








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (4): 9-20.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0497
Previous Articles Next Articles
LI Xiao-ming( ), SHANG Xiu-hua, WANG You-shuang, WU Zhi-hua(
), SHANG Xiu-hua, WANG You-shuang, WU Zhi-hua( )
)
Received:2024-05-28
Online:2025-04-26
Published:2025-04-25
Contact:
WU Zhi-hua
E-mail:lixiaoming203@163.com;wzhua2889@163.com
LI Xiao-ming, SHANG Xiu-hua, WANG You-shuang, WU Zhi-hua. Research Progress in Benzoxazinoids in Plants[J]. Biotechnology Bulletin, 2025, 41(4): 9-20.
分类 Classification | 缩写 Abbreviation | 化学全称 Chemical name | 官能团 Functional groups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | |||||||
| 苯并噁嗪酮 | 异羟肟酸
| DIBOA | 2,4-二羟基-1,4苯并噁嗪-3-酮 | H | H | H | |||
| Benzoxaziones | DIMBOA | 2,4-二羟基-7-甲氧基-1,4苯并噁嗪-4-酮 | H | OCH3 | H | ||||
| TRIBOA | 2,4,7-三羟基-1,4苯并噁嗪-3-酮 | H | OH | H | |||||
| TRIMBOA | 2,4,7-三羟基-8-甲氧基-1,4苯并噁嗪-3-酮 | H | OH | OCH3 | |||||
| DIM2BOA | 2,4-二羟基-7,8-二甲氧基-1,4苯并噁嗪-3-酮 | H | OCH3 | OCH3 | |||||
| 内酰胺 | HBOA | 2-羟基-2H-1,4-苯并噁嗪-3(4H)-酮 | H | H | H | ||||
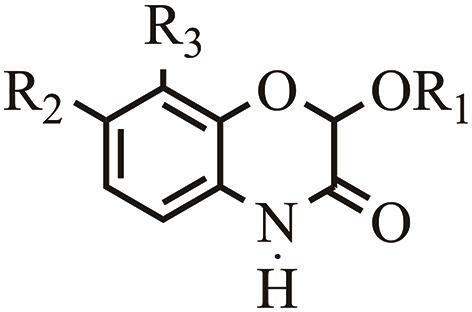 | DHBOA | 2,7-二羟基-1,4-苯并噁嗪-3-酮 | H | OH | H | ||||
| HMBOA | 2-羟基-7-甲氧基-2H-1,4-苯并噁嗪-3(4H)-酮 | H | OCH3 | H | |||||
| HM2BOA | 2-羟基-6,7-二甲氧基-2H-1,4-苯并噁嗪-3(4H)-酮 | H | OCH3 | OCH3 | |||||
甲基肟酸
| HDMBOA | 2-羟基-4,7-二甲氧基-1,4-苯并噁嗪-3-酮 | H | OCH3 | H | ||||
| HDM2BOA | 2-羟基-4,7,8-三甲氧基-1,4-苯并噁嗪-3-酮 | H | OCH3 | OCH3 | |||||
苯并噁嗪唑啉酮 Benzoxazolinones | 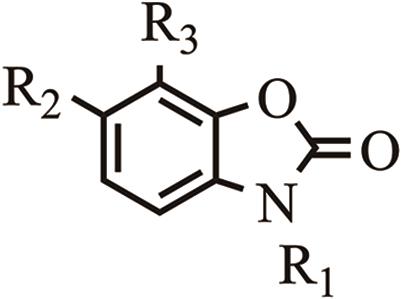 | BOA | 2-苯并噁唑啉-2(3H)酮 | H | H | H | |||
| MBOA | 6-甲氧基-苯并噁唑啉-2-酮 | H | OCH3 | H | |||||
| M2BOA | 2-羟基-2H-1,4-苯并噁嗪-3(4)酮 | H | OCH3 | OCH3 | |||||
Table 1 Classification and chemical names of BXs compounds
分类 Classification | 缩写 Abbreviation | 化学全称 Chemical name | 官能团 Functional groups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | |||||||
| 苯并噁嗪酮 | 异羟肟酸
| DIBOA | 2,4-二羟基-1,4苯并噁嗪-3-酮 | H | H | H | |||
| Benzoxaziones | DIMBOA | 2,4-二羟基-7-甲氧基-1,4苯并噁嗪-4-酮 | H | OCH3 | H | ||||
| TRIBOA | 2,4,7-三羟基-1,4苯并噁嗪-3-酮 | H | OH | H | |||||
| TRIMBOA | 2,4,7-三羟基-8-甲氧基-1,4苯并噁嗪-3-酮 | H | OH | OCH3 | |||||
| DIM2BOA | 2,4-二羟基-7,8-二甲氧基-1,4苯并噁嗪-3-酮 | H | OCH3 | OCH3 | |||||
| 内酰胺 | HBOA | 2-羟基-2H-1,4-苯并噁嗪-3(4H)-酮 | H | H | H | ||||
 | DHBOA | 2,7-二羟基-1,4-苯并噁嗪-3-酮 | H | OH | H | ||||
| HMBOA | 2-羟基-7-甲氧基-2H-1,4-苯并噁嗪-3(4H)-酮 | H | OCH3 | H | |||||
| HM2BOA | 2-羟基-6,7-二甲氧基-2H-1,4-苯并噁嗪-3(4H)-酮 | H | OCH3 | OCH3 | |||||
甲基肟酸
| HDMBOA | 2-羟基-4,7-二甲氧基-1,4-苯并噁嗪-3-酮 | H | OCH3 | H | ||||
| HDM2BOA | 2-羟基-4,7,8-三甲氧基-1,4-苯并噁嗪-3-酮 | H | OCH3 | OCH3 | |||||
苯并噁嗪唑啉酮 Benzoxazolinones |  | BOA | 2-苯并噁唑啉-2(3H)酮 | H | H | H | |||
| MBOA | 6-甲氧基-苯并噁唑啉-2-酮 | H | OCH3 | H | |||||
| M2BOA | 2-羟基-2H-1,4-苯并噁嗪-3(4)酮 | H | OCH3 | OCH3 | |||||
| 基因 Gene | 定位 Location | 编码酶 Encoding enzyme | 生化功能 Biochemical function |
|---|---|---|---|
| BX1 | 叶绿体 | 吲哚-3-甘油磷酸裂解酶 | Indole-3-glycerophosphate→Indole |
| BX2 | 内质网 | 细胞色素P450单加氧酶CYP71C4 | Indole→DIBOA |
| BX3 | 内质网 | 细胞色素P450单加氧酶CYP71C2 | |
| BX4 | 内质网 | 细胞色素P450单加氧酶CYP71C1 | |
| BX5 | 内质网 | 细胞色素P450单加氧酶CYP71C3 | |
| BX6 | 细胞质 | 2-酮戊二酸依赖型双加氧酶 | GDIBOA→GTRIBOA |
| BX7 | 细胞质 | O-甲基转移酶 | GTRI(M)BOA→GDIMBOA/GDIM2BOA |
| BX8 | 液泡 | UDP-葡萄糖基转移酶 | DIBOA→GDIBOA |
| BX9 | 液泡 | UDP-葡萄糖基转移酶 | |
| BX10 | 细胞质 | O-甲基转移酶 | GDIMBOA→GHDMBOA |
| BX11 | 细胞质 | O-甲基转移酶 | |
| BX12 | 细胞质 | O-甲基转移酶 | |
| BX13 | 细胞质 | 2-酮戊二酸依赖型双加氧酶 | GDIMBOA→GTRIMBOA |
| BX14 | 细胞质 | O-甲基转移酶 | GDIM2BOA→GHDM2BOA |
Table 2 Location and name of BXs biosynthesis genes and encoding enzyme
| 基因 Gene | 定位 Location | 编码酶 Encoding enzyme | 生化功能 Biochemical function |
|---|---|---|---|
| BX1 | 叶绿体 | 吲哚-3-甘油磷酸裂解酶 | Indole-3-glycerophosphate→Indole |
| BX2 | 内质网 | 细胞色素P450单加氧酶CYP71C4 | Indole→DIBOA |
| BX3 | 内质网 | 细胞色素P450单加氧酶CYP71C2 | |
| BX4 | 内质网 | 细胞色素P450单加氧酶CYP71C1 | |
| BX5 | 内质网 | 细胞色素P450单加氧酶CYP71C3 | |
| BX6 | 细胞质 | 2-酮戊二酸依赖型双加氧酶 | GDIBOA→GTRIBOA |
| BX7 | 细胞质 | O-甲基转移酶 | GTRI(M)BOA→GDIMBOA/GDIM2BOA |
| BX8 | 液泡 | UDP-葡萄糖基转移酶 | DIBOA→GDIBOA |
| BX9 | 液泡 | UDP-葡萄糖基转移酶 | |
| BX10 | 细胞质 | O-甲基转移酶 | GDIMBOA→GHDMBOA |
| BX11 | 细胞质 | O-甲基转移酶 | |
| BX12 | 细胞质 | O-甲基转移酶 | |
| BX13 | 细胞质 | 2-酮戊二酸依赖型双加氧酶 | GDIMBOA→GTRIMBOA |
| BX14 | 细胞质 | O-甲基转移酶 | GDIM2BOA→GHDM2BOA |
| 序号 Serial No. | 昆虫 Insect | 功能描述 Functional description | 参考 Reference |
|---|---|---|---|
| 1 | 蚜虫 | 利用蚜虫侵染玉米自交系后转录和代谢水平均发生显著变化,其中ZmBX1、ZmBX2和ZmBX6基因的突变增加了蚜虫的繁殖 | [ [ |
