








Biotechnology Bulletin ›› 2023, Vol. 39 ›› Issue (11): 168-181.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0625
Previous Articles Next Articles
WANG Chen-yu1( ), ZHOU Chu-yuan1, HE Di1, FAN Zi-hao2, WANG Meng-meng1, YANG Liu-yan1(
), ZHOU Chu-yuan1, HE Di1, FAN Zi-hao2, WANG Meng-meng1, YANG Liu-yan1( )
)
Received:2023-06-30
Online:2023-11-26
Published:2023-12-20
Contact:
YANG Liu-yan
E-mail:18851732535@163.com;yangly@nju.edu.cn
WANG Chen-yu, ZHOU Chu-yuan, HE Di, FAN Zi-hao, WANG Meng-meng, YANG Liu-yan. Role and Mechanism of Polyphosphate in the Microbial Response to Environmental Stresses[J]. Biotechnology Bulletin, 2023, 39(11): 168-181.
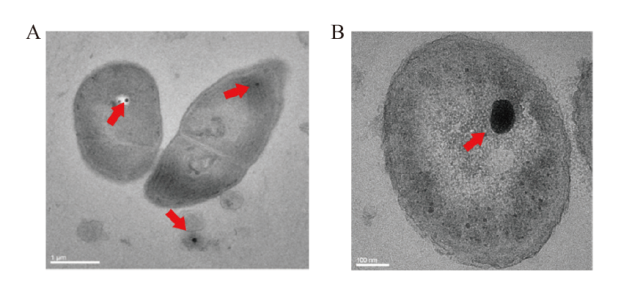
Fig. 1 Transmission electron micrograph of intracellular polyP granules in microorganism cells A: polyP granules of Limnothrix(cross-section); B: polyP granules of Citrobacter fumigatus
| 胁迫类型 Type of stress | PolyP抗胁迫机制 Mechanism of polyP-mediated resistance to stress | 文献 References |
|---|---|---|
| 高温 High temperature | (1)使不耐热的α-螺旋蛋白质转化成耐热的β-中间体Converting thermolabile α-helical proteins into heat-resistant β-intermediates (2)结合热休克蛋白,参与其折叠调节Binding heat shock proteins and regulating their folding | [ [ |
| 酸碱 Acid and alkali | 通过酶解polyP释放质子来中和碱性环境 Neutralisation of the alkaline environment by enzymolysis of polyP to release protons | [ |
| 渗透压 Osmotic pressure | (1)被碱性蛋白调控,影响细胞生长和分裂Regulated by basic proteins, affecting cell growth and division (2)结合Ca2+,改变生物膜延展性和刚性Binding Ca2+ and altering membrane ductility and rigidity | [ [ |
| 重金属离子 Heavy metal ion | (1)螯合重金属离子Chelating heavy metal ions (2)对于未知重金属配体起中介作用Mediating role for unknown heavy metal ligands | [ [ |
| 紫外线 Ultraviolet ray | (1)改变细胞壁结构,合成外膜或EPS Changing the structure of the cell wall and synthesizing the outer membrane or EPS (2)结合蛋白质,促进其折叠或防止其自我聚集 Binding proteins to promote their folding or prevents their self-aggregation (3)结合水,促进悬浮和凝胶Binding water and promoting suspension and gelation | [ [ [ |
| 贫营养 Oligotrophic | (1)积聚在蛋白质周围,防止其失活Accumulating around proteins to prevent their inactivation (2)维持ADP和ATP调节的电子流Maintenance of ADP- and ATP-regulated electron flow (3)受(p)ppGpp、RpoS和PhoB综合调节Regulated by a combination of(p)ppGpp, RpoS and PhoB | [ [ [ |
| 干旱 Droughts | 分泌并保留细胞壁外的EPS,结合大量水,增加持水性 Secreting and retaining EPS outside the cell wall, and binding a large amount of water to increase water-holding capacity | [ |
Table 1 Mechanisms of microbial intracellular polyP-mediated resistance to abiotic stresses
| 胁迫类型 Type of stress | PolyP抗胁迫机制 Mechanism of polyP-mediated resistance to stress | 文献 References |
|---|---|---|
| 高温 High temperature | (1)使不耐热的α-螺旋蛋白质转化成耐热的β-中间体Converting thermolabile α-helical proteins into heat-resistant β-intermediates (2)结合热休克蛋白,参与其折叠调节Binding heat shock proteins and regulating their folding | [ [ |
| 酸碱 Acid and alkali | 通过酶解polyP释放质子来中和碱性环境 Neutralisation of the alkaline environment by enzymolysis of polyP to release protons | [ |
| 渗透压 Osmotic pressure | (1)被碱性蛋白调控,影响细胞生长和分裂Regulated by basic proteins, affecting cell growth and division (2)结合Ca2+,改变生物膜延展性和刚性Binding Ca2+ and altering membrane ductility and rigidity | [ [ |
| 重金属离子 Heavy metal ion | (1)螯合重金属离子Chelating heavy metal ions (2)对于未知重金属配体起中介作用Mediating role for unknown heavy metal ligands | [ [ |
| 紫外线 Ultraviolet ray | (1)改变细胞壁结构,合成外膜或EPS Changing the structure of the cell wall and synthesizing the outer membrane or EPS (2)结合蛋白质,促进其折叠或防止其自我聚集 Binding proteins to promote their folding or prevents their self-aggregation (3)结合水,促进悬浮和凝胶Binding water and promoting suspension and gelation | [ [ [ |
| 贫营养 Oligotrophic | (1)积聚在蛋白质周围,防止其失活Accumulating around proteins to prevent their inactivation (2)维持ADP和ATP调节的电子流Maintenance of ADP- and ATP-regulated electron flow (3)受(p)ppGpp、RpoS和PhoB综合调节Regulated by a combination of(p)ppGpp, RpoS and PhoB | [ [ [ |
| 干旱 Droughts | 分泌并保留细胞壁外的EPS,结合大量水,增加持水性 Secreting and retaining EPS outside the cell wall, and binding a large amount of water to increase water-holding capacity | [ |
| [1] | Frank C, Jendrossek D. Acidocalcisomes and polyphosphate granules are different subcellular structures in Agrobacterium tumefaciens[J]. Appl Environ Microbiol, 2020, 86(8): e02759-e02719. |
| [2] |
Müller WEG, Schröder HC, Wang XH. Inorganic polyphosphates as storage for and generator of metabolic energy in the extracellular matrix[J]. Chem Rev, 2019, 119(24): 12337-12374.
