








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (12): 291-298.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0245
Previous Articles Next Articles
JI Zhong-xiang( ), LUORENG Zhuo-ma, LI Yu-hang, WANG Yu-mei, HU Xi-min, LI Yan-qing, WANG Xing-ping(
), LUORENG Zhuo-ma, LI Yu-hang, WANG Yu-mei, HU Xi-min, LI Yan-qing, WANG Xing-ping( )
)
Received:2024-03-13
Online:2024-12-26
Published:2025-01-15
Contact:
WANG Xing-ping
E-mail:13083726975@163.com;wxp@nxu.edu.cn
JI Zhong-xiang, LUORENG Zhuo-ma, LI Yu-hang, WANG Yu-mei, HU Xi-min, LI Yan-qing, WANG Xing-ping. Roles of miR-3604 on the Receptivity, Proliferation, and Apoptosis of Bovine Endometrial Epithelial Cells[J]. Biotechnology Bulletin, 2024, 40(12): 291-298.
| 引物名称Primer name | 序列号Serial number | 引物序列Primer sequence(5'-3') | 产物长度Product length/bp |
|---|---|---|---|
| miR-3604 | NR_036382.1 | F:AGTGCAGGGTCCGAGGTATT R:GCCTCATAACCAATGTGCAG | 63 |
| HOXA10 | NM_001105017.1 | F:TTTCCTTGGCAAAGAGAAGGGCTTG R:GACCTTACACAAACTGGAAGAGAC | 188 |
| IL-6 | NM_173923.2 | F:CACTCCATTCGCTGTCT R:GTGTCTCCTTGCTGCTT | 227 |
| VEGF | NM_001316955.1 | F:GTCTACCAGCGCAGCTTCTG R:TGCTGGCTTTGGTGAGGTT | 212 |
| LIF | NM_173931.1 | F:GGTCTTGGCGGCAGGAGTT R:GTGGCACAGGTGGCGTTGA | 102 |
| CASP3 | NM_001077840.1 | F:AAGATTTAGTGCCGATGC R:GACCACCAAGTTCTAGGATA | 175 |
| BAX | NM_173894.1 | F:GCAAACTGGTGCTCAAGG R:GCACTCCAGCCACAAAGA | 238 |
| GAPDH | NM_001034034.2 | F:GGCATCGTGGAGGGACTTATG R:GCCAGTGAGCTTCCCGTTGAG | 186 |
| RPS18 | NM_001033614.2 | F:GTGGTGTTGAGGAAAGCAGACA R:TGATCACACGTTCCACCTCATC | 79 |
Table 1 Primer information for RT-qPCR
| 引物名称Primer name | 序列号Serial number | 引物序列Primer sequence(5'-3') | 产物长度Product length/bp |
|---|---|---|---|
| miR-3604 | NR_036382.1 | F:AGTGCAGGGTCCGAGGTATT R:GCCTCATAACCAATGTGCAG | 63 |