| 2 | 玉米螟虫 | 玉米中4个转录因子ZmWRKY75、ZmMYB61、ZmNAC35和ZmGRAS37的表达与ZmBX1的表达模式 相关,可能解释蚜虫侵染后苯并噁嗪类水平的变化; 叶片摄食抗性与羟肟酸浓度呈正相关,DIMBOA具有与羟肟酸浓度相当的摄食威慑作用,影响幼虫神经系统酶和解毒酶的活性; 西南玉米螟的抗性与HDMBOA的存在相关,对饲料中的幼虫具有毒性作用; DIMBOA和MBOA均增加了幼虫的死亡率和化蛹的发育时间,且与浓度水平呈正相关 | [ [ [ |
| 3 | 甜菜夜蛾 | 甜菜夜蛾侵染玉米后基因表达和代谢图谱在1 h内发生迅速改变,ZmBX1和ZmBX2的突变显著改善了幼虫的生长 | [ |
| 4 | 斜纹夜蛾 | 经过DIMBOA预处理的幼虫对各类杀虫剂产生高抗性,转录组分析发现解毒酶对斜纹夜蛾基因表达发挥重要作用 | [ |
| 5 | 玉米根虫 | 玉米根中施加DIMBOA会增加玉米根虫的死亡率,根系BXs含量与抗毒性呈显著正相关 | [ |
| 6 | 玉米线虫 | 玉米根中BXs化合物的浓度与线虫序列读数的相对丰度呈负相关,但与线虫类群之间存在正相关 | [ |
| 7 | 草地贪夜蛾 | 草地贪夜蛾通过UDP-葡萄糖基转移酶进行立体选择性重新葡萄糖基化,对DIMBOA进行解毒; UGT33和UGT40参与苯并噁嗪的糖基化,并参与对草地贪夜蛾幼虫的解毒; 模拟草地贪夜蛾取食后BXs含量增加,启动子和共表达分析表明,转录因子ZmbHLH57与ZmWRKY34调控系统叶片中的BXs生物合成基因 | [ [ [ |
| 8 | 小麦球茎蝇 | MBOA对幼虫的吸引呈剂量依赖性,并可能促进分泌物活性,而DIMBOA引起的反应较弱,因为土壤中MBOA比DIMBOA更稳定 | [ |
Table 3 Effects of BXs compounds on insects
| 序号 Serial No. | 昆虫 Insect | 功能描述 Functional description | 参考 Reference |
|---|---|---|---|
| 1 | 蚜虫 | 利用蚜虫侵染玉米自交系后转录和代谢水平均发生显著变化,其中ZmBX1、ZmBX2和ZmBX6基因的突变增加了蚜虫的繁殖 | [ [ |
| 2 | 玉米螟虫 | 玉米中4个转录因子ZmWRKY75、ZmMYB61、ZmNAC35和ZmGRAS37的表达与ZmBX1的表达模式 相关,可能解释蚜虫侵染后苯并噁嗪类水平的变化; 叶片摄食抗性与羟肟酸浓度呈正相关,DIMBOA具有与羟肟酸浓度相当的摄食威慑作用,影响幼虫神经系统酶和解毒酶的活性; 西南玉米螟的抗性与HDMBOA的存在相关,对饲料中的幼虫具有毒性作用; DIMBOA和MBOA均增加了幼虫的死亡率和化蛹的发育时间,且与浓度水平呈正相关 | [ [ [ |
| 3 | 甜菜夜蛾 | 甜菜夜蛾侵染玉米后基因表达和代谢图谱在1 h内发生迅速改变,ZmBX1和ZmBX2的突变显著改善了幼虫的生长 | [ |
| 4 | 斜纹夜蛾 | 经过DIMBOA预处理的幼虫对各类杀虫剂产生高抗性,转录组分析发现解毒酶对斜纹夜蛾基因表达发挥重要作用 | [ |
| 5 | 玉米根虫 | 玉米根中施加DIMBOA会增加玉米根虫的死亡率,根系BXs含量与抗毒性呈显著正相关 | [ |
| 6 | 玉米线虫 | 玉米根中BXs化合物的浓度与线虫序列读数的相对丰度呈负相关,但与线虫类群之间存在正相关 | [ |
| 7 | 草地贪夜蛾 | 草地贪夜蛾通过UDP-葡萄糖基转移酶进行立体选择性重新葡萄糖基化,对DIMBOA进行解毒; UGT33和UGT40参与苯并噁嗪的糖基化,并参与对草地贪夜蛾幼虫的解毒; 模拟草地贪夜蛾取食后BXs含量增加,启动子和共表达分析表明,转录因子ZmbHLH57与ZmWRKY34调控系统叶片中的BXs生物合成基因 | [ [ [ |
| 8 | 小麦球茎蝇 | MBOA对幼虫的吸引呈剂量依赖性,并可能促进分泌物活性,而DIMBOA引起的反应较弱,因为土壤中MBOA比DIMBOA更稳定 | [ |
| 序号 Serial No. | 菌属 Strain genus | 功能描述 Functional description | 参考 Reference |
|---|---|---|---|
| 1 | 禾谷镰刀菌 | DIMBOA消除了禾谷镰刀菌毒素的产生,对真菌生长没有明显影响,并强烈影响转录调节因子Tri6的表达,还抑制Tri5的表达 | [ |
| 2 | 轮枝镰刀菌 | 轮枝镰刀菌对MBOA和BOA具有高度耐受性,并且可以主动将这些化合物转化为无毒代谢物 | [ |
| 3 | 双极杆菌 | 玉米叶枯病的侵染程度与叶片中的羟肟酸浓度呈负相关,DIMBOA抑制了真菌的发育; 小麦感染叶斑病后BXs生物合成及精氨酸和脯氨酸代谢相关基因和差异代谢物酚酸、生物碱和黄酮类化合物高度富集 | [ [ |
| 4 | 从枝菌根 | DIMBOA强烈抑制菌丝体的生长,其中ZmBX9表达量显著升高 | [ |
| 5 | 叶锈菌 | GDIBOA和DIMBOA是黑麦对褐锈病防御反应的重要组成部分 | [ |
| 6 | 尖孢镰刀菌 | 外源添加DIMBOA和MBOA降低了植物细胞壁降解酶的活性和镰刀菌酸含量,显著减弱了镰刀菌的致病性; BXs化合物对镰刀菌的菌丝生长、孢子萌发和孢子形成和根部分泌物表现出显著的化感作用, 还表现出破坏镰刀菌的菌丝体膜和细胞壁的结构完整性和稳定性 | [ [ |
| 7 | 异旋孢腔菌/ 炭疽菌 | ZmPep1刺激ZmBX1基因的表达,叶子中GHDMBOA的积累,预处理发现ZmPep1可增强南方叶枯病和茎腐病的抵抗力 | [ |
Table 4 Effects of BXs compounds on pathogenic bacteria
| 序号 Serial No. | 菌属 Strain genus | 功能描述 Functional description | 参考 Reference |
|---|---|---|---|
| 1 | 禾谷镰刀菌 | DIMBOA消除了禾谷镰刀菌毒素的产生,对真菌生长没有明显影响,并强烈影响转录调节因子Tri6的表达,还抑制Tri5的表达 | [ |
| 2 | 轮枝镰刀菌 | 轮枝镰刀菌对MBOA和BOA具有高度耐受性,并且可以主动将这些化合物转化为无毒代谢物 | [ |
| 3 | 双极杆菌 | 玉米叶枯病的侵染程度与叶片中的羟肟酸浓度呈负相关,DIMBOA抑制了真菌的发育; 小麦感染叶斑病后BXs生物合成及精氨酸和脯氨酸代谢相关基因和差异代谢物酚酸、生物碱和黄酮类化合物高度富集 | [ [ |
| 4 | 从枝菌根 | DIMBOA强烈抑制菌丝体的生长,其中ZmBX9表达量显著升高 | [ |
| 5 | 叶锈菌 | GDIBOA和DIMBOA是黑麦对褐锈病防御反应的重要组成部分 | [ |
| 6 | 尖孢镰刀菌 | 外源添加DIMBOA和MBOA降低了植物细胞壁降解酶的活性和镰刀菌酸含量,显著减弱了镰刀菌的致病性; BXs化合物对镰刀菌的菌丝生长、孢子萌发和孢子形成和根部分泌物表现出显著的化感作用, 还表现出破坏镰刀菌的菌丝体膜和细胞壁的结构完整性和稳定性 | [ [ |
| 7 | 异旋孢腔菌/ 炭疽菌 | ZmPep1刺激ZmBX1基因的表达,叶子中GHDMBOA的积累,预处理发现ZmPep1可增强南方叶枯病和茎腐病的抵抗力 | [ |
| 序号 Serial No. | 非生物因子 Abiotic factor | 功能描述 Functional description | 参考 Reference |
|---|---|---|---|
| 1 | 光照 | 蓝光通过受体信号转导被照侧β-葡萄糖苷酶的活性,随后DIMBOA和MBOA的上调导致表皮细胞层的生长抑制; 光周期和温度可刺激冬小麦中MBOA含量,并随着植株的年龄增长而降低 | [ [ |
| 2 | 温度 | 4℃处理BXs浓度和基因表达量均有所下降,控制春化期持续时间可显著影响黑麦中BXs的含量; ZmBX12被认为是影响玉米雄蕊和雌蕊开花的驯化位点,向更温暖区域种植ZmBX12表达量更高,玉米幼苗中GHDMBOA含量更高 | [ [ |
| 3 | 水分 | 水分是影响叶片防御的重要因素,受到ABA的正向调节,激活DIMBOA的防御作用 | [ |
| 4 | 干旱 | 干旱条件下玉米秸秆的DIMBOA浓度会增加,GHDMBOA浓度显著降低; 干旱条件下非靶代谢分析揭示ZmBX12和ZmGLK44基因在调控玉米生物合成和抗旱性中的作用机制 | [ [ |
| 5 | 盐度 | 盐胁迫导致GIMBOA水平显著积累,且DIMBOA、GHDMBOA和GDIM2BOA水平增加,表明盐度对BXs生物合成具有很强的诱导作用 | [ |
| 6 | 机械损伤 | 机械损伤处理6 h后,第2叶的DIMBOA含量显著增加;根系中DIMBOA和ZmBX6和ZmBX9基因表达量明显下降 | [ |
Table 5 BXs responses to various abiotic stresses
| 序号 Serial No. | 非生物因子 Abiotic factor | 功能描述 Functional description | 参考 Reference |
|---|---|---|---|
| 1 | 光照 | 蓝光通过受体信号转导被照侧β-葡萄糖苷酶的活性,随后DIMBOA和MBOA的上调导致表皮细胞层的生长抑制; 光周期和温度可刺激冬小麦中MBOA含量,并随着植株的年龄增长而降低 | [ [ |
| 2 | 温度 | 4℃处理BXs浓度和基因表达量均有所下降,控制春化期持续时间可显著影响黑麦中BXs的含量; ZmBX12被认为是影响玉米雄蕊和雌蕊开花的驯化位点,向更温暖区域种植ZmBX12表达量更高,玉米幼苗中GHDMBOA含量更高 | [ [ |
| 3 | 水分 | 水分是影响叶片防御的重要因素,受到ABA的正向调节,激活DIMBOA的防御作用 | [ |
| 4 | 干旱 | 干旱条件下玉米秸秆的DIMBOA浓度会增加,GHDMBOA浓度显著降低; 干旱条件下非靶代谢分析揭示ZmBX12和ZmGLK44基因在调控玉米生物合成和抗旱性中的作用机制 | [ [ |
| 5 | 盐度 | 盐胁迫导致GIMBOA水平显著积累,且DIMBOA、GHDMBOA和GDIM2BOA水平增加,表明盐度对BXs生物合成具有很强的诱导作用 | [ |