doi: 10.1021/acs.chemrev.9b00460 pmid: 31738523 |
| [3] |
Asady B, Dick CF, Ehrenman K, et al. A single Na+-Pi cotransporter in Toxoplasma plays key roles in phosphate import and control of parasite osmoregulation[J]. PLoS Pathog, 2020, 16(12): e1009067.
doi: 10.1371/journal.ppat.1009067 URL |
| [4] | Potapenko E, Cordeiro CD, Huang GZ, et al. Pyrophosphate stimulates the phosphate-sodium symporter of Trypanosoma brucei acidocalcisomes and Saccharomyces cerevisiae vacuoles[J]. mSphere, 2019, 4(2): e00045-e00019. |
| [5] |
Nunes DCOS, Costa MS, Bispo-da-Silva LB, et al. Mitochondrial dysfunction on Leishmania(Leishmania)amazonensis induced by ketoconazole: insights into drug mode of action[J]. Mem Inst Oswaldo Cruz, 2022, 117: e210157.
doi: 10.1590/0074-02760210157 URL |
| [6] |
Gezelius K. Inorganic polyphosphates and enzymes of polyphosphate metabolism in the cellular slime mold Dictyostelium discoideum[J]. Arch Microbiol, 1974, 98(4): 311-329.
pmid: 4367840 |
| [7] |
Negreiros RS, Lander N, Huang GZ, et al. Inorganic polyphosphate interacts with nucleolar and glycosomal proteins in trypanosomatids[J]. Mol Microbiol, 2018, 110(6): 973-994.
doi: 10.1111/mmi.14131 pmid: 30230089 |
| [8] |
Werner TP, Amrhein N, Freimoser FM. Inorganic polyphosphate occurs in the cell wall of Chlamydomonas reinhardtii and accumulates during cytokinesis[J]. BMC Plant Biol, 2007, 7: 51.
doi: 10.1186/1471-2229-7-51 |
| [9] |
Das S, Lengweiler UD, Seebach D, et al. Proof for a nonproteinaceous calcium-selective channel in Escherichia coli by total synthesis from(R)-3-hydroxybutanoic acid and inorganic polyphosphate[J]. Proc Natl Acad Sci USA, 1997, 94(17): 9075-9079.
pmid: 9256437 |
| [10] | Racki LR, Tocheva EI, Dieterle MG, et al. Polyphosphate granule biogenesis is temporally and functionally tied to cell cycle exit during starvation in Pseudomonas aeruginosa[J]. Proc Natl Acad Sci USA, 2017, 114(12): E2440-E2449. |
| [11] |
Watanabe T, Kitamura Y, Aizawa H, et al. Fluorometric quantification of human platelet polyphosphate using 4', 6-diamidine-2-phenylindole dihydrochloride: applications in the Japanese population[J]. Int J Mol Sci, 2021, 22(14): 7257.
doi: 10.3390/ijms22147257 URL |
| [12] |
Kornberg A, Rao NN, Ault-Riché D. Inorganic polyphosphate: a molecule of many functions[J]. Annu Rev Biochem, 1999, 68: 89-125.
pmid: 10872445 |
| [13] |
Trilisenko L, Zvonarev A, Valiakhmetov A, et al. The reduced level of inorganic polyphosphate mobilizes antioxidant and manganese-resistance systems in Saccharomyces cerevisiae[J]. Cells, 2019, 8(5): 461.
doi: 10.3390/cells8050461 URL |
| [14] |
Baijal K, Downey M. Targeting polyphosphate kinases in the fight against Pseudomonas aeruginosa[J]. mBio, 2021, 12(4): e0147721.
doi: 10.1128/mBio.01477-21 URL |
| [15] |
Solovchenko A, Gorelova O, Karpova O, et al. Phosphorus feast and famine in cyanobacteria: is luxury uptake of the nutrient just a consequence of acclimation to its shortage?[J]. Cells, 2020, 9(9): 1933.
