








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (11): 312-320.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0228
Previous Articles Next Articles
DUAN Zi-peng1,2( ), SUN Man-li1,2, CHEN Yan-feng1,2, DENG Tong-xing1,2, JIN Shao-ju1,2, FAN Wen-juan1,2(
), SUN Man-li1,2, CHEN Yan-feng1,2, DENG Tong-xing1,2, JIN Shao-ju1,2, FAN Wen-juan1,2( ), CHEN Xu-dong1,2(
), CHEN Xu-dong1,2( )
)
Received:2024-03-08
Online:2024-11-26
Published:2024-12-19
Contact:
FAN Wen-juan, CHEN Xu-dong
E-mail:573373763@qq.com;fwj81@163.com
DUAN Zi-peng, SUN Man-li, CHEN Yan-feng, DENG Tong-xing, JIN Shao-ju, FAN Wen-juan, CHEN Xu-dong. Astaxanthin Promotes the Proliferation and Differentiation of Chicken Muscle Stem Cells via AMPK/mTOR Signaling Pathway[J]. Biotechnology Bulletin, 2024, 40(11): 312-320.
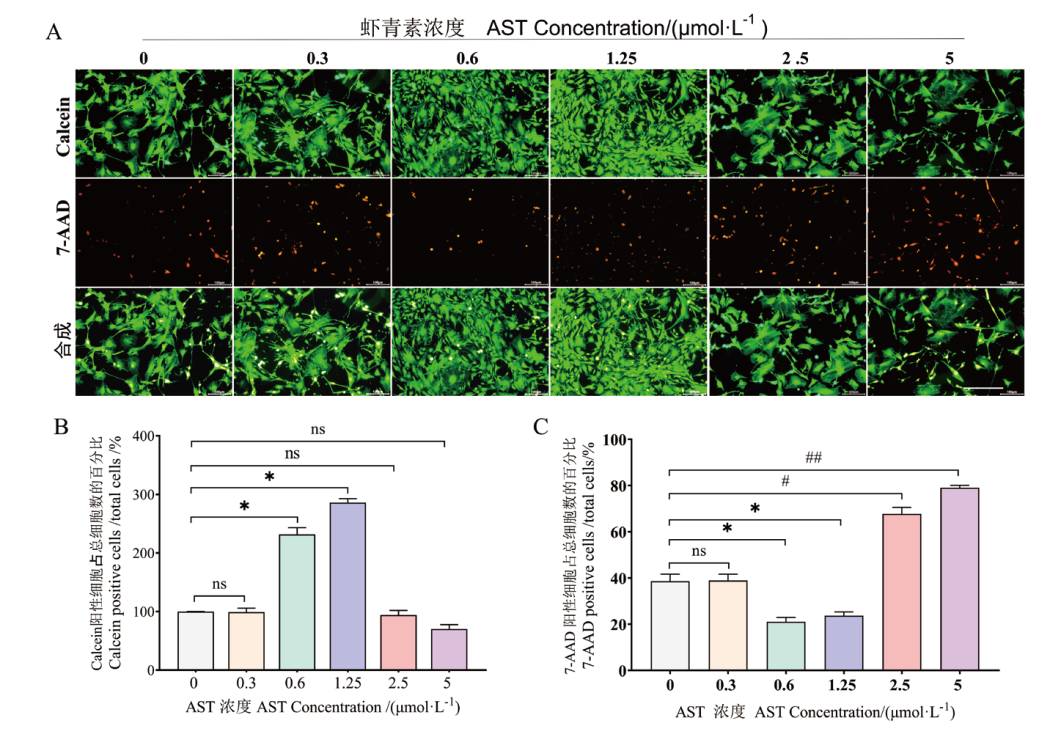
Fig. 1 Effects of different concentrations of AST on Ch-MuSCs cell activity and cell viability A: Under fluorescence microscope, live cells were stained with Calcein AM(green)and dead cells were stained with 7-AAD(red). Scale =100 μm. B: Relative percentage of Calcein AM positive cells in Ch-MuSCs after treatment with AST for 48 h. C: Relative percentage of 7-AAD-stained positive cells in Ch-MuSCs after treatment with AST for 48 h. Compared with control(0), *P < 0.05,# P <0.01,## P <0.001, ns indicates not statistically significant, the same below