| 6 | 机械损伤 | 机械损伤处理6 h后,第2叶的DIMBOA含量显著增加;根系中DIMBOA和ZmBX6和ZmBX9基因表达量明显下降 | [ |
| 序号 Serial No. | 激素 Phytohormone | 功能描述 Functional description | 参考 Reference |
|---|---|---|---|
| 1 | 生长素AUX | ZmAuXRP1可能作为资源调节剂,调节IAA和BX生物合成途径之间分支点上的IGL和吲哚通量; DIMBOA在抑制生长素-1-萘乙酸与膜结合或溶解受体结合方面活性高于MBOA,从而抑制生长素结合活性及诱导生长能力 | [ [ |
| 2 | 脱落酸ABA | ABA诱导BXs基因在叶片中转录,与植物的渗透胁迫有关,斜纹夜蛾的地下攻击引发了ABA局部积累和BXs含量增加 | [ |
| 3 | 水杨酸SA | 不同浓度的SA处理玉米叶片,DIMBOA含量和多酚氧化酶活性显著增强 | [ |
| 4 | 茉莉酸JA | 外施茉莉酸使Bt玉米第1叶DIMBOA含量明显增加,表明导入抗性和自身化学防御过程之间的互作关系是协同的; 茉莉酸对小麦和薏米的GHDMBOA积累具有诱导作用,GDIMBOA含量相应降低,而黑麦中没有 这种变化 | [ [ |
| 5 | 细胞分裂素CTK | 玉米幼苗外源施加细胞分裂素后导致韧皮部汁液中BXs含量增加,DIMBOA能够增强CTK的活性,并与多酚氧化酶或过氧化酶反应形成的电子受体前体 | [ |
| 6 | 乙烯ETH | ZmMPK6以乙烯依赖性方式抑制DIMBOA/GDIMBOA的积累,可能控制乙烯信号通路中的MYB转录因子调控ZmBX1的转录,影响下游BXs基因的表达 | [ |
| 7 | 水杨酸甲酯/茉莉酸甲酯MeSA/MeJA | 外源施加MeSA和MeJA对DIMBOA含量具有化学诱导作用,在一定浓度范围内诱导抗性强度与浓度呈正相关,超过最佳浓度,随着浓度增大而DIMBOA含量下降 | [ |
| 8 | 赤霉素GA | BXs抑制了大麦种子中α-淀粉酶活性,同样还抑制赤霉素诱导的大麦去胚种子中α-淀粉酶的活性 | [ |
Table 6 BXs involved in plants hormone signaling pathways
| 序号 Serial No. | 激素 Phytohormone | 功能描述 Functional description | 参考 Reference |
|---|---|---|---|
| 1 | 生长素AUX | ZmAuXRP1可能作为资源调节剂,调节IAA和BX生物合成途径之间分支点上的IGL和吲哚通量; DIMBOA在抑制生长素-1-萘乙酸与膜结合或溶解受体结合方面活性高于MBOA,从而抑制生长素结合活性及诱导生长能力 | [ [ |
| 2 | 脱落酸ABA | ABA诱导BXs基因在叶片中转录,与植物的渗透胁迫有关,斜纹夜蛾的地下攻击引发了ABA局部积累和BXs含量增加 | [ |
| 3 | 水杨酸SA | 不同浓度的SA处理玉米叶片,DIMBOA含量和多酚氧化酶活性显著增强 | [ |
| 4 | 茉莉酸JA | 外施茉莉酸使Bt玉米第1叶DIMBOA含量明显增加,表明导入抗性和自身化学防御过程之间的互作关系是协同的; 茉莉酸对小麦和薏米的GHDMBOA积累具有诱导作用,GDIMBOA含量相应降低,而黑麦中没有 这种变化 | [ [ |
| 5 | 细胞分裂素CTK | 玉米幼苗外源施加细胞分裂素后导致韧皮部汁液中BXs含量增加,DIMBOA能够增强CTK的活性,并与多酚氧化酶或过氧化酶反应形成的电子受体前体 | [ |
| 6 | 乙烯ETH | ZmMPK6以乙烯依赖性方式抑制DIMBOA/GDIMBOA的积累,可能控制乙烯信号通路中的MYB转录因子调控ZmBX1的转录,影响下游BXs基因的表达 | [ |
| 7 | 水杨酸甲酯/茉莉酸甲酯MeSA/MeJA | 外源施加MeSA和MeJA对DIMBOA含量具有化学诱导作用,在一定浓度范围内诱导抗性强度与浓度呈正相关,超过最佳浓度,随着浓度增大而DIMBOA含量下降 | [ |
| 8 | 赤霉素GA | BXs抑制了大麦种子中α-淀粉酶活性,同样还抑制赤霉素诱导的大麦去胚种子中α-淀粉酶的活性 | [ |
| 1 | Hartmann T. From waste products to ecochemicals: fifty years research of plant secondary metabolism [J]. Phytochemistry, 2007, 68(22/23/24): 2831-2846. |
| 2 | Fernie AR, Pichersky E. Focus issue on metabolism: metabolites, metabolites everywhere [J]. Plant Physiol, 2015, 169(3): 1421-1423. |
| 3 | Erb M, Kliebenstein DJ. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy [J]. Plant Physiol, 2020, 184(1): 39-52. |
| 4 | Li YQ, Kong DX, Fu Y, et al. The effect of developmental and environmental factors on secondary metabolites in medicinal plants [J]. Plant Physiol Biochem, 2020, 148: 80-89. |
| 5 | Adhikari KB, Tanwir F, Gregersen PL, et al. Benzoxazinoids: Cereal phytochemicals with putative therapeutic and health-protecting properties [J]. Mol Nutr Food Res, 2015, 59(7): 1324-1338. |
| 6 | Yang L, Wen KS, Ruan X, et al. Response of plant secondary metabolites to environmental factors [J]. Molecules, 2018, 23(4): 762. |
| 7 | Bennett RN, Wallsgrove RM. Secondary metabolites in plant defence mechanisms [J]. New Phytol, 1994, 127(4): 617-633. |
| 8 | Patra B, Schluttenhofer C, Wu YM, et al. Transcriptional regulation of secondary metabolite biosynthesis in plants [J]. Biochim Biophys Acta, 2013, 1829(11): 1236-1247. |
| 9 | Ma ZQ, Li SS, Zhang MJ, et al. Light intensity affects growth, photosynthetic capability, and total flavonoid accumulation of Anoectochilus plants [J]. HortScience, 2010, 45(6): 863-867. |
| 10 | Sanchita, Sharma A. Gene expression analysis in medicinal plants under abiotic stress conditions [M]//Plant Metabolites and Regulation Under Environmental Stress. Amsterdam: Elsevier, 2018: 407-414. |
| 11 | Wink M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective [J]. Phytochemistry, 2003, 64(1): 3-19. |
| 12 | 朱三明, 郑敏敏, 田恬, 等. 植物次生代谢途径与调控研究进展 [J]. 植物生理学报, 2023, 59(12): 2188-2216. |
| Zhu SM, Zheng MM, Tian T, et al. Research progress on plant secondary metabolism and regulation [J]. Plant Physiol J, 2023, 59(12): 2188-2216. | |
| 13 | 朱璐, 袁冲, 刘义飞. 植物次生代谢产物生物合成基因簇研究进展 [J]. 植物学报, 2024, 59(1): 134-143. |
| Zhu L, Yuan C, Liu YF. Research progress on plant secondary metabolite biosynthetic gene clusters [J]. Chin Bull Bot, 2024, 59(1): 134-143. | |
| 14 | Dixon RA, Strack D. Phytochemistry meets genome analysis, and beyond [J]. Phytochemistry, 2003, 62(6): 815-816. |
| 15 | de Bruijn WJC, Vincken JP, Duran K, et al. Mass spectrometric characterization of benzoxazinoid glycosides from Rhizopus-elicited wheat (Triticum aestivum) seedlings [J]. J Agric Food Chem, 2016, 64(32): 6267-6276. |