doi: 10.3390/cells9091933 URL |
| [16] |
Ghosh R, Barman S, Mandal NC. Phosphate deficiency induced biofilm formation of Burkholderia on insoluble phosphate granules plays a pivotal role for maximum release of soluble phosphate[J]. Sci Rep, 2019, 9(1): 5477.
doi: 10.1038/s41598-019-41726-9 |
| [17] |
Hothorn M, Neumann H, Lenherr ED, et al. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase[J]. Science, 2009, 324(5926): 513-516.
doi: 10.1126/science.1168120 pmid: 19390046 |
| [18] |
Ruiz FA, Marchesini N, Seufferheld M, et al. The polyphosphate bodies of Chlamydomonas reinhardtii possess a proton-pumping pyrophosphatase and are similar to acidocalcisomes[J]. J Biol Chem, 2001, 276(49): 46196-46203.
doi: 10.1074/jbc.M105268200 pmid: 11579086 |
| [19] |
Docampo R, Moreno SN. The acidocalcisome[J]. Mol Biochem Parasitol, 2001, 114(2): 151-159.
doi: 10.1016/S0166-6851(01)00246-8 URL |
| [20] | 金文育, 姚炜民, 欧林坚, 等. 米氏凯伦藻胞内多聚磷酸盐对环境磷变化的响应研究[J]. 海洋与湖沼, 2022, 53(2): 340-345. |
| Jin WY, Yao WM, Ou LJ, et al. Response of intracellular polyphosphate in Karenia mikimotoi to the variation of phosphorus in the environment[J]. Oceanol Limnol Sin, 2022, 53(2): 340-345. | |
| [21] |
Sanz-Luque E, Bhaya D, Grossman AR. Polyphosphate: a multifunctional metabolite in cyanobacteria and algae[J]. Front Plant Sci, 2020, 11: 938.
doi: 10.3389/fpls.2020.00938 pmid: 32670331 |
| [22] |
Mullan A, Quinn JP, McGrath JW. Enhanced phosphate uptake and polyphosphate accumulation in Burkholderia cepacia grown under low pH conditions[J]. Microb Ecol, 2002, 44(1): 69-77.
doi: 10.1007/s00248-002-3004-x pmid: 12187377 |
| [23] |
Wacey D, Sirantoine E, Saunders M, et al. 1 billion-year-old cell contents preserved in monazite and xenotime[J]. Sci Rep, 2019, 9(1): 9068.
doi: 10.1038/s41598-019-45575-4 pmid: 31227773 |
| [24] |
Swift DT, Forciniti D. Accumulation of lead by Anabaena cylindrica: mathematical modeling and an energy dispersive X-ray study[J]. Biotechnol Bioeng, 1997, 55(2): 408-418.
pmid: 18636499 |
| [25] |
Zhu JL, Wei RP, Wang X, et al. Polyphosphate accelerates transformation of nonstructural carbohydrates to improve growth of ppk-expressing transgenic rice in phosphorus deficiency culture[J]. Rice Sci, 2023, 30(3): 235-246.
doi: 10.1016/j.rsci.2023.03.007 |
| [26] |
Wei RP, Wang X, Zhang W, et al. The improved phosphorus utilization and reduced phosphorus consumption of ppk-expressing transgenic rice[J]. Field Crops Res, 2020, 248: 107715.
doi: 10.1016/j.fcr.2020.107715 URL |
| [27] |
Zhang Y, Jing J, Liu T, et al. A molecularly engineered bioderived polyphosphate for enhanced flame retardant, UV-blocking and mechanical properties of poly(lactic acid)[J]. Chem Eng J, 2021, 411: 128493.
doi: 10.1016/j.cej.2021.128493 URL |
| [28] |
Gao JP, Chao DY, Lin HX. Understanding abiotic stress tolerance mechanisms: recent studies on stress response in rice[J]. J Integr Plant Biol, 2007, 49(6): 742-750.
doi: 10.1111/jipb.2007.49.issue-6 URL |
| [29] | Camci İY, Doruk T, Avİcan Ü, et al. Deletion of polyphosphate kinase gene(ppk)has a stimulatory effect on actinorhodin production by Streptomyces coelicolor A3(2)[J]. Turk J Biol, 2012, 36: 373-380. |
| [30] |
Cavalcanti Luna MA, Vieira ER, Okada K, et al. Copper-induced adaptation, oxidative stress and its tolerance in Aspergillus niger UCP1261[J]. Electron J Biotechnol, 2015, 18(6): 418-427.
doi: 10.1016/j.ejbt.2015.09.006 URL |
| [31] |
Cheng YY, Sun BL. Polyphosphate kinase affects oxidative stress response by modulating cAMP receptor protein and rpoS expression in Salmonella typhimurium[J]. J Microbiol Biotechnol, 2009, 19(12): 1527-1535.
doi: 10.4014/jmb URL |
| [32] | Diaz JM, Ingall ED, Snow SD, et al. Potential role of inorganic polyphosphate in the cycling of phosphorus within the hypoxic water column of Effingham Inlet, British Columbia[J]. Global Biogeochem Cycles, 2012, 26(2): 2040. |
| [33] |
Cherry DS, Guthrie RK. Effects of Flavobacterium lutescens growth on populations of Escherichia coli and Streptococcus faecalis in water following thermal loading[J]. Microb Ecol, 1975, 2(3): 186-193.
