








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (9): 237-247.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1577
Previous Articles Next Articles
LI Ying( ), LONG Chang-mei, JIANG Biao, HAN Li-zhen(
), LONG Chang-mei, JIANG Biao, HAN Li-zhen( )
)
Received:2021-12-21
Online:2022-09-26
Published:2022-10-11
Contact:
HAN Li-zhen
E-mail:1363393909@qq.com;lzhan1@gzu.edu.cn
LI Ying, LONG Chang-mei, JIANG Biao, HAN Li-zhen. Colonization on the Peanuts of Two Plant-growth Promoting Rhizobacteria Strains and Effects on the Bacterial Community Structure of Rhizosphere[J]. Biotechnology Bulletin, 2022, 38(9): 237-247.

Fig.1 PCR verification of positive transformants of GFP-labeled strains M:1 000 bp marker;1:positive control;2 and 4:original strain of P9 and P10,respectively;3 and 5:positive transformants of GFP-labeled P9 and P10,respectively
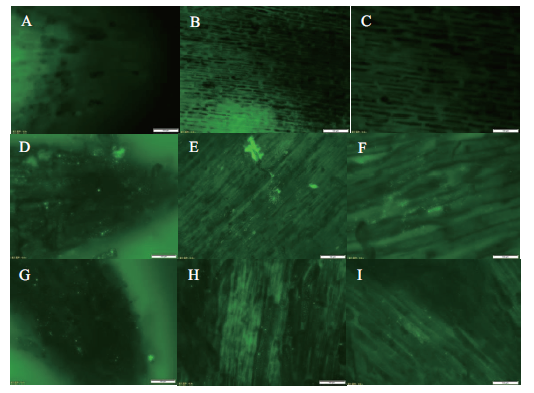
Fig.2 Distribution of two GFP-labeled PGPR strains on the root tips of peanut A:Root tip of CK;B and C:meristem region of root tip of CK;D:root tip of P9 treated group;E and F:meristem region of P9 treated group;G:root tip of P10 treated group;H and I:meristem region of P10 treated group. All are the images of microscope objectives 40,except that B,E and H are of objectives 20

Fig.3 Colony morphology and cell morphology of two rifampicin-labeled PGPR strains A and B are the colonies of P9r and P10r on rifampicin LB plate respectively;C and D are the cell morphologies of P9r and P10r under scanning electron microscopy,respectively
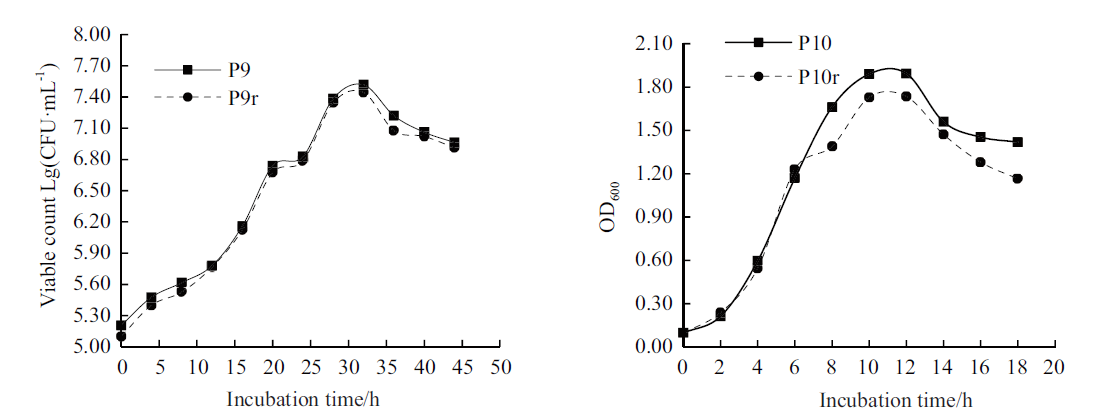
Fig. 4 Growth curves of two PGPR strains and their rifampicin-labeled strains P9 and P10 are original strains,P9r and P10r are rifampicin-labeled strains,the same below
| 菌株 Strain | 可溶磷含量 Soluble phorsphorus content/(µg·mL-1) | IAA含量 IAA content /(mg·L-1) | ACC脱氨酶活性 ACC deaminase activity/(μmol·(h·mg)-1) | 铁载体相对含量 Siderophore relative content/% |
|---|---|---|---|---|
| P9 | 307.84±10.81a | 38.72±0.52a | 1.00±0.09a | 83.40±0.40a |
| P9r | 297.28±1.40a | 36.35±1.46a | 0.99±0.02a | 83.20±0.50a |
| P10 | 74.09±3.71b | 44.91±1.3b | 26.31±1.53b | 83.80±1.60a |
| P10r | 67.26±0.53b | 40.31±1.13bc | 25.96±1.69b | 81.90±2.10a |
Table 1 Growth-promoting characteristics of two PGPR strains and their rifampicin-labeled strains
| 菌株 Strain | 可溶磷含量 Soluble phorsphorus content/(µg·mL-1) | IAA含量 IAA content /(mg·L-1) | ACC脱氨酶活性 ACC deaminase activity/(μmol·(h·mg)-1) | 铁载体相对含量 Siderophore relative content/% |
|---|---|---|---|---|
| P9 | 307.84±10.81a | 38.72±0.52a | 1.00±0.09a | 83.40±0.40a |