Fig. 2 Effects of different concentrations of AST on the proliferation and cytotoxicity of Ch-Muscs A: EdU-labeled(red)indicates the proliferation of cells, DAPI marks nuclei. B: Percentage of EdU positive cells. C: The cell proliferation were estimated by MTT assay with AST for 48 h

Fig. 3 Effect of AST on the myogenic differentiation ability of Ch-Muscs A: Titin(red)and MyoD(green)immunofluorescence double labeling, showing differentiated muscle tubes and muscle nuclei. Bar=100 μm. B: Quantitative analysis of myotube length; C: number of nuclei per muscular duct

Fig. 4 Key protein levels in the PI3K/Akt signaling pathway measured by Western blot analysis A: Results of Western blot. B: Quantitative analysis of Western blots for AKT and p-AKT. C: Quantitative analysis of Western blots forPI3K and p-PI3K
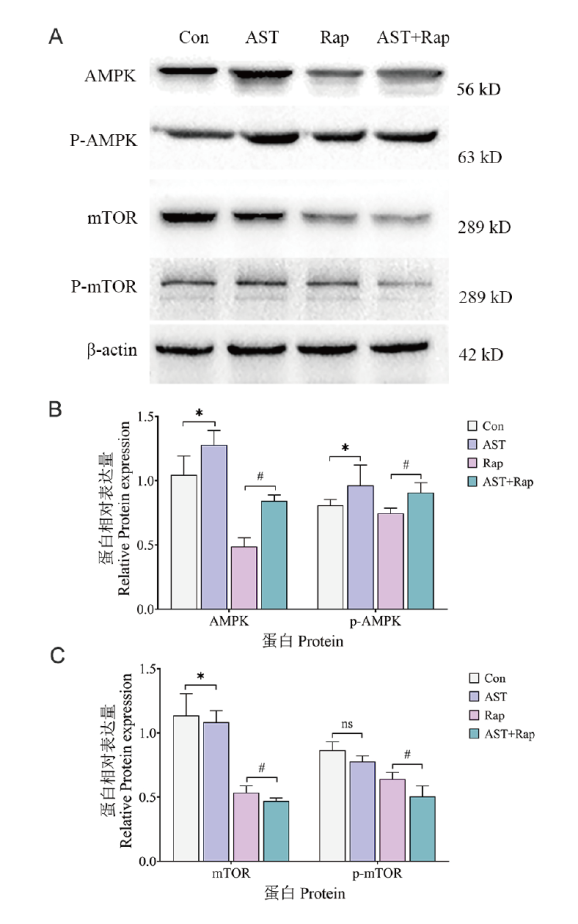
Fig. 5 Expressions of AMPK, p-AMPK, mTOR, and p-mTOR A: Results of Western blot. B: Quantitative analysis of Western blot for AMPK and p-AMPK. C: Quantitative analysis of Western blot for mTOR and p-mTOR
| [1] |
胡荣蓉, 丁世杰, 郭赟, 等. Trolox对猪肌肉干细胞增殖及分化的影响[J]. 中国农业科学, 2021, 54(24): 5290-5301.
doi: 10.3864/j.issn.0578-1752.2021.24.011 |
|
Hu RR, Ding SJ, Guo Y, et al. Effects of trolox on proliferation and differentiation of pig muscle stem cells[J]. Sci Agric Sin, 2021, 54(24): 5290-5301.