doi: 10.1007/BF02010438 pmid: 24241333 |
| [34] |
McDevitt-Irwin JM, Baum JK, Garren M, et al. Responses of coral-associated bacterial communities to local and global stressors[J]. Front Mar Sci, 2017, 4: 262.
doi: 10.3389/fmars.2017.00262 URL |
| [35] | Guthrie RK, Cherry DS, Singleton FL. Alteration of microbial populations in thermal stress[J]. J Water Pollut Control Fed, 1976, 48(5): 962-965. |
| [36] |
Guan NZ, Li JH, Shin HD, et al. Microbial response to environmental stresses: from fundamental mechanisms to practical applications[J]. Appl Microbiol Biotechnol, 2017, 101(10): 3991-4008.
doi: 10.1007/s00253-017-8264-y pmid: 28409384 |
| [37] |
Guyot S, Gervais P, Young M, et al. Surviving the heat: heterogeneity of response in Saccharomyces cerevisiae provides insight into thermal damage to the membrane[J]. Environ Microbiol, 2015, 17(8): 2982-2992.
doi: 10.1111/emi.2015.17.issue-8 URL |
| [38] |
Nicolaou SA, Gaida SM, Papoutsakis ET. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: from biofuels and chemicals, to biocatalysis and bioremediation[J]. Metab Eng, 2010, 12(4): 307-331.
doi: 10.1016/j.ymben.2010.03.004 pmid: 20346409 |
| [39] |
Gao LM, Liu YQ, Sun H, et al. Advances in mechanisms and modifications for rendering yeast thermotolerance[J]. J Biosci Bioeng, 2016, 121(6): 599-606.
doi: S1389-1723(15)00403-X pmid: 26685013 |
| [40] |
Achbergerová L, Nahálka J. Polyphosphate - an ancient energy source and active metabolic regulator[J]. Microb Cell Fact, 2011, 10: 63.
doi: 10.1186/1475-2859-10-63 pmid: 21816086 |
| [41] |
Solovchenko A, Khozin-Goldberg I, Selyakh I, et al. Phosphorus starvation and luxury uptake in green microalgae revisited[J]. Algal Res, 2019, 43: 101651.
doi: 10.1016/j.algal.2019.101651 URL |
| [42] |
Cremers CM, Knoefler D, Gates S, et al. Polyphosphate: a conserved modifier of amyloidogenic processes[J]. Mol Cell, 2016, 63(5): 768-780.
doi: 10.1016/j.molcel.2016.07.016 pmid: 27570072 |
| [43] |
Marijan D, Tse R, Elliott K, et al. Stress-specific aggregation of proteins in the amyloid bodies[J]. FEBS Lett, 2019, 593(22): 3162-3172.
doi: 10.1002/1873-3468.13597 pmid: 31512750 |
| [44] |
Gray MJ, Wholey WY, Wagner NO, et al. Polyphosphate is a primordial chaperone[J]. Mol Cell, 2014, 53(5): 689-699.
doi: 10.1016/j.molcel.2014.01.012 pmid: 24560923 |
| [45] |
Yoo NG, Dogra S, Meinen BA, et al. Polyphosphate stabilizes protein unfolding intermediates as soluble amyloid-like oligomers[J]. J Mol Biol, 2018, 430(21): 4195-4208.
doi: S0022-2836(18)30625-9 pmid: 30130556 |
| [46] | 陈成, 郑超群, 王梦梦, 等. 低浓度硝态氮促进微囊藻累积多聚磷酸盐[J]. 湖泊科学, 2022, 34(3): 766-776. |
| Chen C, Zheng CQ, Wang MM, et al. Low concentration nitrate-nitrogen improves polyphosphate accumulation in Microcystis[J]. J Lake Sci, 2022, 34(3): 766-776. | |
| [47] |
Baker-Austin C, Dopson M. Life in acid: pH homeostasis in acidophiles[J]. Trends Microbiol, 2007, 15(4): 165-171.
pmid: 17331729 |
| [48] |
Guan NZ, Liu L, Shin HD, et al. Systems-level understanding of how Propionibacterium acidipropionici respond to propionic acid stress at the microenvironment levels: mechanism and application[J]. J Biotechnol, 2013, 167(1): 56-63.
doi: 10.1016/j.jbiotec.2013.06.008 URL |
| [49] | 杨柳燕, 肖琳. 湖泊蓝藻水华暴发、危害与控制[M]. 北京: 科学出版社, 2011. |
| Yang LY, Xiao L. Outbreak, harm and control of cyanobacteria bloom in lakes[M]. Beijing: Science Press, 2011. | |
| [50] | 魏峥, 聂琰晖, 刘乐庭, 等. 多聚磷酸盐在原核和真核生物中的研究进展[J]. 生理科学进展, 2009, 40(3): 197-202. |
| Wei Z, Nie YH, Liu LT, et al. Progress in functional polyphosphate in prokaryotic and eukaryotic living organisms[J]. Prog Physiol Sci, 2009, 40(3): 197-202. | |
| [51] |
Bremer E, Krämer R. Responses of microorganisms to osmotic stress[J]. Annu Rev Microbiol, 2019, 73: 313-334.