| HOXA10 | NM_001105017.1 | F:TTTCCTTGGCAAAGAGAAGGGCTTG R:GACCTTACACAAACTGGAAGAGAC | 188 |
| IL-6 | NM_173923.2 | F:CACTCCATTCGCTGTCT R:GTGTCTCCTTGCTGCTT | 227 |
| VEGF | NM_001316955.1 | F:GTCTACCAGCGCAGCTTCTG R:TGCTGGCTTTGGTGAGGTT | 212 |
| LIF | NM_173931.1 | F:GGTCTTGGCGGCAGGAGTT R:GTGGCACAGGTGGCGTTGA | 102 |
| CASP3 | NM_001077840.1 | F:AAGATTTAGTGCCGATGC R:GACCACCAAGTTCTAGGATA | 175 |
| BAX | NM_173894.1 | F:GCAAACTGGTGCTCAAGG R:GCACTCCAGCCACAAAGA | 238 |
| GAPDH | NM_001034034.2 | F:GGCATCGTGGAGGGACTTATG R:GCCAGTGAGCTTCCCGTTGAG | 186 |
| RPS18 | NM_001033614.2 | F:GTGGTGTTGAGGAAAGCAGACA R:TGATCACACGTTCCACCTCATC | 79 |
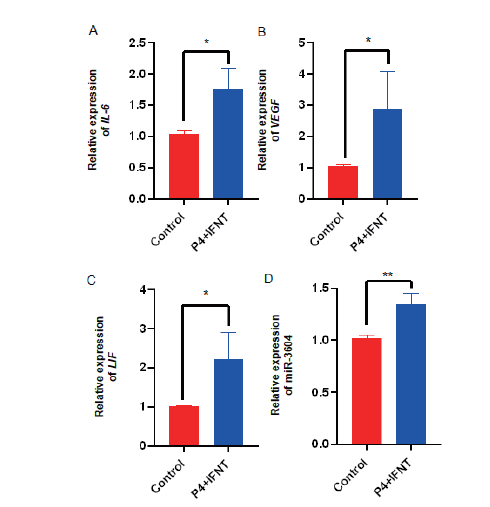
Fig. 1 Expressions of cell receptivity marker genes and miR-3604 in bEECs receptivity models A, B and C: Expressions of receptive marker genes IL-6, VEGF, and LIF. D: Expression of miR-3604, *P <0.05, **P <0.01, the same below
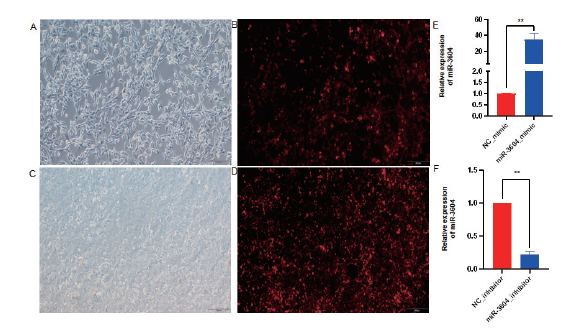
Fig. 2 Overexpression and interference efficiency detection of miR-3604 in bEECs A, B, C and D: Fluorescence observation of bEECs transfected with miR-3604(100×); E: mimic transfection efficiency; F: inhibitor transfection efficiency
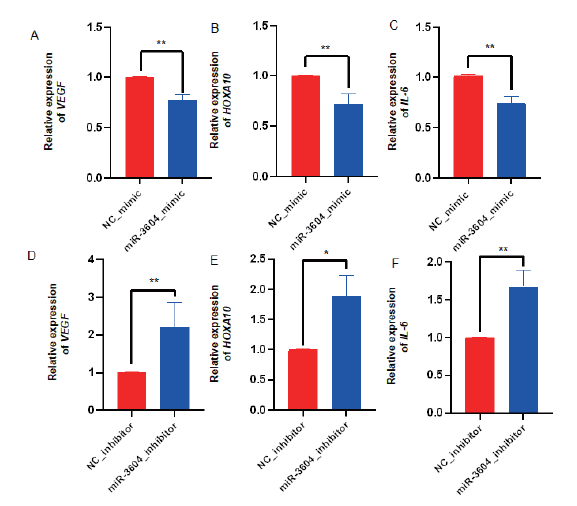
Fig. 3 Effect of miR-3604 on the expression of receptivity marker gene in bEECs A, B and C: The expressions of VEGF, HOXA10, and IL-6 in the miR-3604 overexpression group. D, E and F: The expressions of VEGF, HOXA10, and IL-6 in the miR-3604 inhibitor group
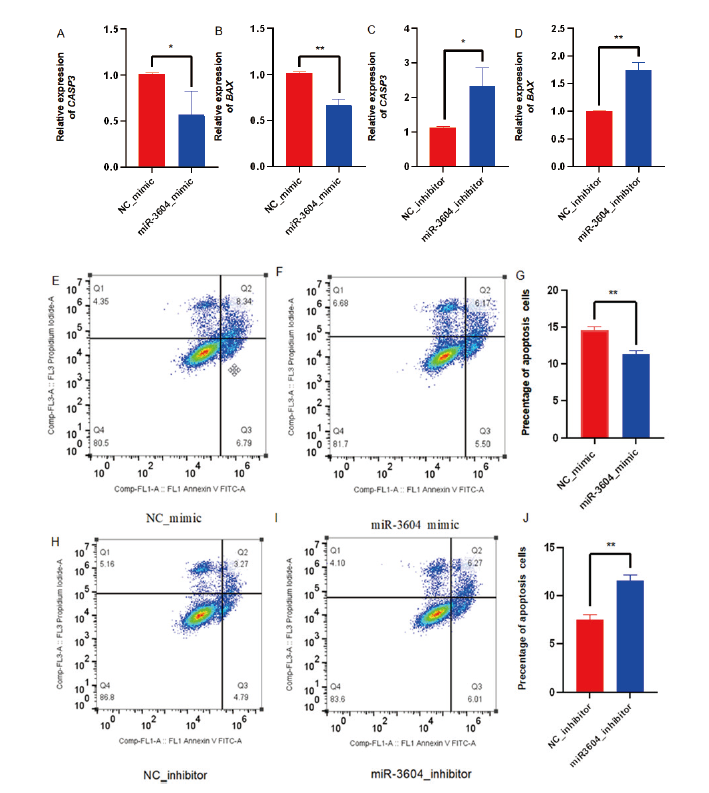
Fig. 4 Effect of miR-3604 on the apoptosis of receptive bEECs A-D: Expressions of CASP3 and BAX genes related to cell apoptosis detected by RT-qPCR. E-J: Cell apoptosis rate detected by flow cytometry
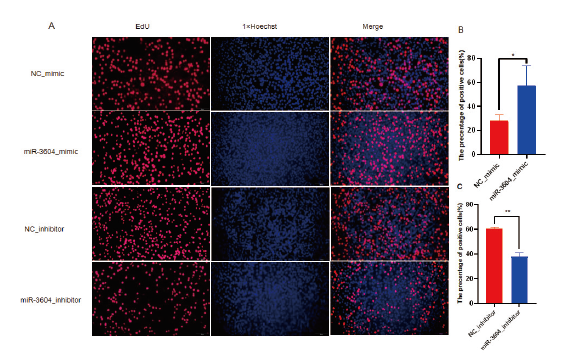
