糖 |
| pTf16 | tig | araB | Chl+ | L-阿拉伯糖 |
| pG-Tf2 | groES-groEL-tig | Pzt-1 | Chl+ | 四环素 |
Table 3 Chaperone protein information
| 质粒Plasmid | 分子伴侣组合Chaperone | 启动子Promoter | 抗性标签Resistant marker | 诱导物Inducer |
|---|---|---|---|---|
| pG-KJE8 | dnaK-dnaJ-grpE groES-groEL | araB Pzt-1 | Chl+ | L-阿拉伯糖 四环素 |
| pGro7 | groES-groEL | araB | Chl+ | L-阿拉伯糖 |
| pKJE7 | dnaK-dnaJ-grpE | araB | Chl+ | L-阿拉伯糖 |
| pTf16 | tig | araB | Chl+ | L-阿拉伯糖 |
| pG-Tf2 | groES-groEL-tig | Pzt-1 | Chl+ | 四环素 |

Fig. 1 Gene structure mapping of recombinant strains and colony PCR validation A: Genetic structure of colonies of recombinant strains; B: PCR validation of recombinant strains

Fig. 2 SDS-PAGE analysis of induced expressions of recombinant strains M: Protein molecular weight marker. Lanes 1-2: BL21(DE3)-SMR-MEcBO supernatant, precipitate. Lane 3-4: BL21(DE3)-pCold II-MBP-EcBO supernatant, precipitate. Lanes 5-6: BL21(DE3)-pTrc99a-EcBO supernatant, precipitation. Lanes 7-8: BL21(DE3)-pBad24-EcBO supernatant, precipitate. Lanes 9-10: BL21(DE3)-pET28a-sumo-EcBO supernatant, precipitate
| 质粒 Plasmid | 启动子强度 Promoter strength | 启动子 Promoter | 特点 Peculiarity | 可溶性蛋白比例Proportion of soluble protein/% | l-SLR产量 l-SLR production/(mg· L-1) | 参考文献References |
|---|---|---|---|---|---|---|
| pMal-c4x | 强 | Tac | 由Trp和Lac合成的人工杂交启动子,效率高于Lac启动子 | 67.9 | 20.89 | [ |
| pET-28a-sumo | 最强 | T7 | 效率高,受T7RNA聚合酶调节,不被大肠杆菌RNA 聚合酶调节 | 47.24 | 0 | [ |