doi: 10.1146/annurev-micro-020518-115504 pmid: 31180805 |
| [52] |
De Angelis M, Gobbetti M. Environmental stress responses in Lactobacillus: a review[J]. Proteomics, 2004, 4(1): 106-122.
pmid: 14730676 |
| [53] | 马芮, 苏莉, 宋宇昊, 等. 多聚磷酸盐: 菌体内多功能调控子和环境压力守护者[J]. 微生物学通报, 2017, 44(7): 1736-1746. |
| Ma R, Su L, Song YH, et al. Inorganic polyphosphate: the multifunctional regulator and the guardian of environmental stresses in bacteria[J]. Microbiol China, 2017, 44(7): 1736-1746. | |
| [54] |
Liu W, Li YL, Feng Y, et al. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil[J]. Sci Rep, 2020, 10(1): 858.
doi: 10.1038/s41598-020-57954-3 pmid: 31965039 |
| [55] | 姚雪丹, 付建红, 徐彤, 等. 重金属胁迫下的微生物代谢组学研究进展[J]. 生物资源, 2020, 42(6): 678-685. |
| Yao XD, Fu JH, Xu T, et al. Metabolomics of microorganisms in response to heavy metal stress[J]. Biotic Resour, 2020, 42(6): 678-685. | |
| [56] |
White RA III, Soles SA, Gavelis G, et al. The complete genome and physiological analysis of the eurythermal firmicute Exiguobacterium chiriqhucha strain RW2 isolated from a freshwater microbialite, widely adaptable to broad thermal, pH, and salinity ranges[J]. Front Microbiol, 2019, 9: 3189.
doi: 10.3389/fmicb.2018.03189 URL |
| [57] |
Xu LZJ, Wu J, Xia WJ, et al. Adaption and restoration of anammox biomass to Cd(II)stress: performance, extracellular polymeric substance and microbial community[J]. Bioresour Technol, 2019, 290: 121766.
doi: 10.1016/j.biortech.2019.121766 URL |
| [58] |
Haider FU, Farooq M, Naveed M, et al. Influence of biochar and microorganism co-application on stabilization of cadmium(Cd)and improved maize growth in Cd-contaminated soil[J]. Front Plant Sci, 2022, 13: 983830.
doi: 10.3389/fpls.2022.983830 URL |
| [59] |
Darnall DW, Greene B, Henzl MT, et al. Selective recovery of gold and other metal ions from an algal biomass[J]. Environ Sci Technol, 1986, 20(2): 206-208.
doi: 10.1021/es00144a018 URL |
| [60] |
Komine Y, Eggink LL, Park H, et al. Vacuolar Granules in Chlamydomonas reinhardtii: polyphosphate and a 70-kDa polypeptide as major components[J]. Planta, 2000, 210(6): 897-905.
pmid: 10872220 |
| [61] |
Chu FF, Chu PN, Cai PJ, et al. Phosphorus plays an important role in enhancing biodiesel productivity of Chlorella vulgaris under nitrogen deficiency[J]. Bioresour Technol, 2013, 134: 341-346.
doi: 10.1016/j.biortech.2013.01.131 URL |
| [62] |
刘沙沙, 付建平, 蔡信德, 等. 重金属污染对土壤微生物生态特征的影响研究进展[J]. 生态环境学报, 2018, 27(6): 1173-1178.
doi: 10.16258/j.cnki.1674-5906.2018.06.024 |
| Liu SS, Fu JP, Cai XD, et al. Effect of heavy metals pollution on ecological characteristics of soil microbes: a review[J]. Ecol Environ Sci, 2018, 27(6): 1173-1178. | |
| [63] |
Moura KAF, Lizieri C, Wittig Franco M, et al. Physiological and thylakoid ultrastructural changes in cyanobacteria in response to toxic manganese concentrations[J]. Ecotoxicology, 2019, 28(8): 1009-1021.
doi: 10.1007/s10646-019-02098-y pmid: 31471822 |
| [64] |
Sicko-Goad L, Jensen TE, Ayala RP. Phosphate metabolism in blue-green bacteria. V. Factors affecting phosphate uptake in Plectonema boryanum[J]. Can J Microbiol, 1978, 24(2): 105-108.
pmid: 25703 |
| [65] |
Samadani M, Dewez D. Effect of mercury on the polyphosphate level of Alga Chlamydomonas reinhardtii[J]. Environ Pollut, 2018, 240: 506-513.
doi: S0269-7491(17)33603-5 pmid: 29754100 |
| [66] |
Tsednee M, Castruita M, Salomé PA, et al. Manganese co-localizes with calcium and phosphorus in Chlamydomonas acidocalcisomes and is mobilized in manganese-deficient conditions[J]. J Biol Chem, 2019, 294(46): 17626-17641.
doi: 10.1074/jbc.RA119.009130 URL |
| [67] |
Penen F, Isaure MP, Dobritzsch D, et al. Pools of cadmium in Chlamydomonas reinhardtii revealed by chemical imaging and XAS spectroscopy[J]. Metallomics, 2017, 9(7): 910-923.