Fig. 5 Effect of miR-3604 on the proliferation of receptive bEECs A: Fluorescence observation of proliferating cells. B and C: Percentage of EdU positive cells
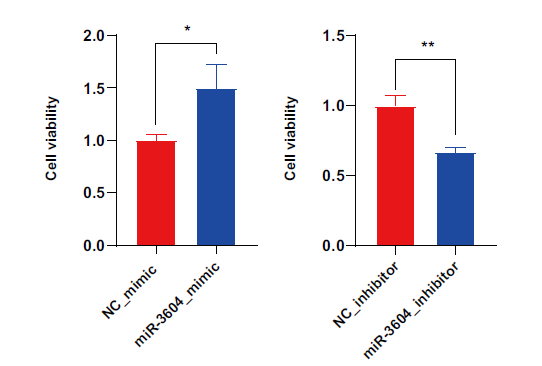
Fig. 6 Effects of miR-3604 on the viability of the receptive bEECs A: Effect of miR-3604 overexpression on the viability of bEECs. B: Effect of miR-3604 interference on the viability of bEECs
| [1] | Kaya A, Dogan S, Vargovic P, et al. Sperm proteins ODF2 and PAWP as markers of fertility in breeding bulls[J]. Cell Tissue Res, 2022, 387(1): 159-171. |
| [2] | 秦雪, 冯瑞, 李琦, 等. 氨离子对奶牛子宫内膜上皮细胞凋亡和容受性的影响[J]. 中国兽医学报, 2022, 42(3): 535-540. |
| Qin X, Feng R, Li Q, et al. Effects of ammonia on apoptosis and receptivity of endometrial epithelial cells of dairy cows[J]. Chin J Vet Sci, 2022, 42(3): 535-540. | |
| [3] |
Zhang S, Lin HY, Kong SB, et al. Physiological and molecular determinants of embryo implantation[J]. Mol Aspects Med, 2013, 34(5): 939-980.
doi: 10.1016/j.mam.2012.12.011 pmid: 23290997 |
| [4] |
Lonergan P. Influence of progesterone on oocyte quality and embryo development in cows[J]. Theriogenology, 2011, 76(9): 1594-1601.
doi: 10.1016/j.theriogenology.2011.06.012 pmid: 21855985 |
| [5] |
余婕, 胡修忠, 向敏, 等. 精氨酸对干扰素-tau处理牛子宫内膜上皮细胞基因表达的影响[J]. 中国畜牧兽医, 2021, 48(7): 2495-2503.
doi: 10.16431/j.cnki.1671-7236.2021.07.025 |
| Yu J, Hu XZ, Xiang M, et al. Effects of arginine on expression of genes in bovine endometrial epithelial cells treated with interferon-tau[J]. China Anim Husb Vet Med, 2021, 48(7): 2495-2503. | |
| [6] | Shekibi M, Heng S, Nie GY. MicroRNAs in the regulation of endometrial receptivity for embryo implantation[J]. Int J Mol Sci, 2022, 23(11): 6210. |
| [7] |
Lyu SJ, Zhai YY, Zhu XT, et al. Bta-miR-200b promotes endometrial epithelial cell apoptosis by targeting MYB in cattle[J]. Theriogenology, 2023, 195: 77-84.