doi: 10.3864/j.issn.0578-1752.2021.24.011 |
|
| [2] | Dong WX, Chen WH, Zou HB, et al. Ginsenoside Rb1 prevents oxidative stress-induced apoptosis and mitochondrial dysfunction in muscle stem cells via NF- κ B pathway[J]. Oxid Med Cell Longev, 2022, 2022: 9159101. |
| [3] | Kumar S, Kumar R, Diksha, et al. Astaxanthin: a super antioxidant from microalgae and its therapeutic potential[J]. J Basic Microbiol, 2022, 62(9): 1064-1082. |
| [4] | Queen CJJ, Sparks SA, Marchant DC, et al. The effects of astaxanthin on cognitive function and neurodegeneration in humans: a critical review[J]. Nutrients, 2024, 16(6): 826. |
| [5] | Chen Y, Ling CJ, Chen MT, et al. Astaxanthin ameliorates worsened muscle dysfunction of MDX mice fed with a high-fat diet through reducing lipotoxicity and regulating gut microbiota[J]. Nutrients, 2023, 16(1): 33. |
| [6] |
Kjøbsted R, Hingst JR, Fentz J, et al. AMPK in skeletal muscle function and metabolism[J]. FASEB J, 2018, 32(4): 1741-1777.
doi: 10.1096/fj.201700442R pmid: 29242278 |
| [7] | Xu JH, Velleman SG. Critical role of the mTOR pathway in poultry skeletal muscle physiology and meat quality: an opinion paper[J]. Front Physiol, 2023, 14: 1228318. |
| [8] |
Ozaki Y, Ohashi K, Otaka N, et al. Myonectin protects against skeletal muscle dysfunction in male mice through activation of AMPK/PGC1α pathway[J]. Nat Commun, 2023, 14(1): 4675.
doi: 10.1038/s41467-023-40435-2 pmid: 37542026 |
| [9] |
周琳, 梁轩铭, 赵磊. 天然类胡萝卜素的生物合成研究进展[J]. 生物技术通报, 2022, 38(7): 119-127.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-1184 |
| Zhou L, Liang XM, Zhao L. Biosynthesis of natural carotenoids: progress and perspective[J]. Biotechnol Bull, 2022, 38(7): 119-127. | |
| [10] | Inoue H, Shimamoto S, Takahashi H, et al. Effects of astaxanthin-rich dried cell powder from Paracoccus carotinifaciens on carotenoid composition and lipid peroxidation in skeletal muscle of broiler chickens under thermo-neutral or realistic high temperature conditions[J]. Anim Sci J, 2019, 90(2): 229-236. |
| [11] | Cao YR, Yang L, Qiao X, et al. Dietary astaxanthin: an excellent carotenoid with multiple health benefits[J]. Crit Rev Food Sci Nutr, 2023, 63(18): 3019-3045. |
| [12] | Nishida Y, Berg PC, Shakersain B, et al. Astaxanthin: past, present, and future[J]. Mar Drugs, 2023, 21(10): 514. |
| [13] | Medoro A, Davinelli S, Milella L, et al. Dietary astaxanthin: a promising antioxidant and anti-inflammatory agent for brain aging and adult neurogenesis[J]. Mar Drugs, 2023, 21(12): 643. |
| [14] | Fang JH, Li M, Zhang GQ, et al. Vitamin C enhances the ex vivo proliferation of porcine muscle stem cells for cultured meat production[J]. Food Funct, 2022, 13(9): 5089-5101. |
| [15] |
Motohashi N, Asakura A. Muscle satellite cell heterogeneity and self-renewal[J]. Front Cell Dev Biol, 2014, 2: 1.
doi: 10.3389/fcell.2014.00001 pmid: 25364710 |
| [16] |
Mouradian S, Cicciarello D, Lacoste N, et al. LSD1 controls a nuclear checkpoint in Wnt/β-Catenin signaling to regulate muscle stem cell self-renewal[J]. Nucleic Acids Res, 2024, 52(7): 3667-3681.