| P9r | 297.28±1.40a | 36.35±1.46a | 0.99±0.02a | 83.20±0.50a |
| P10 | 74.09±3.71b | 44.91±1.3b | 26.31±1.53b | 83.80±1.60a |
| P10r | 67.26±0.53b | 40.31±1.13bc | 25.96±1.69b | 81.90±2.10a |

Fig.6 Colonizaiton of two rifampicin-labeled strains on root and stem surface observed by scanning electron microscope A:Un-inoculated root of peanut;B:P9r treated root;C:P10r treated root;D:P9r+P10r treated root;E:uninoculated stems;F:P9r treated stems;G:P10r treated stems;and H:P9r+P10r treated stems
| 处理 Treatment | 鲜重 Fresh weight/g | 株高 Plant height /cm | 总根长 Total root length /cm | 根重 Root weight /g | 叶绿素含量 Cholorphyll content /(mg.g-1FW) |
|---|---|---|---|---|---|
| CK | 2.91±0.14a | 8.92±0.37a | 7.48±0.37a | 0.524±0.05a | 3.72±0.20a |
| P9r | 3.23±0.28ab | 11.68±0.22b | 11.78±1.23b | 0.580±0.06a | 4.39±0.39a |
| P10r | 3.03±0.09b | 12.53±0.31bc | 10.88±0.18b | 0.524±0.05a | 4.35±0.31a |
| P9r+P10r | 4.20±0.32c | 14.28±0.54d | 12.08±0.45bc | 0.718±0.06bc | 4.37±0.26a |
| P9 | 3.84±0.16bc | 13.18±0.30cd | 11.37±0.50b | 0.624±0.03ab | 4.65±0.14a |
| P10 | 3.79±0.27bc | 14.23±0.22d | 13.83±0.54c | 0.739±0.02bc | 4.38±0.39a |
| P9+P10 | 4.92±0.23d | 16.78±0.61e | 12.60±0.27bc | 0.843±0.01c | 4.62±0.36a |
Table 2 Effects of two rifampicin-labeled and their original PGPR strains on the growth of peanuts
| 处理 Treatment | 鲜重 Fresh weight/g | 株高 Plant height /cm | 总根长 Total root length /cm | 根重 Root weight /g | 叶绿素含量 Cholorphyll content /(mg.g-1FW) |
|---|---|---|---|---|---|
| CK | 2.91±0.14a | 8.92±0.37a | 7.48±0.37a | 0.524±0.05a | 3.72±0.20a |
| P9r | 3.23±0.28ab | 11.68±0.22b | 11.78±1.23b | 0.580±0.06a | 4.39±0.39a |
| P10r | 3.03±0.09b | 12.53±0.31bc | 10.88±0.18b | 0.524±0.05a | 4.35±0.31a |
| P9r+P10r | 4.20±0.32c | 14.28±0.54d | 12.08±0.45bc | 0.718±0.06bc | 4.37±0.26a |
| P9 | 3.84±0.16bc | 13.18±0.30cd | 11.37±0.50b | 0.624±0.03ab | 4.65±0.14a |
| P10 | 3.79±0.27bc | 14.23±0.22d | 13.83±0.54c | 0.739±0.02bc | 4.38±0.39a |
| P9+P10 | 4.92±0.23d | 16.78±0.61e | 12.60±0.27bc | 0.843±0.01c | 4.62±0.36a |

Fig.7 Multi-samples Shannon curves and principal component analysis(PCA)of bacterial communities in peanut rhizosphere soil Left:Shannon curves. Right:PCA analysis. A,B,C,D represents CK,P9-inoculated group,P10-inoculated group,P9+P10 inoculated group,respectively

Fig. 8 Relative abundances of bacteria communities at phy-lum and genus level in rhizosphere soils of different treated peanuts A:Phylum level. B:Genus level
| [1] |
Vejan P, Abdullah R, Khadiran T, et al. Role of plant growth promoting rhizobacteria in agricultural sustainability-a review[J]. Molecules, 2016, 21(5):573.