| 16 | Hanhineva K, Rogachev I, Aura AM, et al. Qualitative characterization of benzoxazinoid derivatives in whole grain rye and wheat by LC-MS metabolite profiling [J]. J Agric Food Chem, 2011, 59(3): 921-927. |
| 17 | Niemeyer HM. Hydroxamic acids derived from 2-hydroxy-2H-1, 4-benzoxazin-3(4H)-one: key defense chemicals of cereals [J]. J Agric Food Chem, 2009, 57(5): 1677-1696. |
| 18 | Sicker D, Frey M, Schulz M, et al. Role of natural benzoxazinones in the survival strategy of plants [J]. Int Rev Cytol, 2000, 198: 319-346. |
| 19 | Frey M, Chomet P, Glawischnig E, et al. Analysis of a chemical plant defense mechanism in grasses [J]. Science, 1997, 277(5326): 696-699. |
| 20 | Von Rad U, Hüttl R, Lottspeich F, et al. Two glucosyltransferases are involved in detoxification of benzoxazinoids in maize [J]. Plant J, 2001, 28(6): 633-642. |
| 21 | Jonczyk R, Schmidt H, Osterrieder A, et al. Elucidation of the final reactions of DIMBOA-glucoside biosynthesis in maize: characterization of Bx6 and Bx7 [J]. Plant Physiol, 2008, 146(3): 1053-1063. |
| 22 | Sue M, Nakamura C, Nomura T. Dispersed benzoxazinone gene cluster: molecular characterization and chromosomal localization of glucosyltransferase and glucosidase genes in wheat and rye [J]. Plant Physiol, 2011, 157(3): 985-997. |
| 23 | Bakera B, Makowska B, Groszyk J, et al. Structural characteristics of ScBx genes controlling the biosynthesis of hydroxamic acids in rye (Secale cereale L.) [J]. J Appl Genet, 2015, 56(3): 287-298. |
| 24 | Kriechbaumer V, Weigang LD, Fiesselmann A, et al. Characterisation of the tryptophan synthase alpha subunit in maize [J]. BMC Plant Biol, 2008, 8: 44. |
| 25 | Spiteller P, Glawischnig E, Gierl A, et al. Studies on the biosynthesis of 2-hydroxy-1, 4-benzoxazin-3-one (HBOA) from 3-hydroxyindolin-2-one in Zea mays [J]. Phytochemistry, 2001, 57(3): 373-376. |
| 26 | Meihls LN, Handrick V, Glauser G, et al. Natural variation in maize aphid resistance is associated with 2, 4-dihydroxy-7-methoxy-1, 4-benzoxazin-3-one glucoside methyltransferase activity [J]. Plant Cell, 2013, 25(6): 2341-2355. |
| 27 | Handrick V, Robert CAM, Ahern KR, et al. Biosynthesis of 8-O-methylated benzoxazinoid defense compounds in maize [J]. Plant Cell, 2016, 28(7): 1682-1700. |
| 28 | Frey M, Schullehner K, Dick R, et al. Benzoxazinoid biosynthesis, a model for evolution of secondary metabolic pathways in plants [J]. Phytochemistry, 2009, 70(15/16): 1645-1651. |
| 29 | Niculaes C, Abramov A, Hannemann L, et al. Plant protection by benzoxazinoids—recent insights into biosynthesis and function [J]. Agronomy, 2018, 8(8): 143. |
| 30 | Nützmann HW, Huang AC, Osbourn A. Plant metabolic clusters from genetics to genomics [J]. New Phytol, 2016, 211(3): 771-789. |
| 31 | Wisecaver JH, Borowsky AT, Tzin V, et al. A global coexpression network approach for connecting genes to specialized metabolic pathways in plants [J]. Plant Cell, 2017, 29(5): 944-959. |
| 32 | Zheng LL, McMullen MD, Bauer E, et al. Prolonged expression of the BX1 signature enzyme is associated with a recombination hotspot in the benzoxazinoid gene cluster in Zea mays [J]. J Exp Bot, 2015, 66(13): 3917-3930. |
| 33 | Dutartre L, Hilliou F, Feyereisen R. Phylogenomics of the benzoxazinoid biosynthetic pathway of Poaceae: gene duplications and origin of the Bx cluster [J]. BMC Evol Biol, 2012, 12: 64. |
| 34 | Nomura T, Nasuda S, Kawaura K, et al. Structures of the three homoeologous loci of wheat benzoxazinone biosynthetic genes TaBx3 and TaBx4 and characterization of their promoter sequences [J]. Theor Appl Genet, 2008, 116(3): 373-381. |
| 35 | Rokas A, Wisecaver JH, Lind AL. The birth, evolution and death of metabolic gene clusters in fungi [J]. Nat Rev Microbiol, 2018, 16(12): 731-744. |
| 36 | Wittkopp PJ, Kalay G. Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence [J]. Nat Rev Genet, 2011, 13(1): 59-69. |
| 37 | Nomura T, Ishihara A, Yanagita RC, et al. Three genomes differentially contribute to the biosynthesis of benzoxazinones in hexaploid wheat [J]. Proc Natl Acad Sci U S A, 2005, 102(45): 16490-16495. |
| 38 | Tzin V, Fernandez-Pozo N, Richter A, et al. Dynamic maize responses to aphid feeding are revealed by a time series of transcriptomic and metabolomic assays [J]. Plant Physiol, 2015, 169(3): 1727-1743. |