doi: 10.1093/nar/gkae060 pmid: 38321961 |
| [17] | Lian D, Chen MM, Wu HY, et al. The role of oxidative stress in skeletal muscle myogenesis and muscle disease[J]. Antioxidants, 2022, 11(4): 755. |
| [18] | Liu Z, Lin L, Zhu HZ, et al. YAP promotes cell proliferation and stemness maintenance of porcine muscle stem cells under high-density condition[J]. Cells, 2021, 10(11): 3069. |
| [19] | Yoshihara T, Sugiura T, Miyaji N, et al. Effect of a combination of astaxanthin supplementation, heat stress, and intermittent reloading on satellite cells during disuse muscle atrophy[J]. J Zhejiang Univ Sci B, 2018, 19(11): 844-852. |
| [20] | Dose J, Matsugo S, Yokokawa H, et al. Free radical scavenging and cellular antioxidant properties of astaxanthin[J]. Int J Mol Sci, 2016, 17(1): 103. |
| [21] |
Yu TZ, Dohl J, Chen YF, et al. Astaxanthin but not quercetin preserves mitochondrial integrity and function, ameliorates oxidative stress, and reduces heat-induced skeletal muscle injury[J]. J Cell Physiol, 2019, 234(8): 13292-13302.
doi: 10.1002/jcp.28006 pmid: 30609021 |
| [22] | Zhou YS, Baker JS, Chen XP, et al. High-dose astaxanthin supplementation suppresses antioxidant enzyme activity during moderate-intensity swimming training in mice[J]. Nutrients, 2019, 11(6): 1244. |
| [23] | Kawamura A, Aoi W, Abe R, et al. Combined intake of astaxanthin, β-carotene, and resveratrol elevates protein synthesis during muscle hypertrophy in mice[J]. Nutrition, 2020, 69: 110561. |
| [24] |
Yoshihara T, Yamamoto Y, Shibaguchi T, et al. Dietary astaxanthin supplementation attenuates disuse-induced muscle atrophy and myonuclear apoptosis in the rat soleus muscle[J]. J Physiol Sci, 2017, 67(1): 181-190.
doi: 10.1007/s12576-016-0453-4 pmid: 27117878 |
| [25] | Jaime D, Fish LA, Madigan LA, et al. The MuSK-BMP pathway maintains myofiber size in slow muscle through regulation of Akt- mTOR signaling[J]. Res Sq, 2023: rs.3.rs-rs.2613527. |
| [26] | Tang G, Du Y, Guan HC, et al. Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals[J]. Br J Pharmacol, 2022, 179(1): 159-178. |
| [27] | Morissette MR, Cook SA, Buranasombati C, et al. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt[J]. Am J Physiol Cell Physiol, 2009, 297(5): C1124-C1132. |
| [28] | Di CN, Jia W. Food-derived bioactive peptides as momentous food components: can functional peptides passed through the PI3K/Akt/mTOR pathway and NF-κB pathway to repair and protect the skeletal muscle injury?[J]. Crit Rev Food Sci Nutr, 2023: 1-18. |
| [29] |
Kwak HJ, Kim J, Kim SY, et al. Moracin E and M isolated from Morus alba Linné induced the skeletal muscle cell proliferation via PI3K-Akt-mTOR signaling pathway[J]. Sci Rep, 2023, 13(1): 20570.
doi: 10.1038/s41598-023-47411-2 pmid: 37996535 |
| [30] | Chun Y, Kim J. AMPK-mTOR signaling and cellular adaptations in hypoxia[J]. Int J Mol Sci, 2021, 22(18): 9765. |
| [31] | Yan LS, Zhang SF, Luo G, et al. Schisandrin B mitigates hepatic steatosis and promotes fatty acid oxidation by inducing autophagy through AMPK/mTOR signaling pathway[J]. Metabolism, 2022, 131: 155200. |
| [1] | JIAO Dan-rong, MA Meng-xue, HE Bai-shui, XIE Long, ZUO Er-wei. Editing Features of High-fidelity CRISPR/Cas9 System in Poultry Cells [J]. Biotechnology Bulletin, 2024, 40(10): 191-197. |