doi: 10.1039/c7mt00029d pmid: 28598481 |
| [68] |
MacFie SM, Welbourn PM. The cell wall as a barrier to uptake of metal ions in the unicellular green Alga Chlamydomonas reinhardtii(Chlorophyceae)[J]. Arch Environ Contam Toxicol, 2000, 39(4): 413-419.
doi: 10.1007/s002440010122 URL |
| [69] |
Samadani M, Dewez D. Cadmium accumulation and toxicity affect the extracytoplasmic polyphosphate level in Chlamydomonas reinhardtii[J]. Ecotoxicol Environ Saf, 2018, 166: 200-206.
doi: 10.1016/j.ecoenv.2018.09.094 URL |
| [70] |
Samadani M, El-Khoury J, Dewez D. Tolerance capacity of Chlamydomonas VHLR mutants for the toxicity of mercury[J]. Water Air Soil Pollut, 2020, 231(4): 1-12.
doi: 10.1007/s11270-019-4368-6 |
| [71] |
El Bouraie M, Masoud AA. Adsorption of phosphate ions from aqueous solution by modified bentonite with magnesium hydroxide Mg(OH)2[J]. Appl Clay Sci, 2017, 140: 157-164.
doi: 10.1016/j.clay.2017.01.021 URL |
| [72] |
Li SS, Li JH, Xia MS, et al. Adsorption of nitrogen and phosphorus by intact cells and cell wall polysaccharides of Microcystis[J]. J Appl Phycol, 2013, 25(5): 1539-1544.
doi: 10.1007/s10811-013-9992-8 URL |
| [73] |
Sheng GP, Xu J, Li WH, et al. Quantification of the interactions between Ca2+, Hg2+ and extracellular polymeric substances(EPS)of sludge[J]. Chemosphere, 2013, 93(7): 1436-1441.
doi: 10.1016/j.chemosphere.2013.07.076 URL |
| [74] |
Tan X, Gao WP, Duan ZP, et al. Synthesis of novel algal extracellular polymeric substances(EPS)-based hydrogels for the efficient removal and recovery of phosphorus from contaminated waters: development, characterisation, and performance[J]. J Environ Chem Eng, 2023, 11(1): 109044.
doi: 10.1016/j.jece.2022.109044 URL |
| [75] | 杨柳燕, 胡志新, 何连生. 中国湖泊水生态系统区域差异性[M]. 北京: 科学出版社, 2016. |
| Yang LY, Hu ZX, He LS. Regional differences of lake water ecosystem in China[M]. Beijing: Science Press, 2016. | |
| [76] |
Stumpf JD, Foster PL. Polyphosphate kinase regulates error-prone replication by DNA polymerase IV in Escherichia coli[J]. Mol Microbiol, 2005, 57(3): 751-761.
doi: 10.1111/mmi.2005.57.issue-3 URL |
| [77] |
Rodriguez RJ. Polyphosphate present in DNA preparations from filamentous fungal species of Colletotrichum inhibits restriction endonucleases and other enzymes[J]. Anal Biochem, 1993, 209(2): 291-297.
pmid: 8385889 |
| [78] | Kuroda A, Nomura K, Takiguchi N, et al. Inorganic polyphosphate stimulates lon-mediated proteolysis of nucleoid proteins in Escherichia coli[J]. Cell Mol Biol, 2006, 52(4): 23-29. |
| [79] | English BP, Hauryliuk V, Sanamrad A, et al. Single-molecule investigations of the stringent response machinery in living bacterial cells[J]. Proc Natl Acad Sci USA, 2011, 108(31): E365-E373. |
| [80] |
Price-Carter M, Fazzio TG, Vallbona EI, et al. Polyphosphate kinase protects Salmonella enterica from weak organic acid stress[J]. J Bacteriol, 2005, 187(9): 3088-3099.
pmid: 15838036 |
| [81] |
Kulakovskaya TV, Vagabov VM, Kulaev IS. Inorganic polyphosphate in industry, agriculture and medicine: modern state and outlook[J]. Process Biochem, 2012, 47(1): 1-10.
doi: 10.1016/j.procbio.2011.10.028 URL |
| [82] |
Paerl HW, Fulton RS Ⅲ, Moisander PH, et al. Harmful freshwater algal blooms, with an emphasis on cyanobacteria[J]. Sci World J, 2001, 1: 76-113.
pmid: 12805693 |
| [83] |
Schwarz R, Forchhammer K. Acclimation of unicellular cyanobacteria to macronutrient deficiency: emergence of a complex network of cellular responses[J]. Microbiology, 2005, 151(Pt 8): 2503-2514.
doi: 10.1099/mic.0.27883-0 pmid: 16079330 |
| [84] |
Goodenough U, Heiss AA, Roth R, et al. Acidocalcisomes: ultrastructure, biogenesis, and distribution in microbial eukaryotes[J]. Protist, 2019, 170(3): 287-313.
doi: S1434-4610(19)30003-3 pmid: 31154072 |
| [85] |
Chu FF, Shen XF, Lam PKS, et al. Polyphosphate during the regreening of Chlorella vulgaris under nitrogen deficiency[J]. Int J Mol Sci, 2015, 16(10): 23355-23368.