| pCold II | 中等 | CSPA | 低温高效表达 | 52.78 | 0 | [ |
| pTrc99a | 弱 | Trc | 由Trp和Lac合成的人工杂交启动子,效率高于Lac启动子 | 0 | 0 | [ |
| pBad24 | 中等 | araBAD | 表达强度由阿拉伯糖的浓度决定 | 0 | 0 | [ |
Table 4 Protein expressions of promoters and corresponding recombinant strains
| 质粒 Plasmid | 启动子强度 Promoter strength | 启动子 Promoter | 特点 Peculiarity | 可溶性蛋白比例Proportion of soluble protein/% | l-SLR产量 l-SLR production/(mg· L-1) | 参考文献References |
|---|---|---|---|---|---|---|
| pMal-c4x | 强 | Tac | 由Trp和Lac合成的人工杂交启动子,效率高于Lac启动子 | 67.9 | 20.89 | [ |
| pET-28a-sumo | 最强 | T7 | 效率高,受T7RNA聚合酶调节,不被大肠杆菌RNA 聚合酶调节 | 47.24 | 0 | [ |
| pCold II | 中等 | CSPA | 低温高效表达 | 52.78 | 0 | [ |
| pTrc99a | 弱 | Trc | 由Trp和Lac合成的人工杂交启动子,效率高于Lac启动子 | 0 | 0 | [ |
| pBad24 | 中等 | araBAD | 表达强度由阿拉伯糖的浓度决定 | 0 | 0 | [ |
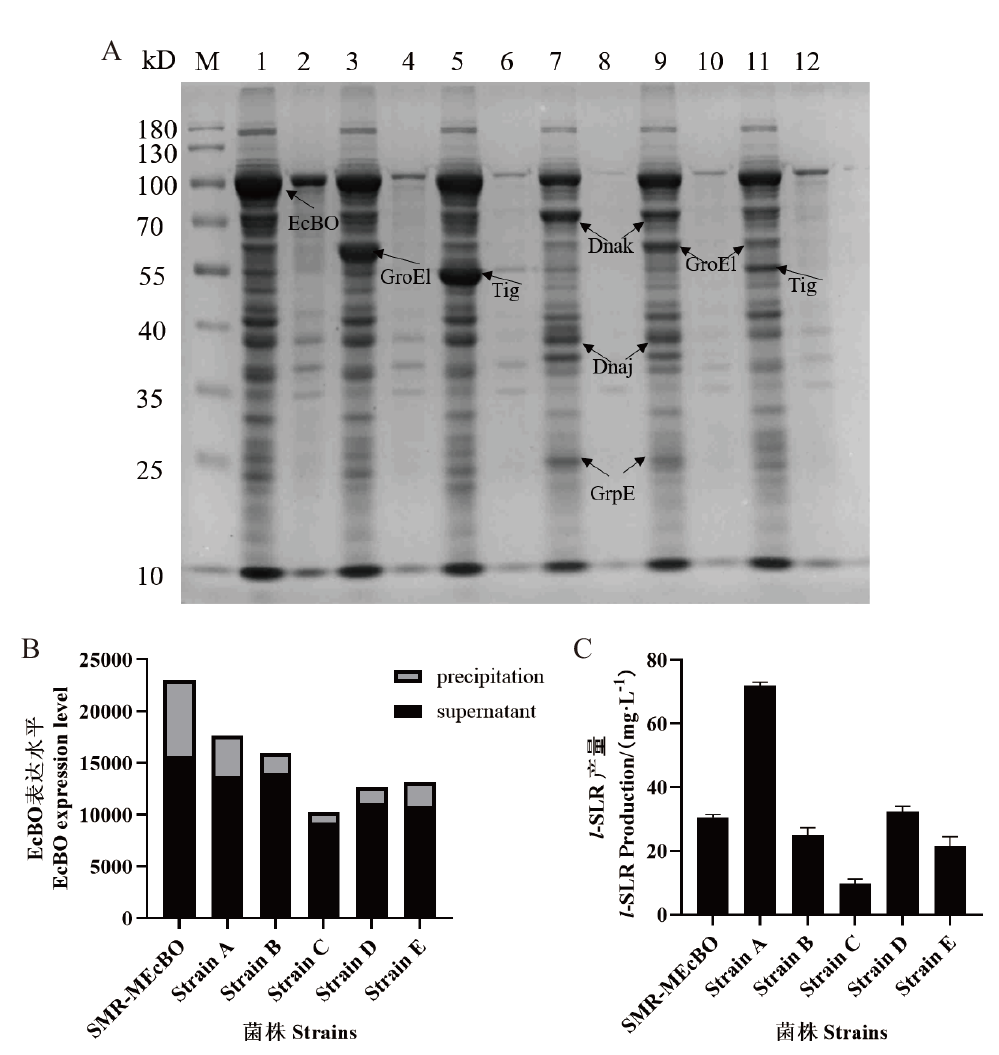
Fig. 3 SDS-PAGE analysis of molecular chaperone protein screening and catalytic activity of EcBO A: M: Protein molecular weight marker. Lanes 1-2: SMR-MEcBO supernatant, precipitate. Lanes 3-4: Strain A supernatant, precipitate. Lanes 5-6: Strain B supernatant, precipitate. Lanes 7-8: Strain C supernatant, precipitate. Lanes 9-10: Strain D supernatant, precipitate. Lanes 11-12: Strain E supernatant, precipitate. B: Effect of chaperone proteins on soluble expression of EcBO. C: Production of l-SLR biosynthesis by chaperone co-expression strains
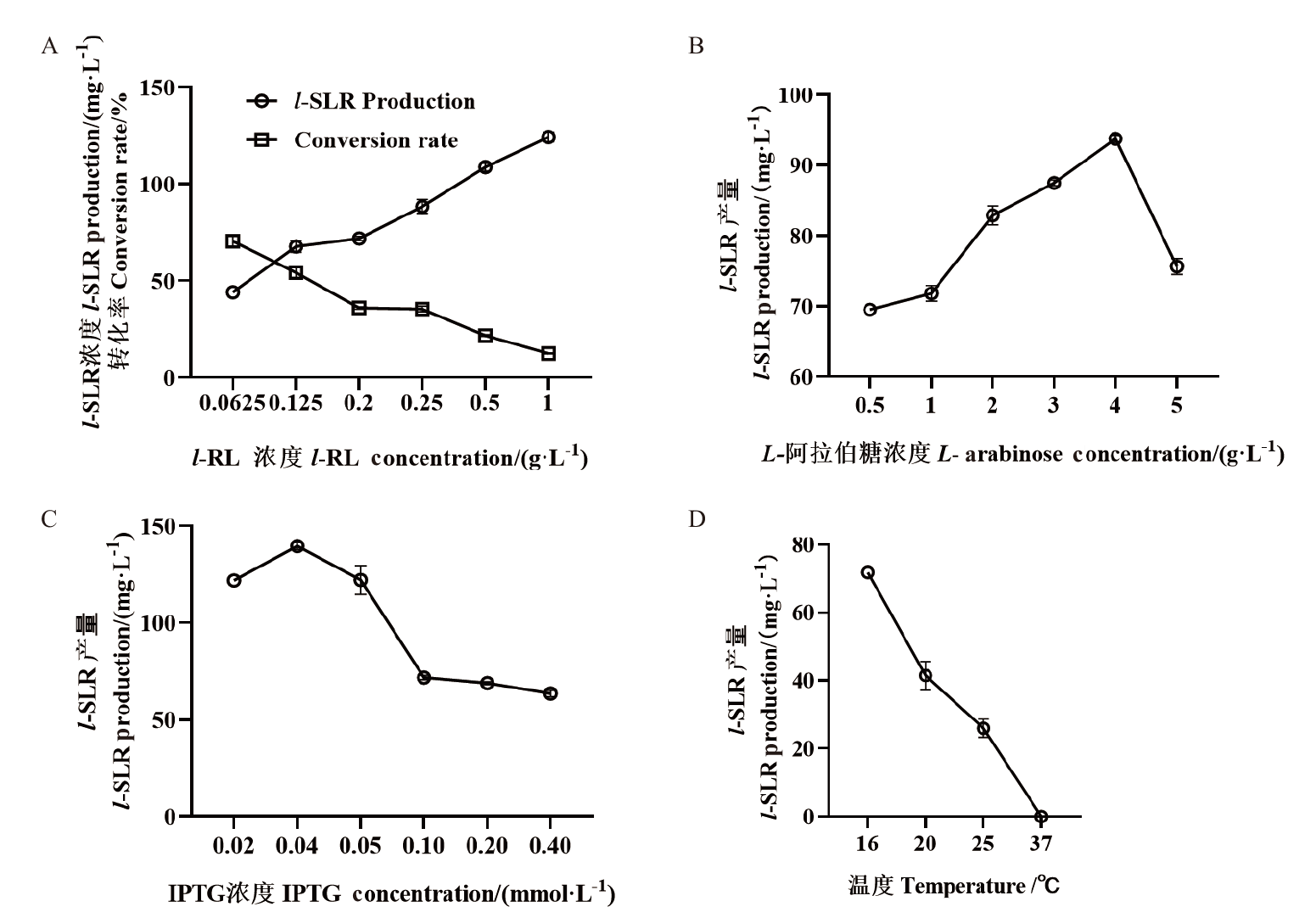