| 39 | Song J, Liu H, Zhuang HF, et al. Transcriptomics and alternative splicing analyses reveal large differences between maize lines B73 and Mo17 in response to aphid Rhopalosiphum padi infestation [J]. Front Plant Sci, 2017, 8: 1738. |
| 40 | Yan F, Liang X, Zhu X. The role of DIMBOA on the feeding of Asian corn borer, Ostrinia furnacalis (Guenée) (Lep., Pyralidae) [J]. J Appl Entomol, 1999, 123(1): 49-53. |
| 41 | Yan FM, Xu CR, Li SG, et al. Effects of DIMBOA on several enzymatic systems in Asian corn borer, Ostrinia furnacalis (Guenée) [J]. J Chem Ecol, 1995, 21(12): 2047-2056. |
| 42 | Hedin PA, Davis FM, Williams WP. 2-hydroxy-4, 7-dimethoxy-1,4-benzoxazin-3-one (N-O-Me-DIMBOA), a possible toxic factor in corn to the southwestern corn borer [J]. J Chem Ecol, 1993, 19(3): 531-542. |
| 43 | Campos F, Donskov N, Arnason TJ, et al. Biological effects and toxicokinetics of DIM BOA in Diadegma terebrans (Hymenoptera: Ichneumonidae), an endoparasitoid of Ostrinia nubilalis (Lepidoptera: Pyralidae) [J]. J Econ Entomol, 1990, 83(2): 356-360. |
| 44 | Tzin V, Hojo Y, Strickler SR, et al. Rapid defense responses in maize leaves induced by Spodoptera exigua caterpillar feeding [J]. J Exp Bot, 2017, 68(16): 4709-4723. |
| 45 | Yang X, Hafeez M, Chen HY, et al. DIMBOA-induced gene expression, activity profiles of detoxification enzymes, multi-resistance mechanisms, and increased resistance to indoxacarb in tobacco cutworm, Spodoptera litura (Fabricius) [J]. Ecotoxicol Environ Saf, 2023, 267: 115669. |
| 46 | Robert CA, Zhang X, Machado RA, et al. Sequestration and activation of plant toxins protect the western corn rootworm from enemies at multiple trophic levels [J]. eLife, 2017, 6: e29307. |
| 47 | Sikder MM, Vestergård M, Kyndt T, et al. Benzoxazinoids selectively affect maize root-associated nematode taxa [J]. J Exp Bot, 2021, 72(10): 3835-3845. |
| 48 | Israni B, Wouters FC, Luck K, et al. The fall armyworm Spodoptera frugiperda utilizes specific UDP-glycosyltransferases to inactivate maize defensive benzoxazinoids [J]. Front Physiol, 2020, 11: 604754. |
| 49 | Israni B, Luck K, Römhild SCW, et al. Alternative transcript splicing regulates UDP-glucosyltransferase-catalyzed detoxification of DIMBOA in the fall armyworm (Spodoptera frugiperda) [J]. Sci Rep, 2022, 12(1): 10343. |
| 50 | Xie YS, Arnason JT, Philogène BJR, et al. Role of 2, 4-dihydroxy-7-methoxy-1, 4-benzoxazin-3-one (DIMBOA) in the resistance of maize to western corn rootworm, Diabrotica virgifera virgifera (Leconte) (Coleoptera: Chrysomelidae) [J]. Can Entomol, 1990, 122(6): 1177-1186. |
| 51 | Malook SU, Qi JF, Hettenhausen C, et al. The oriental armyworm (Mythimna separata) feeding induces systemic defence responses within and between maize leaves [J]. Philos Trans R Soc Lond B Biol Sci, 2019, 374(1767): 20180307. |
| 52 | Klun JA, Tipton CL, Brindley TA. 2, 4-dihydroxy-7-methoxy-1, 4-benzoxazin-3-one (DIMBOA), an active agent in the resistance of maize to the European corn borer [J]. J Econ Entomol, 1967, 60(6): 1529-1533. |
| 53 | Maag D, Köhler A, Robert CAM, et al. Highly localized and persistent induction of Bx1-dependent herbivore resistance factors in maize [J]. Plant J, 2016, 88(6): 976-991. |
| 54 | Givovich A, Morse S, Cerda H, et al. Hydroxamic acid glucosides in honeydew of aphids feeding on wheat [J]. J Chem Ecol, 1992, 18(6): 841-846. |
| 55 | Zhang ZF, Lan H, Cao HH, et al. Impacts of constitutive and induced benzoxazinoids levels on wheat resistance to the grain aphid (Sitobion avenae) [J]. Metabolites, 2021, 11(11): 783. |
| 56 | Batyrshina ZS, Shavit R, Yaakov B, et al. The transcription factor TaMYB31 regulates the benzoxazinoid biosynthetic pathway in wheat [J]. J Exp Bot, 2022, 73(16): 5634-5649. |
| 57 | Etzerodt T, Gislum R, Laursen BB, et al. Correlation of deoxynivalenol accumulation in Fusarium-infected winter and spring wheat cultivars with secondary metabolites at different growth stages [J]. J Agric Food Chem, 2016, 64(22): 4545-4555. |
| 58 | Etzerodt T, Maeda K, Nakajima Y, et al. 2, 4-dihydroxy-7-methoxy-2H-1, 4-benzoxazin-3(4H)-one (DIMBOA) inhibits trichothecene production by Fusarium graminearum through suppression of Tri6 expression [J]. Int J Food Microbiol, 2015, 214: 123-128. |
| 59 | Ridenour JB, Bluhm BH. The novel fungal-specific gene FUG1 has a role in pathogenicity and fumonisin biosynthesis in Fusarium verticillioides [J]. Mol Plant Pathol, 2017, 18(4): 513-528. |