doi: 10.3390/molecules21050573 URL |
| [2] |
Gouda S, Kerry RG, Das G, et al. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture[J]. Microbiol Res, 2018, 206:131-140.
doi: S0944-5013(17)30341-5 pmid: 29146250 |
| [3] |
Santoyo G, Urtis-Flores CA, Loeza-Lara PD, et al. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria(PGPR)[J]. Biology, 2021, 10(6):475.
doi: 10.3390/biology10060475 URL |
| [4] |
Batista BD, Lacava PT, Ferrari A, et al. Screening of tropically derived, multi-trait plant growth- promoting rhizobacteria and evaluation of corn and soybean colonization ability[J]. Microbiol Res, 2018, 206:33-42.
doi: S0944-5013(17)30922-9 pmid: 29146258 |
| [5] |
Yoo SJ, da Jeong Shin, Won HY, et al. Aspergillus terreus JF27 promotes the growth of tomato plants and induces resistance against Pseudomonas syringae pv. tomato[J]. Mycobiology, 2018, 46(2):147-153.
doi: 10.1080/12298093.2018.1475370 URL |
| [6] |
Pathania P, Bhatia R, Khatri M. Cross-competence and affectivity of maize rhizosphere bacteria Bacillus sp. MT7 in tomato rhizosphere[J]. Sci Hortic, 2020, 272:109480.
doi: 10.1016/j.scienta.2020.109480 URL |
| [7] |
Zhai ZY, Du J, Chen LJ, et al. A genetic tool for production of GFP-expressing Rhodopseudomonas palustris for visualization of bacterial colonization[J]. AMB Express, 2019, 9(1):141.
doi: 10.1186/s13568-019-0866-6 URL |
| [8] | Ma YE, Jiao J, Fan XC, et al. Endophytic bacterium Pseudomonas fluorescens RG11 may transform tryptophan to melatonin and promote endogenous melatonin levels in the roots of four grape cultivars[J]. Front Plant Sci, 2017, 7:2068. |
| [9] | Maron PA, Sarr A, Kaisermann A, et al. High microbial diversity promotes soil ecosystem functioning[J]. Appl Environ Microbiol, 2018, 84(9):e02738-e02717. |
| [10] | Bishnoi U. PGPR interaction:an ecofriendly approach promoting the sustainable agriculture system[J]. Adv Bot Res, 2015, 75:81-113. |
| [11] |
Jin YQ, Zhu HF, Luo S, et al. Role of maize root exudates in promotion of colonization of Bacillus velezensis strain S3-1 in rhizosphere soil and root tissue[J]. Curr Microbiol, 2019, 76(7):855-862.
doi: 10.1007/s00284-019-01699-4 URL |
| [12] |
Zhang DS, Sun ZX, Feng LS, et al. Maize plant density affects yield, growth and source-sink relationship of crops in maize/peanut intercropping[J]. Field Crops Res, 2020, 257:107926.
doi: 10.1016/j.fcr.2020.107926 URL |
| [13] |
Zhang H, Han LZ, Jiang B, et al. Identification of a phosphorus-solubilizing Tsukamurella tyrosinosolvens strain and its effect on the bacterial diversity of the rhizosphere soil of peanuts growth-promoting[J]. World J Microbiol Biotechnol, 2021, 37(7):109.
doi: 10.1007/s11274-021-03078-3 URL |
| [14] |
Han LZ, Zhang H, Xu Y, et al. Biological characteristics and salt-tolerant plant growth-promoting effects of an ACC deaminase-producing Burkholderia pyrrocinia strain isolated from the tea rhizosphere[J]. Arch Microbiol, 2021, 203(5):2279-2290.