Fig. 5 Optimization of cultivating conditions for co-expressed strain A A. l-RL concentration. B. L-Arabinos concentration;C. IPTG concentration ;D. temperature
| [1] | 高赟赟, 米桂芸, 刘帅, 等. 左旋金黄紫堇碱抗精神分裂症作用研究[J]. 中国药理学通报, 2016, 32(1): 103-109. |
| Gao YY, Mi GY, Liu S, et al. On antipsychotic effects of l-scoulerine[J]. Chin Pharmacol Bull, 2016, 32(1): 103-109. | |
| [2] | 霍立, 甘永军, 沈毅, 等. 罗通定的合成[J]. 中国医药工业杂志, 2012, 43(5): 323-325. |
| Huo L, Gan YJ, Shen Y, et al. Synthesis of rotundine[J]. Chin J Pharm, 2012, 43(5): 323-325. | |
| [3] | 赵娟, 王洁, 孙德夫, 等. 罗通定的合成工艺改进[J]. 中国药物化学杂志, 2015, 25(5): 378-381. |
| Zhao J, Wang J, Sun DF, et al. Improved synthesis of rotundine[J]. Chin J Med Chem, 2015, 25(5): 378-381. | |
| [4] |
Dittrich H, Kutchan TM. Molecular cloning, expression, and induction of berberine bridge enzyme, an enzyme essential to the formation of benzophenanthridine alkaloids in the response of plants to pathogenic attack[J]. Proc Natl Acad Sci USA, 1991, 88(22): 9969-9973.
doi: 10.1073/pnas.88.22.9969 pmid: 1946465 |
| [5] |
Facchini PJ, Penzes C, Johnson AG, et al. Molecular characterization of berberine bridge enzyme genes from opium poppy[J]. Plant Physiol, 1996, 112(4): 1669-1677.
pmid: 8972604 |
| [6] |
Pyne ME, Kevvai K, Grewal PS, et al. A yeast platform for high-level synthesis of tetrahydroisoquinoline alkaloids[J]. Nat Commun, 2020, 11(1): 3337.