| 60 | Ding XP, Yang M, Huang HC, et al. Priming maize resistance by its neighbors: activating 1, 4-benzoxazine-3-ones synthesis and defense gene expression to alleviate leaf disease [J]. Front Plant Sci, 2015, 6: 830. |
| 61 | Zhang XS, Huang TZ, Wang QC, et al. Mechanisms of resistance to spot blotch in Yunnan iron shell wheat based on metabolome and transcriptomics [J]. Int J Mol Sci, 2022, 23(9): 5184. |
| 62 | Song YY, Cao M, Xie LJ, et al. Induction of DIMBOA accumulation and systemic defense responses as a mechanism of enhanced resistance of mycorrhizal corn (Zea mays L.) to sheath blight [J]. Mycorrhiza, 2011, 21(8): 721-731. |
| 63 | Święcicka M, Dmochowska-Boguta M, Orczyk W, et al. Changes in benzoxazinoid contents and the expression of the associated genes in rye (Secale cereale L.) due to brown rust and the inoculation procedure [J]. PLoS One, 2020, 15(5): e0233807. |
| 64 | Cen ZX, Hu BJ, Yang SY, et al. Mechanism of benzoxazinoids affecting the growth and development of Fusarium oxysporum f. sp. fabae [J]. Plant Mol Biol, 2024, 114(3): 42. |
| 65 | Ma GL, Hu BJ, Yang SY, et al. Benzoxazinoids secreted by wheat root weaken the pathogenicity of Fusarium oxysporum f.sp. fabae by inhibiting linoleic acid and nucleotide metabolisms [J]. Plant Cell Rep, 2024, 43(4): 109. |
| 66 | Huffaker A, Dafoe NJ, Schmelz EA. ZmPep1, an ortholog of Arabidopsis elicitor peptide 1, regulates maize innate immunity and enhances disease resistance [J]. Plant Physiol, 2011, 155(3): 1325-1338. |
| 67 | Venturelli S, Belz RG, Kämper A, et al. Plants release precursors of histone deacetylase inhibitors to suppress growth of competitors [J]. Plant Cell, 2015, 27(11): 3175-3189. |
| 68 | Oikawa A, Ishihara A, Tanaka C, et al. Accumulation of HDMBOA-Glc is induced by biotic stresses prior to the release of MBOA in maize leaves [J]. Phytochemistry, 2004, 65(22): 2995-3001. |
| 69 | Li YH, Xia ZC, Kong CH. Allelobiosis in the interference of allelopathic wheat with weeds [J]. Pest Manag Sci, 2016, 72(11): 2146-2153. |
| 70 | Liu HX, An T, Zhao YF, et al. Benzoxazines in the root exudates responsible for nonhost disease resistance of maize to Phytophthora sojae [J]. Phytopathology, 2022, 112(7): 1537-1544. |
| 71 | Kong CH, Wang P, Gu Y, et al. Fate and impact on microorganisms of rice allelochemicals in paddy soil [J]. J Agric Food Chem, 2008, 56(13): 5043-5049. |
| 72 | Li XJ, Xia ZC, Kong CH, et al. Mobility and microbial activity of allelochemicals in soil [J]. J Agric Food Chem, 2013, 61(21): 5072-5079. |
| 73 | Hazrati H, Fomsgaard IS, Kudsk P. Root-exuded benzoxazinoids: uptake and translocation in neighboring plants [J]. J Agric Food Chem, 2020, 68(39): 10609-10617. |
| 74 | Zhang CL, Li XW, Chen YQ, et al. Effects of Eucalyptus litter and roots on the establishment of native tree species in Eucalyptus plantations in South China [J]. For Ecol Manag, 2016, 375: 76-83. |
| 75 | Williams RA. Mitigating biodiversity concerns in Eucalyptus plantations located in South China [J]. J Biosci Med, 2015, 3(6): 1-8. |
| 76 | Jabeen R, Yamada K, Shigemori H, et al. Induction of ß-glucosidase activity in maize coleoptiles by blue light illumination [J]. J Plant Physiol, 2006, 163(5): 538-545. |
| 77 | Epstein WW, Rowsemitt CN, Berger PJ, et al. Dynamics of 6-methoxybenzoxazolinone in winter wheat: effects of photoperiod and temperature [J]. J Chem Ecol, 1986, 12(10): 2011-2020. |
| 78 | Bakera B, Święcicka M, Stochmal A, et al. Benzoxazinoids biosynthesis in rye (Secale cereale L.) is affected by low temperature [J]. Agronomy, 2020, 10(9): 1260. |
| 79 | Wang XF, Chen QY, Wu YY, et al. Genome-wide analysis of transcriptional variability in a large maize-teosinte population [J]. Mol Plant, 2018, 11(3): 443-459. |
| 80 | Erb M, Köllner TG, Degenhardt J, et al. The role of abscisic acid and water stress in root herbivore-induced leaf resistance [J]. New Phytol, 2011, 189(1): 308-320. |
| 81 | Sutour S, Doan VC, Mateo P, et al. Isolation and structure determination of drought-induced multihexose benzoxazinoids from maize (Zea mays) [J]. J Agric Food Chem, 2024, 72(7): 3427-3435. |
| 82 | Zhang F, Wu JF, Sade N, et al. Genomic basis underlying the metabolome-mediated drought adaptation of maize [J]. Genome Biol, 2021, 22(1): 260. |
| 83 | Erb M, Gordon-Weeks R, Flors V, et al. Belowground ABA boosts aboveground production of DIMBOA and primes induction of chlorogenic acid in maize [J]. Plant Signal Behav, 2009, 4(7): 636-638. |