doi: 10.1007/s00203-021-02204-x URL |
| [15] | 韩丽珍, 刘畅, 周静. 接种促生菌对花生根际土壤微生物及营养元素的影响[J]. 基因组学与应用生物学, 2019, 38(7):3065-3073. |
| Han LZ, Liu C, Zhou J. Effects of inoculation with growth-promoting bacteria on peanut rhizosphere soil microorganism and nutrient elements[J]. Genom Appl Biol, 2019, 38(7):3065-3073. | |
| [16] |
Pérez E, Sulbarán M, Ball MM, et al. Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region[J]. Soil Biol Biochem, 2007, 39(11):2905-2914.
doi: 10.1016/j.soilbio.2007.06.017 URL |
| [17] |
Han DD, Wang LY, Luo YP. Isolation, identification, and the growth promoting effects of two antagonistic actinomycete strains from the rhizosphere of Mikania micrantha Kunth[J]. Microbiol Res, 2018, 208:1-11.
doi: 10.1016/j.micres.2018.01.003 URL |
| [18] | Jaemsaeng R, Jantasuriyarat C, Thamchaipenet A. Positive role of 1-aminocyclopropane-1-carboxylate deaminase-producing endophytic Streptomyces sp. GMKU 336 on flooding resistance of mung bean[J]. Agric Nat Resour, 2018, 52(4):330-334. |
| [19] |
Vora SM, Joshi P, Belwalkar M, et al. Root exudates influence chemotaxis and colonization of diverse plant growth promoting rhizobacteria in the pigeon pea - maize intercropping system[J]. Rhizosphere, 2021, 18:100331.
doi: 10.1016/j.rhisph.2021.100331 URL |
| [20] |
Wang J, Jacobs JL, Roth MG, et al. Temporal dynamics of Fusarium virguliforme colonization of soybean roots[J]. Plant Dis, 2019, 103(1):19-27.
doi: 10.1094/PDIS-03-18-0384-RE pmid: 30358505 |
| [21] |
Coy RM, Held DW, Kloepper JW. Rhizobacterial colonization of bermudagrass by Bacillus spp. in a Marvyn loamy sand soil[J]. Appl Soil Ecol, 2019, 141:10-17.
doi: 10.1016/j.apsoil.2019.04.018 URL |
| [22] | Mirzaei A, Naseri R, Soleymanifard A, et al. Effect of plant growth promoting rhizobacteria(PGPR)on agronomic characteristic and root colonization in fennel[J]. Planta Med, 2011, 77(12): 77:1277. |
| [23] | Palmqvist NGM, Bejai S, Meijer J, et al. Nano titania aided clustering and adhesion of beneficial bacteria to plant roots to enhance crop growth and stress management[J]. Sci Reports, 2015, 5:10146. |
| [24] |
Gamez R, Cardinale M, Montes M, et al. Screening, plant growth promotion and root colonization pattern of two rhizobacteria(Pseudomonas fluorescens Ps006 and Bacillus amyloliquefaciens Bs006)on banana cv. Williams(Musa acuminata Colla)[J]. Microbiol Res, 2019, 220:12-20.
doi: S0944-5013(18)30729-8 pmid: 30744815 |
| [25] |
Compant S, Kaplan H, Sessitsch A, et al. Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN:from the rhizosphere to inflorescence tissues[J]. FEMS Microbiol Ecol, 2008, 63(1):84-93.
doi: 10.1111/j.1574-6941.2007.00410.x URL |
| [26] | 董爱菊, 邱慧珍, 董莉, 等. 类芽孢杆菌QHZ11-gfp在马铃薯植株上的定殖特征及促生效果[J]. 微生物学通报, 2021, 48(11):4075-4086. |
| Dong AJ, Qiu HZ, Dong L, et al. The colonization characteristics of Paenibacillus jamilae QHZ11-gfp in potato plants and its growth-promoting effect[J]. Microbiol China, 2021, 48(11):4075-4086. | |
| [27] | Marín M, Wong I, Mena J, et al. Promoción del crecimiento de plantas de Zea mays L. por Tsukamurella paurometabola Cepa C-924[J]. Biotecnologia Apl, 2013, 30(2):105-110. |
| [28] |
Podile AR, Vukanti RR, Sravani A, et al. Root colonization and quorum sensing are the driving forces of plant growth promoting rhizobacteria(pgpr)for growth promotion[J]. Proc Indian Natl Sci Acad, 2014, 80(2):407.