doi: 10.1038/s41467-020-17172-x pmid: 32620756 |
| [7] |
Zhao WL, Liu MY, Shen C, et al. Biosynthesis of plant-specific alkaloids tetrahydroprotoberberines in engineered Escherichia coli[J]. Green Chem, 2021, 23(16): 5944-5955.
doi: 10.1039/D1GC01670A URL |
| [8] | 张正晖, 曹铭铭, 李珺, 等. 微生物高效分泌蛋白质的策略与应用[J]. 化工进展, 2018, 37(8): 3129-3137. |
| Zhang ZH, Cao MM, Li J, et al. Strategies and application of highly efficient secretion of proteins by microorganisms[J]. Chem Ind Eng Prog, 2018, 37(8): 3129-3137. | |
| [9] |
Peng H, Yang M, Huang WS, et al. Soluble expression and purification of a crab antimicrobial peptide scygonadin in different expression plasmids and analysis of its antimicrobial activity[J]. Protein Expr Purif, 2010, 70(1): 109-115.
doi: 10.1016/j.pep.2009.09.008 URL |
| [10] | 黄津伟, 高阳, 李道凡, 等. 启动子优化提高重组大肠杆菌PHA产量[J]. 基因组学与应用生物学, 2019, 38(7): 3090-3096. |
| Huang JW, Gao Y, Li DF, et al. Optimized promoters to enhance the production of PHA in recombinant Escherichia coli[J]. Genom Appl Biol, 2019, 38(7): 3090-3096. | |
| [11] | 林影, 韩双艳, 袁清焱, 等. 酶高效表达体系构建及高通量筛选关键技术[J]. 生物产业技术, 2019(3): 44-53. |
| Lin Y, Han SY, Yuan QY, et al. Key technologies of construction of efficient enzyme expression system and highthroughput screening[J]. Biotechnol & Bus, 2019(3): 44-53. | |
| [12] |
Laskey RA, Honda BM, Mills AD, et al. Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA[J]. Nature, 1978, 275(5679): 416-420.