| 84 | 冯远娇, 金琼, 王建武. 机械损伤对Bt玉米化学防御的系统诱导效应 [J]. 植物生态学报, 2010, 34(6): 695-703. |
| Feng YJ, Jin Q, Wang JW. Systemic induced effects of mechanical wounding on the chemical defense of Bt corn (Zea mays) [J]. Chin J Plant Ecol, 2010, 34(6): 695-703. | |
| 85 | Vaughan MM, Huffaker A, Schmelz EA, et al. Interactive effects of elevated [CO2] and drought on the maize phytochemical defense response against mycotoxigenic Fusarium verticillioides [J]. PLoS One, 2016, 11(7): e0159270. |
| 86 | Robert CAM, Mateo P. The chemical ecology of benzoxazinoids [J]. Chimia, 2022, 76(11): 928-938. |
| 87 | Bi HH, Luang S, Li Y, et al. Identification and characterization of wheat drought-responsive MYB transcription factors involved in the regulation of cuticle biosynthesis [J]. J Exp Bot, 2016, 67(18): 5363-5380. |
| 88 | Guyer A, van Doan C, Maurer C, et al. Climate change modulates multitrophic interactions between maize, a root herbivore, and its enemies [J]. J Chem Ecol, 2021, 47(10/11): 889-906. |
| 89 | Petho M. Possible role of cyclic hydroxamic acids in the iron uptake by grasses[J]. Acta Agronomica Hungarica, 1993, 42: 203-214. |
| 90 | Aciksoz SB, Ozturk L, Gokmen OO, et al. Effect of nitrogen on root release of phytosiderophores and root uptake of Fe(III)-phytosiderophore in Fe-deficient wheat plants [J]. Physiol Plant, 2011, 142(3): 287-296. |
| 91 | Hu LF, Wu ZW, Robert CAM, et al. Soil chemistry determines whether defensive plant secondary metabolites promote or suppress herbivore growth [J]. Proc Natl Acad Sci U S A, 2021, 118(43): e2109602118. |
| 92 | Poschenrieder C, Tolrà RP, Barceló J. A role for cyclic hydroxamates in aluminium resistance in maize? [J]. J Inorg Biochem, 2005, 99(9): 1830-1836. |
| 93 | Clark RT, Famoso AN, Zhao KY, et al. High-throughput two-dimensional root system phenotyping platform facilitates genetic analysis of root growth and development [J]. Plant Cell Environ, 2013, 36(2): 454-466. |
| 94 | Guimaraes CT, Simoes CC, Pastina MM, et al. Genetic dissection of Al tolerance QTLs in the maize genome by high density SNP scan [J]. BMC Genomics, 2014, 15(1): 153. |
| 95 | Zhao ZK, Gao XF, Ke Y, et al. A unique aluminum resistance mechanism conferred by aluminum and salicylic-acid-activated root efflux of benzoxazinoids in maize [J]. Plant Soil, 2019, 437(1): 273-289. |
| 96 | Ye JR, Zhong T, Zhang DF, et al. The auxin-regulated protein ZmAuxRP1 coordinates the balance between root growth and stalk rot disease resistance in maize [J]. Mol Plant, 2019, 12(3): 360-373. |
| 97 | Venis MA, Watson PJ. Naturally occuring modifiers of auxin-receptor interaction in corn: identification as benzoxazolinones [J]. Planta, 1978, 142(1): 103-107. |
| 98 | Feng YJ, Wang XY, Du TT, et al. Effects of salicylic acid concentration and post-treatment time on the direct and systemic chemical defense responses in maize (Zea mays L.) following exogenous foliar application [J]. Molecules, 2022, 27(20): 6917. |
| 99 | Feng YJ, Wang XY, Du TT, et al. Effects of exogenous salicylic acid application to aboveground part on the defense responses in Bt (Bacillus thuringiensis) and Non-Bt corn (Zea mays L.) seedlings [J]. Plants (Basel), 2022, 11(16): 2162. |
| 100 | 冯远娇, 王建武, 骆世明. 外源茉莉酸处理对Bt玉米直接防御物质含量及其相关基因表达的影响 [J]. 中国农业科学, 2007, 40(11): 2481-2487. |
| Feng YJ, Wang JW, Luo SM. Effects of exogenous jasmonic acid on concentrations of direct defense chemicals and expression of related genes in Bt (Bacillus thuringiensis) corn (Zea mays) [J]. Sci Agric Sin, 2007, 40(11): 2481-2487. | |
| 101 | Oikawa A, Ishihara A, Iwamura H. Induction of HDMBOA-Glc accumulation and DIMBOA-Glc 4-O-methyltransferase by jasmonic acid in poaceous plants [J]. Phytochemistry, 2002, 61(3): 331-337. |
| 102 | Frébortová J, Novák O, Frébort I, et al. Degradation of cytokinins by maize cytokinin dehydrogenase is mediated by free radicals generated by enzymatic oxidation of natural benzoxazinones [J]. Plant J, 2010, 61(3): 467-481. |
| 103 | Zhang CP, Li J, Li S, et al. ZmMPK6 and ethylene signalling negatively regulate the accumulation of anti-insect metabolites DIMBOA and DIMBOA-Glc in maize inbred line A188 [J]. New Phytol, 2021, 229(4): 2273-2287. |
| 104 | 赵媛. 小麦幼苗中丁布的含量、异株克生活性与诱导效应研究 [D]. 杨凌: 西北农林科技大学, 2005. |
| Zhao Y. Study on the content, allogram activity and induction effect of Butachlor in wheat seedlings [D]. Yangling: Northwest A & F University, 2005. | |