doi: 10.16943/ptinsa/2014/v80i2/55117 URL |
| [29] |
Ju WL, Jin XL, Liu L, et al. Rhizobacteria inoculation benefits nutrient availability for phytostabilization in copper contaminated soil:drivers from bacterial community structures in rhizosphere[J]. Appl Soil Ecol, 2020, 150:103450.
doi: 10.1016/j.apsoil.2019.103450 URL |
| [30] |
Carrión VJ, Perez-Jaramillo J, Cordovez V, et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome[J]. Science, 2019, 366(6465):606-612.
doi: 10.1126/science.aaw9285 pmid: 31672892 |
| [31] |
Bogas AC, Ferreira AJ, Araújo WL, et al. Endophytic bacterial diversity in the phyllosphere of Amazon Paullinia cupana associated with asymptomatic and symptomatic anthracnose[J]. SpringerPlus, 2015, 4:258.
doi: 10.1186/s40064-015-1037-0 URL |
| [32] |
Mhedbi-Hajri N, Jacques MA, Koebnik R. Adhesion mechanisms of plant-pathogenic Xanthomonadaceae[J]. Adv Exp Med Biol, 2011, 715:71-89.
doi: 10.1007/978-94-007-0940-9_5 pmid: 21557058 |
| [33] |
Lee HJ, Jeong SE, Cho MS, et al. Flavihumibacter solisilvae sp. nov., isolated from forest soil[J]. Int J Syst Evol Microbiol, 2014, 64(Pt_8):2897-2901.
doi: 10.1099/ijs.0.063669-0 URL |
| [34] |
Black M, Moolhuijzen P, Chapman B, et al. The genetics of symbiotic nitrogen fixation:comparative genomics of 14 rhizobia strains by resolution of protein clusters[J]. Genes, 2012, 3(1):138-166.
doi: 10.3390/genes3010138 URL |
| [35] |
Molina-Santiago C, Pearson JR, Navarro Y, et al. The extracellular matrix protects Bacillus subtilis colonies from Pseudomonas invasion and modulates plant co-colonization[J]. Nat Commun, 2019, 10(1):1919.
doi: 10.1038/s41467-019-09944-x pmid: 31015472 |
| [36] |
Miljaković D, Marinković J, Balešević-Tubić S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops[J]. Microorganisms, 2020, 8(7):1037.
doi: 10.3390/microorganisms8071037 URL |
| [37] |
Xu JB, Feng YZ, Wang YL, et al. Effect of rhizobacterium Rhodopseudomonas palustris inoculation on Stevia rebaudiana plant growth and soil microbial community[J]. Pedosphere, 2018, 28(5):793-803.
doi: 10.1016/S1002-0160(18)60043-8 URL |
| [38] | Anwar S, Ali B, Sajid I. Screening of rhizospheric actinomycetes for various in-vitro and in-vivo plant growth promoting(PGP)traits and for agroactive compounds[J]. Front Microbiol, 2016, 7:1334. |
| [39] | 邵美琪, 赵卫松, 苏振贺, 等. 盐胁迫下枯草芽孢杆菌NCD-2对番茄促生作用及对土壤微生物群落结构的影响[J]. 中国农业科学, 2021, 54(21):4573-4584. |
| Shao MQ, Zhao WS, Su ZH, et al. Effect of Bacillus subtilis NCD-2 on the growth of tomato and the microbial community structure of rhizosphere soil under salt stress[J]. Sci Agric Sin, 2021, 54(21):4573-4584. | |
| [40] | Ulanova D, Goo KS. Diversity of actinomycetes isolated from subseafloor sediments after prolonged low-temperature storage[J]. Folia Microbiol(Praha), 2015, 60(3):211-216. |
| [1] | XU Yang, ZHANG Guan-chu, DING Hong, QIN Fei-fei, ZHANG Zhi-meng, DAI Liang-xiang. Effects of Soil Types on Bacterial Community Diversity on the Rhizosphere Soil of Arachis hypogaea and Yield [J]. Biotechnology Bulletin, 2022, 38(6): 221-234. |
| [2] | LI Si-si, ZHANG Bo-yuan, FU Yun-hui, ZHOU Jia, QU Jian-hang. Condition Optimization of an Efficient Phosphate-dissolving Bacterial Strain and Its Phosphate-dissolving Characteristics [J]. Biotechnology Bulletin, 2022, 38(12): 274-286. |