doi: 10.1038/275416a0 |
| [13] | 方红辉, 倪晔, 周婕妤, 等. 糖基转移酶UGT73C5在大肠杆菌中的可溶性表达研究[J]. 食品与发酵工业, 2023, 49(2): 1-7. |
| Fang HH, Ni Y, Zhou JY, et al. Soluble expression of glycosyltransferase UGT73C5 in Escherichia coli[J]. Food Ferment Ind, 2023, 49(2): 1-7. | |
| [14] | 李琦, 朱文慧, 姜云鹏, 等. 伴侣蛋白提高木糖苷酶的可溶性表达及其协同酶解木聚糖[J]. 林产化学与工业, 2021, 41(5): 15-22. |
| Li Q, Zhu WH, Jiang YP, et al. Enhancement of soluble expression of β-xylosidase by molecular chaperone and its synergistic enzymatic hydrolysis of xylan[J]. Chem Ind For Prod, 2021, 41(5): 15-22. | |
| [15] | 邓通, 周海胜, 吴坚平, 等. 基于分子伴侣策略提高NADPH依赖型醇脱氢酶的异源可溶性表达[J]. 中国生物工程杂志, 2020, 40(8): 24-32. |
| Deng T, Zhou HS, Wu JP, et al. Enhance soluble heteroexpression of a NADPH-dependent alcohol dehydrogenase based on the chaperone strategy[J]. China Biotechnol, 2020, 40(8): 24-32. | |
| [16] |
Amann E, Brosius J, Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli[J]. Gene, 1983, 25(2/3): 167-178.
doi: 10.1016/0378-1119(83)90222-6 URL |
| [17] |
Wang WY, Li Y, Wang YQ, et al. Bacteriophage T7 transcription system: an enabling tool in synthetic biology[J]. Biotechnol Adv, 2018, 36(8): 2129-2137.
doi: S0734-9750(18)30164-2 pmid: 30290194 |
| [18] | 曾发姣, 舒燕, 缪东, 等. pColdTMTF载体原核表达FocA可溶性蛋白的研究[J]. 湖南农业科学, 2016(7): 12-14, 17. |
| Zeng FJ, Shu Y, Miao D, et al. Prokaryotic expression of soluble FocA protein with pCold TM TF vector[J]. Hunan Agric Sci, 2016(7): 12-14, 17. | |
| [19] | 焦志勇, 陈旻湖, 李国庆, 等. 重组原核表达质粒pTrc99A-ureB/hlyE的构建和表达[J]. 中国人兽共患病杂志, 2003, 19(3): 34-36, 67. |
| Jiao ZY, Chen MH, Li GQ, et al. Cloning and expressing in E.coli of recombinant prokaryotic expression plasmid pTrc99A-ureB/hlyE[J]. Chin J Zoonoses, 2003, 19(3): 34-36, 67. | |
| [20] |
Yang J, Kim N, Park W, et al. Optimization of protein trans-splicing in an inducible plasmid display system for high-throughput screening and selection of soluble proteins[J]. Enzyme Microb Technol, 2022, 153: 109914.
doi: 10.1016/j.enzmictec.2021.109914 URL |
| [21] |
Seddi R, Chaix JC, Puigserver A, et al. Expression of a soluble and activatable form of bovine procarboxypeptidase A in Escherichia coli[J]. Protein Expr Purif, 2003, 27(2): 220-228.
doi: 10.1016/S1046-5928(02)00573-9 URL |
| [22] | 黄海荣, 董旭, 张宗德, 等. 应用分子伴侣共表达系统表达结核分枝杆菌编码蛋白[J]. 中国防痨杂志, 2009, 31(2): 76-79. |
| Huang HR, Dong X, Zhang ZD, et al. Co-expression of protein of M. tuberculosis with molecular chaperone[J]. Chin J Antituberc, 2009, 31(2): 76-79. | |
| [23] |
Kim JS, Nakagawa A, Yamazaki Y, et al. Improvement of reticuline productivity from dopamine by using engineered Escherichia coli[J]. Biosci Biotechnol Biochem, 2013, 77(10): 2166-2168.