doi: 10.1016/j.theriogenology.2022.10.006 pmid: 36332375 |
| [8] | Feng F, Li YX, Wang JP, et al. LncRNA CA12-AS1 targets miR-133a to promote LPS-induced inflammatory response in bovine mammary epithelial cells[J]. Int J Biol Macromol, 2024, 261(Pt 1): 129710. |
| [9] | Ostovar T, Zadehbagheri S, Hekmatimoghaddam SH. Comparison of different types of liposomal nano structures for microRNA transfection to human mesenchymal stem cell line S1939[J]. Nucleosides Nucleotides Nucleic Acids, 2023, 42(3): 217-233. |
| [10] |
Huang H, Li XY, Wang ZM, et al. Anti-inflammatory effect of selenium on lead-induced testicular inflammation by inhibiting NLRP3 inflammasome activation in chickens[J]. Theriogenology, 2020, 155: 139-149.
doi: S0093-691X(20)30365-4 pmid: 32673849 |
| [11] | 赵丽, 杨洋, 温传俊. 茎-环RT-PCR法定量miRNA-421的引物设计[J]. 南京师大学报: 自然科学版, 2012, 35(2): 83-88. |
| Zhao L, Yang Y, Wen CJ. Stem-loop real-time quantitative PCR for quantification of miRNA-421 by specific primers[J]. J Nanjing Norm Univ Nat Sci Ed, 2012, 35(2): 83-88. | |
| [12] |
Tranguch S, Daikoku T, Guo Y, et al. Molecular complexity in establishing uterine receptivity and implantation[J]. Cell Mol Life Sci, 2005, 62(17): 1964-1973.
doi: 10.1007/s00018-005-5230-0 pmid: 16143898 |
| [13] |
Lonergan P, Sánchez JM. Symposium review: Progesterone effects on early embryo development in cattle[J]. J Dairy Sci, 2020, 103(9): 8698-8707.
doi: S0022-0302(20)30510-5 pmid: 32622590 |
| [14] | 李琦, 秦雪, 冯瑞, 等. 孕酮对奶牛子宫内膜上皮细胞凋亡和容受性的影响[J]. 中国兽医学报, 2022, 42(9): 1915-1922. |
| Li Q, Qin X, Feng R, et al. Effects of progesterone on apoptosis and receptivity of endometrial epithelial cells in dairy cows[J]. Chin J Vet Sci, 2022, 42(9): 1915-1922. | |
| [15] |
Garcia-Ispierto I, López-Helguera I, Serrano-Pérez B, et al. Progesterone supplementation during the time of pregnancy recognition after artificial insemination improves conception rates in high-producing dairy cows[J]. Theriogenology, 2016, 85(7): 1343-1347.
doi: 10.1016/j.theriogenology.2015.12.021 pmid: 26786244 |
| [16] | Roberts RM. Interferon-tau, a Type 1 interferon involved in maternal recognition of pregnancy[J]. Cytokine Growth Factor Rev, 2007, 18(5/6): 403-408. |
| [17] |
Dorniak P, Bazer FW, Spencer TE. Physiology and Endocrinology Symposium: biological role of interferon tau in endometrial function and conceptus elongation[J]. J Anim Sci, 2013, 91(4): 1627-1638.
doi: 10.2527/jas.2012-5845 pmid: 23097402 |
| [18] |
Schulte MMB, Tsai JH, Moley KH. Obesity and PCOS: the effect of metabolic derangements on endometrial receptivity at the time of implantation[J]. Reprod Sci, 2015, 22(1): 6-14.
doi: 10.1177/1933719114561552 pmid: 25488942 |
| [19] |
Diedrich K, Fauser BCJM, Devroey P, et al. The role of the endometrium and embryo in human implantation[J]. Hum Reprod Update, 2007, 13(4): 365-377.
doi: 10.1093/humupd/dmm011 pmid: 17548368 |
| [20] |
Zhang L, Liu XR, Liu JZ, et al. MiR-26a promoted endometrial epithelium cells(EECs)proliferation and induced stromal cells(ESCs)apoptosis via the PTEN-PI3K/AKT pathway in dairy goats[J]. J Cell Physiol, 2018, 233(6): 4688-4706.