| [2] | YANG Lan, ZHANG Chen-xi, FAN Xue-wei, WANG Yang-guang, WANG Chun-xiu, LI Wen-ting. Gene Cloning, Expression Pattern, and Promoter Activity Analysis of Chicken BMP15 [J]. Biotechnology Bulletin, 2023, 39(4): 304-312. |
| [3] | CHEN Chu-wen, LI Jie, ZHAO Rui-peng, LIU Yuan, WU Jin-bo, LI Zhi-xiong. Cloning, Tissue Expression Profile and Function Prediction of GPX3 Gene in Tibetan Chicken [J]. Biotechnology Bulletin, 2023, 39(3): 311-320. |
| [4] | ZHOU Lin, LIANG Xuan-ming, ZHAO Lei. Biosynthesis of Natural Carotenoids:Progress and Perspective [J]. Biotechnology Bulletin, 2022, 38(7): 119-127. |
| [5] | YANG Xin-ran, WANG Jian-fang, MA Xin-hao, ZAN Lin-sen. Expression Analyses of m6A Methylase Genes in Bovine Adipogenesis [J]. Biotechnology Bulletin, 2022, 38(7): 70-79. |
| [6] | DU Zhen-wei, ZHU Shuai-peng, MA Xiang-fei, LI Dong-hua, SUN Gui-rong. Cloning,Expression and Bioinformatics Analysis of the CDS Region of Chicken CEBPA Gene [J]. Biotechnology Bulletin, 2021, 37(8): 203-212. |
| [7] | ZHANG Chen, ZUO Qi-sheng, ZOU Yi-chen, ZHAO Juan-juan, ZHANG Ya-ni, LI Bi-chun. Study on the Function of Glycolysis in Inducing Chicken PGCLC in vitro Formation [J]. Biotechnology Bulletin, 2021, 37(6): 163-170. |
| [8] | YIN Jun-lei, ZHANG Yan-fang, ZOU Fan-yu, PAN Peng-tao, DUAN Yan-hong, QIU Shu-xing. Construction and Immunoprotection of sptP Deletion Mutant of Salmonella Pullorum [J]. Biotechnology Bulletin, 2021, 37(2): 122-128. |
| [9] | WANG Jing, DAI Dong, WU Shu-geng, ZHANG Hai-jun, QI Guang-hai. Advances in Successional Development and Early Establishment of the Chicken Intestinal Microbiota [J]. Biotechnology Bulletin, 2020, 36(2): 1-8. |
| [10] | YANG Lei, YE Zhou-jie, LI Zhao-long, SHEN Yang-kun, FU Ya-juan. Effects of TET2 on T Cell Proliferation by Electroporation [J]. Biotechnology Bulletin, 2020, 36(1): 229-237. |
| [11] | YU Hai-liang, ZOU Wen-bin, WANG Xiao-hui, LIN Yu-xin, DAI Guo-jun, ZHANG Tao, ZHANG Gen-xi, XIE Kai-zhou, WANG Jin-yu, SHI Hui-qiang. RNA Sequencing Analysis of Cecum Tissues of Jinghai Yellow Chickens Infected by E. tenella [J]. Biotechnology Bulletin, 2019, 35(11): 64-71. |
| [12] | ZHANG Ya-nan, LIN Ya-qiu, XU Qing, XU Ya-ou, HE Qing-hua. Correlation Analysis Between IRX3 Gene Expression and Intramuscular Fat Deposition in Tibetan Chicken [J]. Biotechnology Bulletin, 2018, 34(9): 219-223. |
| [13] | LI Ping ,ZHANG Gui-ping, HU Jian-ran. Effects of Total Flavonoids from Forsythia suspense on the Proliferation of Gastric Cancer Cell MGC80-3 [J]. Biotechnology Bulletin, 2018, 34(6): 199-203. |
| [14] | GUO Hong-yan, GAO Han, WU Qi, SUN Xiao-jie, LIU Xiu-cai, ZHAO Li-qun. Construction of SGK3 Gene Lentiviral RNA Interference Vector and Effects on Cell proliferation and Apoptosis of Breast Cancer Cell Line MB-474 [J]. Biotechnology Bulletin, 2018, 34(1): 247-252. |
| [15] | LI Dong-hua, WANG Xin-lei ,LI Zhuan-jian ,SUN Gui-rong ,KANG Xiang-tao, YAN Feng-bin. Research Advances on Whole Genome Sequencing of Chicken [J]. Biotechnology Bulletin, 2017, 33(7): 35-39. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||