doi: 10.1271/bbb.130552 URL |
| [24] | 刘微, 姚杨, 马萧萧, 等. 融合表达载体pET22b-SUMO-FGFR4的构建及其在大肠杆菌中表达条件的优化[J]. 吉林大学学报: 医学版, 2016, 42(4): 642-647. |
| Liu W, Yao Y, Ma XX, et al. Construction of fusion expression vector pET22b-SUMO-FGFR4 and optimization of expression conditions in E.coli[J]. J Jilin Univ Med Ed, 2016, 42(4): 642-647. | |
| [25] | 张春鸽, 赵灿, 束长龙, 等. 不同启动子表达Cry1Ie蛋白的特性分析[J]. 生物技术通报, 2012(11): 192-196. |
| Zhang CG, Zhao C, Shu CL, et al. Analysis of expressed Cry1Ie protein initiated by different promoters[J]. Biotechnol Bull, 2012(11): 192-196. | |
| [26] |
Clare DK, Bakkes PJ, van Heerikhuizen H, et al. Chaperonin complex with a newly folded protein encapsulated in the folding chamber[J]. Nature, 2009, 457(7225): 107-110.
doi: 10.1038/nature07479 |
| [27] |
Tang YC, Chang HC, Roeben A, et al. Structural features of the GroEL-GroES nano-cage required for rapid folding of encapsulated protein[J]. Cell, 2006, 125(5): 903-914.
doi: 10.1016/j.cell.2006.04.027 URL |
| [28] | 郑岩. 不同分子伴侣活性对新生肽链的影响[D]. 哈尔滨: 哈尔滨工业大学, 2020. |
| Zheng Y. The effects of different chaperone activities on the folding of nascent chains[D]. Harbin:Harbin Institute of Technology, 2020. | |
| [29] | 张言慧, 高先岭, 黄魁, 等. 重组大肠杆菌发酵表达及代谢调控研究进展[J]. 食品与药品, 2021, 23(1): 85-91. |
| Zhang YH, Gao XL, Huang K, et al. Research progress on fermentation expression and metabolism regulation of recombinant Escherichia coli[J]. Food Drug, 2021, 23(1): 85-91. | |
| [30] |
Sørensen HP, Mortensen KK. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli[J]. Microb Cell Fact, 2005, 4(1): 1.
doi: 10.1186/1475-2859-4-1 pmid: 15629064 |
| [1] | DONG Cong, GAO Qing-hua, WANG Yue, LUO Tong-yang, WANG Qing-qing. Increasing the Expression of FAD-dependent Glucose Dehydrogenase by Recombinant Pichia pastoris Using a Combined Strategy [J]. Biotechnology Bulletin, 2023, 39(6): 316-324. |
| [2] | SUO Qing-qing, WU Nan, YANG Hui, LI Li, WANG Xi-feng. Prokaryotic Expression,Antibody Preparation and Application of Rice Caffeoyl Coenzyme A-O-methyltransferase Gene [J]. Biotechnology Bulletin, 2022, 38(8): 135-141. |
| [3] | QIN Xue-jing, WANG Yu-han, CAO Yi-bo, ZHANG Ling-yun. Prokaryotic Expression and Preparation of Polyclonal Antibody of PwHAP5 Gene in Picea wilsonii [J]. Biotechnology Bulletin, 2022, 38(8): 142-149. |
| [4] | WANG Guang-li, FAN Chan, WANG Hui, LU Hui-fang, XIA Ling-yin, HUANG Jian, MIN Xun. Prokaryotic Expression,Purification,Identification,and Polyclonal Antibody Preparation of Vibrio cholerae Hemolysin HlyA [J]. Biotechnology Bulletin, 2022, 38(7): 269-277. |
| [5] | WANG Qiao-ju, HU Yu-meng, WEN Ya-ya, SONG Li, MENG Chuang, PAN Zhi-ming, JIAO Xin-an. Expression and Activity Identification of SARS-CoV-2 S1 Protein [J]. Biotechnology Bulletin, 2022, 38(3): 157-163. |