doi: 10.1002/jcp.26252 pmid: 29115668 |
| [21] | Eskandari E, Eaves CJ. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis[J]. J Cell Biol, 2022, 221(6): e202201159. |
| [22] |
Feng R, Qin X, Li Q, et al. Progesterone regulates inflammation and receptivity of cells via the NF-κB and LIF/STAT3 pathways[J]. Theriogenology, 2022, 186: 50-59.
doi: 10.1016/j.theriogenology.2022.04.005 pmid: 35430548 |
| [23] | Hajipour H, Sambrani R, Ghorbani M, et al. Sildenafil citrate-loaded targeted nanostructured lipid carrier enhances receptivity potential of endometrial cells via LIF and VEGF upregulation[J]. Naunyn Schmiedebergs Arch Pharmacol, 2021, 394(11): 2323-2331. |
| [24] | Park HR, Choi HJ, Kim BS, et al. Paeoniflorin enhances endometrial receptivity through leukemia inhibitory factor[J]. Biomolecules, 2021, 11(3): 439. |
| [25] |
Bi Y, Huang WY, Yuan LF, et al. HOXA10 improves endometrial receptivity by upregulating E-cadherin[J]. Biol Reprod, 2022, 106(5): 992-999.
doi: 10.1093/biolre/ioac007 pmid: 35044439 |
| [26] | Luo HL, Kimura K, Aoki M, et al. Vascular endothelial growth factor(VEGF)promotes the early development of bovine embryo in the presence of cumulus cells[J]. J Vet Med Sci, 2002, 64(11): 967-971. |
| [27] | Azimi-Nezhad M. Vascular endothelial growth factor from embryonic status to cardiovascular pathology[J]. Rep Biochem Mol Biol, 2014, 2(2): 59-69. |
| [28] | Wooldridge LK, Ealy AD. Interleukin-6 increases inner cell mass numbers in bovine embryos[J]. BMC Dev Biol, 2019, 19(1): 2. |
| [29] | Ashary N, Laheri S, Modi D. Homeobox genes in endometrium: from development to decidualization[J]. Int J Dev Biol, 2020, 64(1/2/3): 227-237. |
| [30] | Gebert LFR, MacRae IJ. Regulation of microRNA function in animals[J]. Nat Rev Mol Cell Biol, 2019, 20(1): 21-37. |
| [31] |
Li Q, Liu WM, Chiu PCN, et al. MiR-let-7a/g enhances uterine receptivity via suppressing Wnt/β-catenin under the modulation of ovarian hormones[J]. Reprod Sci, 2020, 27(5): 1164-1174.
doi: 10.1007/s43032-019-00115-3 pmid: 31942710 |
| [32] | Revel A, Achache H, Stevens J, et al. MicroRNAs are associated with human embryo implantation defects[J]. Hum Reprod, 2011, 26(10): 2830-2840. |
| [33] |
Akbar R, Ullah K, Rahman TU, et al. MiR-183-5p regulates uterine receptivity and enhances embryo implantation[J]. J Mol Endocrinol, 2020, 64(1): 43-52.
doi: 10.1530/JME-19-0184 pmid: 31786540 |
| [34] | Balaguer N, Moreno I, Herrero M, et al. MicroRNA-30d deficiency during preconception affects endometrial receptivity by decreasing implantation rates and impairing fetal growth[J]. Am J Obstet Gynecol, 2019, 221(1): 46.e1-46.46.e16. |
| [35] | Kang YJ, Lees M, Matthews LC, et al. MiR-145 suppresses embryo-epithelial juxtacrine communication at implantation by modulating maternal IGF1R[J]. J Cell Sci, 2015, 128(4): 804-814. |
| [36] |
Chen C, Zhao Y, Yu Y, et al. MiR-125b regulates endometrial receptivity by targeting MMP26 in women undergoing IVF-ET with elevated progesterone on HCG priming day[J]. Sci Rep, 2016, 6: 25302.