| [3] | LIU Chuan-he, HE Han, HE Xiu-gu, LIU Kai, SHAO Xue-hua, LAI Duo, KUANG Shi-zi, XIAO Wei-qiang. Analysis of Differential Metabolites and Bacterial Community Structure in Soils of a Pineapple Orchard in Different Continuous-cropping Years [J]. Biotechnology Bulletin, 2021, 37(8): 162-175. |
| [4] | WANG Wei-xiong, SHEN Bo, JIA Hong-bai, QIAO Jun-qing, NIU Ben. Application of Rhizospheric Biocontrol Consortia and the Potential Mechanisms of Their Enhancing Efficacy on Disease-suppressive Effect [J]. Biotechnology Bulletin, 2020, 36(9): 31-41. |
| [5] | WANG Jing, DAI Dong, WU Shu-geng, ZHANG Hai-jun, QI Guang-hai. Advances in Successional Development and Early Establishment of the Chicken Intestinal Microbiota [J]. Biotechnology Bulletin, 2020, 36(2): 1-8. |
| [6] | WANG Yong-yan, ZHAO Bing-he, LIANG Guang-yu, LI Yun, XU Yang-cang. Bacterial Community Characteristics of Cultured Seawater with Microecological Preparations in Different Seasons [J]. Biotechnology Bulletin, 2020, 36(2): 126-133. |
| [7] | CHEN Nan, YU Fei, HE Yan-liu, BU Ning. Labeling and Tracing of Green Fluorescent Protein in Fungal Endophyte with Growth-promoting Activity to Rice Seedlings [J]. Biotechnology Bulletin, 2017, 33(3): 100-105. |
| [8] | SUN Zhen, ZHENG Liang, QIU Hao-bin. Research Advances on Colonization of Plant Growth-promoting Rhizobacteria [J]. Biotechnology Bulletin, 2017, 33(2): 8-15. |
| [9] | LIU Ye, LIU Xiao-dan, ZHANG Lin-li, WU Yue, WANG Guo-wen, WANG iang, JIANG Ying. Screening,Identification of Multifunctional Peanut Root-promoting Rhizobacteria and Its Promoting Effects on Peanuts(Arachis hypogaea L.) [J]. Biotechnology Bulletin, 2017, 33(10): 125-134. |
| [10] | LIU Xiao-yu, MA Yu-chao. Green Fluorescent Protein Marker of Biocontrol Streptomyces SSD49 and Its Colonization on the Populus tomentosa Somaclone [J]. Biotechnology Bulletin, 2016, 32(9): 197-202. |
| [11] | FENG Yan-fang, GENG Li-li, HAN Rong, ZHANG Jie. Cloning and Expression Pattern Analysis of NBS-LRR Like-gene in Peanut [J]. Biotechnology Bulletin, 2016, 32(8): 90-95. |
| [12] | WANG Jun-qiang, WANG Jing-jing, WANG Qi, MA Gui-zhen, BAO Zeng-hai, WANG Shu-fang, ZHOU Xiang-hong. Colonization of Double-resistance Strain of Marine Bacterium L1-9 and Its Biocontrol Effect on Fusarium Wilt of Cucumber [J]. Biotechnology Bulletin, 2016, 32(6): 193-198. |
| [13] | ZHONG Rui-chun ,LI Ting-ting, ,TANG Rong-hua, WANG Xing-jun,, LI Cui ,HOU Lei,ZHAO Chuan-zhi,. Cloning and Expression Analysis of Temperature Induced Lipocalin Gene AhTIL1 of Peanut(Arachis hypogaea) [J]. Biotechnology Bulletin, 2016, 32(4): 102-109. |
| [14] | Wang Ruiqin, Zhou Guoying, Liu Junang, Li Dongqin, Meng Qingmin. Colonization of Bacillus subtilis Y13 in Camellia oleifera Leaves and Its Effect on Native Bacteria [J]. Biotechnology Bulletin, 2014, 0(6): 162-167. |
| [15] | Yao Qingshou, Jiang Jigang, Wu Yuyong, Liang Chengbang. Effect of Biomass and Resveratrol Content of Peanut Hairy Root Strains in Different Media [J]. Biotechnology Bulletin, 2014, 0(5): 174-178. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||