doi: 10.3390/ijms161023355 URL |
| [86] |
Jensen TE, Sicko LM. Phosphate metabolism in blue-green algae. I. Fine structure of the polyphosphate overplus phenomenon in Plectonema boryanum[J]. Can J Microbiol, 1974, 20(9): 1235-1239.
pmid: 4371465 |
| [87] |
Liss E, Langen P. Experiments on polyphosphate overcompensation in yeast cells after phosphate deficiency[J]. Archiv für Mikrobiologie, 1962, 41: 383-392.
doi: 10.1007/BF00422195 URL |
| [88] |
Aksoy M, Pootakham W, Grossman AR. Critical function of a Chlamydomonas reinhardtii putative polyphosphate polymerase subunit during nutrient deprivation[J]. Plant Cell, 2014, 26(10): 4214-4229.
doi: 10.1105/tpc.114.129270 URL |
| [89] | Hiraishi A, Kitamura H. Changes in the polyphosphate content of photosynthetically grown Rhodobacter sphaeroides due to nutrient limitation[J]. Agric Biol Chem, 1985, 49(11): 3343-3345. |
| [90] |
Sanz-Luque E, Saroussi S, Huang W, et al. Metabolic control of acclimation to nutrient deprivation dependent on polyphosphate synthesis[J]. Sci Adv, 2020, 6(40): eabb5351.
doi: 10.1126/sciadv.abb5351 URL |
| [91] |
Wild R, Gerasimaite R, Jung JY, et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains[J]. Science, 2016, 352(6288): 986-990.
doi: 10.1126/science.aad9858 pmid: 27080106 |
| [92] |
Gerasimaite R, Pavlovic I, Capolicchio S, et al. Inositol pyrophosphate specificity of the SPX-dependent polyphosphate polymerase VTC[J]. ACS Chem Biol, 2017, 12(3): 648-653.
doi: 10.1021/acschembio.7b00026 pmid: 28186404 |
| [93] |
Potrykus K, Cashel M. (p)ppGpp: still magical?[J]. Annu Rev Microbiol, 2008, 62: 35-51.
doi: 10.1146/annurev.micro.62.081307.162903 pmid: 18454629 |
| [94] |
Rao NN, Kornberg A. Inorganic polyphosphate regulates responses of Escherichia coli to nutritional stringencies, environmental stresses and survival in the stationary phase[J]. Prog Mol Subcell Biol, 1999, 23: 183-195.
pmid: 10448677 |
| [95] |
Alpert P. Constraints of tolerance: why are desiccation-tolerant organisms so small or rare?[J]. J Exp Biol, 2006, 209(Pt 9): 1575-1584.
doi: 10.1242/jeb.02179 URL |
| [96] |
Wang Q, Li J, Yang J, et al. Diversity of endophytic bacterial and fungal microbiota associated with the medicinal lichen Usnea longissima at high altitudes[J]. Front Microbiol, 2022, 13: 958917.
doi: 10.3389/fmicb.2022.958917 URL |
| [97] |
Knowles EJ, Castenholz RW. Effect of exogenous extracellular polysaccharides on the desiccation and freezing tolerance of rock-inhabiting phototrophic microorganisms[J]. FEMS Microbiol Ecol, 2008, 66(2): 261-270.
doi: 10.1111/j.1574-6941.2008.00568.x pmid: 18710394 |
| [98] |
Jasso-Chávez R, Campos-García ML, Vega-Segura A, et al. Microaerophilia enhances heavy metal biosorption and internal binding by polyphosphates in photosynthetic Euglena gracilis[J]. Algal Res, 2021, 58: 102384.
doi: 10.1016/j.algal.2021.102384 URL |
| [99] | Kiyono M, Pan-Hou H. Genetic engineering of bacteria for environmental remediation of mercury[J]. J Heath Sci, 2006, 52(3): 199-204. |
| [100] |
Correa Deza MA, Grillo-Puertas M, Salva S, et al. Inorganic salts and intracellular polyphosphate inclusions play a role in the thermotolerance of the immunobiotic Lactobacillus rhamnosus CRL 1505[J]. PLoS One, 2017, 12(6): e0179242.
doi: 10.1371/journal.pone.0179242 URL |
| [101] |
Mullan A, McGrath JW, Adamson T, et al. Pilot-scale evaluation of the application of low pH-inducible polyphosphate accumulation to the biological removal of phosphate from wastewaters[J]. Environ Sci Technol, 2006, 40(1): 296-301.
doi: 10.1021/es0509782 URL |
| [102] |
Feng GX, Dong SY, Huang M, et al. Biogenic polyphosphate nanoparticles from a marine cyanobacterium Synechococcus sp. PCC 7002: production, characterization, and anti-inflammatory properties in vitro[J]. Mar Drugs, 2018, 16(9): 322.
doi: 10.3390/md16090322 URL |
| [103] |
Gao FZ, Wu HH, Zeng MY, et al. Overproduction, purification, and characterization of nanosized polyphosphate bodies from Synechococcus sp. PCC 7002[J]. Microb Cell Fact, 2018, 17(1): 27.
doi: 10.1186/s12934-018-0870-6 |
| [104] |
Christ JJ, Smith SA, Willbold S, et al. Biotechnological synthesis of water-soluble food-grade polyphosphate with Saccharomyces cerevisiae[J]. Biotechnol Bioeng, 2020, 117(7): 2089-2099.