doi: 10.1038/srep25302 pmid: 27143441 |
| [37] | Yu SL, Kang YJ, Jeong DU, et al. The miR-182-5p/NDRG1 axis controls endometrial receptivity through the NF-κB/ZEB1/E-cadherin pathway[J]. Int J Mol Sci, 2022, 23(20): 12303. |
| [38] | Salilew-Wondim D, Gebremedhn S, Hoelker M, et al. The role of microRNAs in mammalian fertility: from gametogenesis to embryo implantation[J]. Int J Mol Sci, 2020, 21(2): 585. |
| [39] | Kolanska K, Bendifallah S, Canlorbe G, et al. Role of miRNAs in normal endometrium and in endometrial disorders: comprehensive review[J]. J Clin Med, 2021, 10(16): 3457. |
| [1] | DUAN Zi-peng, SUN Man-li, CHEN Yan-feng, DENG Tong-xing, JIN Shao-ju, FAN Wen-juan, CHEN Xu-dong. Astaxanthin Promotes the Proliferation and Differentiation of Chicken Muscle Stem Cells via AMPK/mTOR Signaling Pathway [J]. Biotechnology Bulletin, 2024, 40(11): 312-320. |
| [2] | YANG Xin-ran, WANG Jian-fang, MA Xin-hao, ZAN Lin-sen. Expression Analyses of m6A Methylase Genes in Bovine Adipogenesis [J]. Biotechnology Bulletin, 2022, 38(7): 70-79. |
| [3] | YIN Xiao-meng, CAO Xue-wei, WANG Fu-jun, ZHAO Jian, ZHANG Hui-zhan. Celastrol and Apoptin Mutant Exert Synergistic Anti-tumor Effects by Enhancing Nur77-induced Apoptosis Pathway [J]. Biotechnology Bulletin, 2020, 36(7): 119-129. |
| [4] | YANG Lei, YE Zhou-jie, LI Zhao-long, SHEN Yang-kun, FU Ya-juan. Effects of TET2 on T Cell Proliferation by Electroporation [J]. Biotechnology Bulletin, 2020, 36(1): 229-237. |
| [5] | LI Ping ,ZHANG Gui-ping, HU Jian-ran. Effects of Total Flavonoids from Forsythia suspense on the Proliferation of Gastric Cancer Cell MGC80-3 [J]. Biotechnology Bulletin, 2018, 34(6): 199-203. |
| [6] | ZHAI Yi-zhou ,LU Mei-ya ,ZHAO Jian ,WANG Fu-jun. Screening of a Gelonin Fusion Protein with High Cell-penetrating Efficiency and Its Anti-tumor Activity and Apoptosis Pathway [J]. Biotechnology Bulletin, 2018, 34(6): 204-212. |
| [7] | GUO Hong-yan, GAO Han, WU Qi, SUN Xiao-jie, LIU Xiu-cai, ZHAO Li-qun. Construction of SGK3 Gene Lentiviral RNA Interference Vector and Effects on Cell proliferation and Apoptosis of Breast Cancer Cell Line MB-474 [J]. Biotechnology Bulletin, 2018, 34(1): 247-252. |
| [8] | HU Jian-ran LI Ping LEI Hai-ying LIU Xian-rui. Effects of Polysaccharides from Lu Dangshen(Codonopsis pilosula)on Proliferation and Migration of Human Cervical SiHa [J]. Biotechnology Bulletin, 2017, 33(5): 159-163. |
| [9] | Liu Fengxia, Liu Wenjuan, Li Jianyong, Chen Shenguo. A Study on the Regulation Mechanism of ERK1/2 Promoting Proliferation and Inhibiting Apoptosis in Esophageal Squamous Carcinoma [J]. Biotechnology Bulletin, 2015, 31(10): 242-248. |
| [10] | He Dongyang,Ma Chao,Gao Zhenyue,Wang Shuzhen,Chen Yijun. The Comparison of Expression Efficiency of Human CD137L in Different Expression System [J]. Biotechnology Bulletin, 2014, 30(9): 178-184. |
| [11] | Zhang Xingfu, Du Ruiping, Gao Min, Ao Changjin, Lu Dexun, Cao Qina. Effects of Self-compounding DMEM/F12 Medium on Biological Function in Primary Cultured Bovine Mammary Epithelial Cells [J]. Biotechnology Bulletin, 2013, 0(3): 114-119. |
| [12] | Wu Wei Liu Chaoqi. Calreticulin and Apoptosis Mediated by Endoplasmic Reticulum Stress [J]. Biotechnology Bulletin, 2013, 29(10): 24-27. |
| [13] | Hui Huandong, Liu Yong, Liu Shaojun. Effect of Overexpressed Exogenous Necdin on the Cell Proliferation of P19 Embryonal Carcinoma Cells [J]. Biotechnology Bulletin, 2013, 29(10): 165-169. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||