| [6] | ZHAO Bao-ding, LV Jia, SHEN Yu-yu, GUI Ling, CHEN Zhong-xiu, CHEN Jie, LU Fu-ping, LI Ming. Efficient Transformation of Uridine by Escherichia coli Based on Signal Peptide and Molecular Chaperone Strategy [J]. Biotechnology Bulletin, 2022, 38(11): 238-249. |
| [7] | SHEN Jun-qiang, ZHANG Li-ping, YU Rui-ming, WANG Yong-lu, PAN Li, LIU Xia, LIU Xin-sheng. Porcine Kobuvirus Structural Proteins VP0 and VP1 Prokaryotic Expression and Establishment of Indirect ELISA Method [J]. Biotechnology Bulletin, 2022, 38(10): 243-253. |
| [8] | DUAN Xu-guo, ZHANG Yu-hua, HUANG Ting-ting, DING Qian, LUAN Shu-yue, ZHU Qiu-yu. Synergetic Enhancing the Soluble Expression of Thermotoga maritima α-Glucan Phosphorylase by Chemical Chaperones and Induction Condition Optimization [J]. Biotechnology Bulletin, 2021, 37(8): 233-242. |
| [9] | SHAN Cao-mei, YE Lei, ZHANG Lian-hu, KUANG Wei-gang, SUN Xiao-tang, MA Jian, CUI Ru-qiang. Cloning,and Functional Analysis of Gene OsRAI1 Resistant to Hirschmanniella mucronate in Rice [J]. Biotechnology Bulletin, 2021, 37(7): 146-155. |
| [10] | ZENG Fu-yuan, SU Ze-hui, ZHOU Shi-hui, XIE Miao, PANG Huan-ying. Prokaryotic Expression of the PEPCK Protein of Vibrio alginolyticus and Identification of Its Acetylation and Succinylation [J]. Biotechnology Bulletin, 2021, 37(5): 84-91. |
| [11] | ZHANG Xi-xi, ZHANG Yi-qing, LI Yu-lin, HAN Xiao, WANG Guo-qiang, WANG Xiao-jun, WANG Xu-dong, WANG Yun-long. Prokaryotic Expression,Purification and Application of N Protein C-terminal Recombinant Protein in Novel Coronavirus(SARS-CoV-2) [J]. Biotechnology Bulletin, 2021, 37(5): 92-97. |
| [12] | BAI Fu-mei, LI Zhi-min, WANG Xiao-qin, HU Zi-wei, BAO Ling-ling, LI Zhi-min. Biochemical Characterization and Structural Analysis of N-acetylornithine Transaminase from Synechocystis sp. PCC6803 [J]. Biotechnology Bulletin, 2021, 37(5): 98-107. |
| [13] | QU Huan, LI Cheng, CHEN Rui, LIAO Yi-jie, CAO San-jie, WEN Yi-ping, YAN Qi-gui, HUANG Xiao-bo. Truncated Expression of the S1-CTD Fragment of Porcine Deltacoronavirus and Establishment of an Indirect ELISA for Detecting Its Antibody [J]. Biotechnology Bulletin, 2021, 37(5): 273-280. |
| [14] | PENG Li-zhong, ZHANG Peng, ZHOU Wen-wen, ZENG Xu-hui, ZHANG Xiao-ning. Preparation and Multi-purpose Validation of Sperm-specific Protein Cabs1 Polyclonal Antibody [J]. Biotechnology Bulletin, 2021, 37(3): 261-270. |
| [15] | HE Yang, YU Qiao-ling, WANG Jun, QIN Chuan-jie, LI Hua-tao. Advances in Prokaryotic Expression Gene of Tilapia [J]. Biotechnology Bulletin, 2021, 37(2): 195-202. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||