| 105 | 郑永权. 小麦幼苗中丁布的含量、活性与诱导效应研究 [D]. 武汉: 华中师范大学, 2004. |
| Zheng YQ. Study on the content, activity and induction effect of butachlor in wheat seedlings [D]. Wuhan: Central China Normal University, 2004. | |
| 106 | Kato-Noguchi H. Effects of four benzoxazinoids on gibberellin-induced alpha-amylase activity in barley seeds [J]. J Plant Physiol, 2008, 165(18): 1889-1894. |
| 107 | Nakajima E, Hasegawa K, Yamada K, et al. Effects of the auxin-inhibiting substances raphanusanin and benzoxazolinone on apical dominance of pea seedlings [J]. Plant Growth Regul, 2001, 35(1): 11-15. |
| 108 | Sue M, Fujii M, Fujimaki T. Increased benzoxazinoid (Bx) levels in wheat seedlings via jasmonic acid treatment and etiolation and their effects on Bx genes including Bx6 [J]. Biochem Biophys Rep, 2021, 27: 101059. |
| 109 | Oikawa A, Ishihara A, Hasegawa M, et al. Induced accumulation of 2-hydroxy-4, 7-dimethoxy-1, 4-benzoxazin-3-one glucoside (HDMBOA-Glc) in maize leaves [J]. Phytochemistry, 2001, 56(7): 669-675. |
| [1] | LU Tian-yi, LI Ai-peng, FEI Qiang. Research Progress in the Biosynthesis of Polylactic Acid [J]. Biotechnology Bulletin, 2025, 41(4): 47-60. |
| [2] | NIE Zhu-xin, GUO Jin, QIAO Zi-yang, LI Wei-wei, ZHANG Xue-yan, LIU Chun-yang, WANG Jing. Transcriptome Analysis of the Anthocyanin Biosynthesis in the Fruit Development Processes of Lycium ruthenicum Murr. [J]. Biotechnology Bulletin, 2024, 40(8): 106-117. |
| [3] | MA Xiao-xiang, MA Ze-yuan, LIU Ya-yue, ZHOU Long-jian, HE Yi-fan, ZHANG Yi. Effects of Simulated Mutational Biosynthetic Regulation on the Secondary Metabolites of Aspergillus terreus C23-3 [J]. Biotechnology Bulletin, 2024, 40(8): 275-287. |
| [4] | SHEN Zhen-hui, CAO Yao, YANG Lin-lei, LUO Xiang-ying, ZI Ling-shan, LU Qing-qing, LI Rong-chun. Cloning and Bioinformatics Analysis of the Ergothioneine Biosynthesis Genes in Naematelia aurantialba and Stereum hirsutum [J]. Biotechnology Bulletin, 2024, 40(7): 259-272. |
| [5] | HE Yu-bing, FU Zhen-hao, LI Ren-han, LIU Xiu-xia, LIU Chun-li, YANG Yan-kun, LI Ye, BAI Zhong-hu. Efficient Biosynthesis of 2-Naphthaleneethanol in Metabolically Engineered Saccharomyces cerevisiae [J]. Biotechnology Bulletin, 2024, 40(7): 99-107. |
| [6] | HU Jin-jin, LI Su-zhen, MA Xu-hui, LIU Xiao-qing, XIE Shan-shan, JIANG Hai-yang, CHEN Ru-mei. Regulation of Maize Anthocyanin Biosynthesis Metabolism [J]. Biotechnology Bulletin, 2024, 40(6): 34-44. |
| [7] | YANG Yan, HU Yang, LIU Ni-ru, YIN Lu, YANG Rui, WANG Peng-fei, MU Xiao-peng, ZHANG Shuai, CHENG Chun-zhen, ZHANG Jian-cheng. Cloning and Functional Analysis of MbbZIP43 Gene in ‘Hongmantang’ Red-flesh Apple [J]. Biotechnology Bulletin, 2024, 40(2): 146-159. |
| [8] | JIANG Yu-shan, LAN Qian, WANG Fang, JIANG Liang, PEI Cheng-cheng. Characterization of a Quinoa Mutant Affecting Tyrosine Metabolism [J]. Biotechnology Bulletin, 2024, 40(10): 253-261. |
| [9] | JI Hong-chao, LI Zheng-yan. Research Progress and Prospects in the Structural Annotation of Unknown Secondary Metabolites Based on Mass Spectrometry [J]. Biotechnology Bulletin, 2024, 40(10): 76-85. |
| [10] | WANG Jun-fang, HUANG Qiu-bin, ZHANG Piao-dan, ZHANG Peng-pai. Structure and Biosynthesis of Surfactin as well as Its Role in Biological Control [J]. Biotechnology Bulletin, 2024, 40(1): 100-112. |
| [11] | CHEN Zhi-min, LI Cui, WEI Ji-tian, LI Xin-ran, LIU Yi, GUO Qiang. Research Progress in the Regulation of Chlorogenic Acid Biosynthesis and Its Application [J]. Biotechnology Bulletin, 2024, 40(1): 57-71. |
| [12] | LI Liang, XU Shan-shan, JIANG Yan-jun. Research Progress in the Production of Ergothioneine by Biosynthesis [J]. Biotechnology Bulletin, 2024, 40(1): 86-99. |
| [13] | YE Yun-fang, TIAN Qing-yin, SHI Ting-ting, WANG Liang, YUE Yuan-zheng, YANG Xiu-lian, WANG Liang-gui. Research Progress in the Biosynthesis and Regulation of β-ionone in Plants [J]. Biotechnology Bulletin, 2023, 39(8): 91-105. |
| [14] | WANG Ling, ZHUO Shen, FU Xue-sen, LIU Zi-xuan, LIU Xiao-rong, WANG Zhi-hui, ZHOU Ri-bao, LIU Xiang-dan. Advances in the Biosynthetic Pathways and Related Genes of Lotus Alkaloids [J]. Biotechnology Bulletin, 2023, 39(7): 56-66. |
| [15] | JIANG Qing-chun, DU Jie, WANG Jia-cheng, YU Zhi-he, WANG Yun, LIU Zhong-yu. Expression and Function Analysis of Transcription Factor PcMYB2 from Polygonum cuspidatum [J]. Biotechnology Bulletin, 2023, 39(5): 217-223. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||