doi: 10.1002/bit.v117.7 URL |
| [105] | 赵心清, 彭楠. 新资源微生物:微生物资源的发现和应用[J]. 微生物学报, 2022, 62(11): 4091-4094. |
| Zhao XQ, Peng N. Preface for special issue on new-resource microbes: discovery and applications of microbial resources[J]. Acta Microbiol Sin, 2022, 62(11): 4091-4094. |
| [1] | ZHANG Kun, YAN Chang, TIAN Xin-peng. Research Progress in Microbial Single Cell Separation Methods [J]. Biotechnology Bulletin, 2023, 39(9): 1-11. |
| [2] | JIANG Run-hai, JIANG Ran-ran, ZHU Cheng-qiang, HOU Xiu-li. Research Progress in Mechanisms of Microbial-enhanced Phytoremediation for Lead-contaminated Soil [J]. Biotechnology Bulletin, 2023, 39(8): 114-125. |
| [3] | ZHAO Lin-yan, XU Wu-mei, WANG Hao-ji, WANG Kun-yan, WEI Fu-gang, YANG Shao-zhou, GUAN Hui-lin. Effects of Applying Biochar on the Rhizosphere Fungal Community and Survival Rate of Panax notoginseng Under Continuous Cropping [J]. Biotechnology Bulletin, 2023, 39(7): 219-227. |
| [4] | ZHANG Jing, ZHANG Hao-rui, CAO Yun, HUANG Hong-ying, QU Ping, ZHANG Zhi-ping. Research Progress in Thermophilic Microorganisms for Cellulose Degradation [J]. Biotechnology Bulletin, 2023, 39(6): 73-87. |
| [5] | YU Yang, LIU Tian-hai, LIU Li-xu, TANG Jie, PENG Wei-hong, CHEN Yang, TAN Hao. Study on Aerosol Microbial Community in the Production Workshop of Morel Spawn [J]. Biotechnology Bulletin, 2023, 39(5): 267-275. |
| [6] | ZHANG Hua-xiang, XU Xiao-ting, ZHENG Yun-ting, XIAO Chun-qiao. Roles of Phosphate-solubilizing Microorganisms in the Passivation and Phytoremediation of Heavy Metal Contaminated Soil [J]. Biotechnology Bulletin, 2023, 39(3): 52-58. |
| [7] | LI Xin-yue, ZHOU Ming-hai, FAN Ya-chao, LIAO Sha, ZHANG Feng-li, LIU Chen-guang, SUN Yue, ZHANG Lin, ZHAO Xin-qing. Research Progress in the Improvement of Microbial Strain Tolerance and Efficiency of Biological Manufacturing Based on Transporter Engineering [J]. Biotechnology Bulletin, 2023, 39(11): 123-136. |
| [8] | HU Jin-chao, SHEN Wen-qi, XU Chao-ye, FAN Ya-qi, LU Hao-yu, JIANG Wen-jie, LI Shi-long, JIN Hong-chen, LUO Jian-mei, WANG Min. Research Advances in the Enhancement of Microbial Tolerance to Acid Stress [J]. Biotechnology Bulletin, 2023, 39(11): 137-149. |
| [9] | WAN Qi-wu, BAO Xu-dong, DING Ke, MOU Hua-ming, LUO Yang. Research Progress in Microfluidic Technology in the Detection of Pathogenic Microorganisms [J]. Biotechnology Bulletin, 2023, 39(10): 107-114. |
| [10] | SUN Zhuo, WANG Yan, HAN Zhong-ming, WANG Yun-he, ZHAO Shu-jie, YANG Li-min. Isolation, Identification and Biocontrol Potential of Rhizospheric Fungus of Saposhnikovia divaricata [J]. Biotechnology Bulletin, 2023, 39(1): 264-273. |
| [11] | ZHANG Hao, LIU Miao-miao, LIU Xiao-na, LI Zong-yu, ZHAO Li-li, YANG Qing-xiang. Impact of Endophytic Microorganisms on the Pharmaco-active Compounds Production in Medicinal Plants:A Review [J]. Biotechnology Bulletin, 2022, 38(8): 41-51. |
| [12] | WANG Zheng-yan, HU Hai-sheng, YONG Han-zi, LU Yu-jie. Nutritional Interactions Between Symbiotic Microbiota and Insect Hosts [J]. Biotechnology Bulletin, 2022, 38(7): 99-108. |
| [13] | WANG Xiao-fang, WAN Jin-xin, WEI Zhong, XU Yang-chun, SHEN Qi-rong. Succession of Microbial Communities During Livestock Manure Composting [J]. Biotechnology Bulletin, 2022, 38(5): 13-21. |
| [14] | ZHU Jing, YU Cun. Effects of Trichoderma longibrachiatum on Maize Growth,Soil Fertility and Rhizosphere Microorganism [J]. Biotechnology Bulletin, 2022, 38(4): 230-241. |
| [15] | YANG Lu, XIN Jian-pan, TIAN Ru-nan. Research Progress in the Mitigative Effects of Rhizosphere Microorganisms on Heavy Metal Stress in Plants and Their Mechanisms [J]. Biotechnology Bulletin, 2022, 38(3): 213-225. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||