








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (3): 312-321.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0784
Previous Articles Next Articles
SHAN Xin-yu( ), LI Tai-chun, YANG Ruo-chen, DUAN Xiang-ru, KANG Jia, ZHANG Ying-jie, LIU Yue-qin(
), LI Tai-chun, YANG Ruo-chen, DUAN Xiang-ru, KANG Jia, ZHANG Ying-jie, LIU Yue-qin( )
)
Received:2023-08-14
Online:2024-03-26
Published:2024-04-08
Contact:
LIU Yue-qin
E-mail:1126954588@qq.com;Liuyueqin66@126.com
SHAN Xin-yu, LI Tai-chun, YANG Ruo-chen, DUAN Xiang-ru, KANG Jia, ZHANG Ying-jie, LIU Yue-qin. Effect of γ-aminobutyric Acid on Apoptosis and the Secretion of Steroid Hormone in Ovine Ovarian Granulosa Cells[J]. Biotechnology Bulletin, 2024, 40(3): 312-321.
| 基因Gene | 引物序列Primer sequence(5'-3') | 退火温度Annealing temperature/℃ | 产物长度Size/bp |
|---|---|---|---|
| GAPDH | F: GGTCGGAGTGAACGGATTTG | 60 | 222 |
| R: CTTGACTGTGCCGTGGAACTT | |||
| CCND1 | F:GGCGTTCGTAATCGTGTTTTG | 60 | 185 |
| R:CAGGTAGAAGGAGTGAGGAATGTGT | |||
| PCNA | F:GCCGAGGAGAACAAGCAGA | 60 | 123 |
| R:GAGGGTGGGTTGGAAATGAA | |||
| CCNB1 | F:GGCAGGAGGACTATGACATT | 60 | 222 |
| R:TCAGGCACCCACTTCTCTTTC | |||
| Bax | F:CGAGACCTGAAGCCAGAGAC | 60 | 183 |
| R:GCAAAGATACAGCCAACACC | |||
| Caspase-3 | F:CCCATCCTCACCATCATCAC | 60 | 275 |
| R:GCACAAACACGCACCTCAA | |||
| Bcl-2 | F:ACTTGGACCTGTCGCTGTCC | 60 | 120 |
| R:CCTGCGTTTGGAGTGGTAGA | |||
| CYP11A1 | F:AACATGGAGCTGCAGAGGAT | 60 | 120 |
| R:CCAATGTCCAGCCCATGATG | |||
| StAR | F:TCTTTGAGTTCGGAGGGGTC | 60 | 270 |
| R:GGAGAAATCAAACAGGGGCC | |||
| 3β-HSD | F:TGGACCCGTCGATCTGAAAA | 60 | 283 |
| R:GCGTACAAGAAGTCTGCCTC | |||
| CYP19A1 | F:AATGCTGCCCCAGGTGTT | 60 | 379 |
| R:CGGCGTCTCTGCGATTTT |
Table 1 Primer sequence
| 基因Gene | 引物序列Primer sequence(5'-3') | 退火温度Annealing temperature/℃ | 产物长度Size/bp |
|---|---|---|---|
| GAPDH | F: GGTCGGAGTGAACGGATTTG | 60 | 222 |
| R: CTTGACTGTGCCGTGGAACTT | |||
| CCND1 | F:GGCGTTCGTAATCGTGTTTTG | 60 | 185 |
| R:CAGGTAGAAGGAGTGAGGAATGTGT | |||
| PCNA | F:GCCGAGGAGAACAAGCAGA | 60 | 123 |
| R:GAGGGTGGGTTGGAAATGAA | |||
| CCNB1 | F:GGCAGGAGGACTATGACATT | 60 | 222 |
| R:TCAGGCACCCACTTCTCTTTC | |||
| Bax | F:CGAGACCTGAAGCCAGAGAC | 60 | 183 |
| R:GCAAAGATACAGCCAACACC | |||
| Caspase-3 | F:CCCATCCTCACCATCATCAC | 60 | 275 |
| R:GCACAAACACGCACCTCAA | |||
| Bcl-2 | F:ACTTGGACCTGTCGCTGTCC | 60 | 120 |
| R:CCTGCGTTTGGAGTGGTAGA | |||
| CYP11A1 | F:AACATGGAGCTGCAGAGGAT | 60 | 120 |
| R:CCAATGTCCAGCCCATGATG | |||
| StAR | F:TCTTTGAGTTCGGAGGGGTC | 60 | 270 |
| R:GGAGAAATCAAACAGGGGCC | |||
| 3β-HSD | F:TGGACCCGTCGATCTGAAAA | 60 | 283 |
| R:GCGTACAAGAAGTCTGCCTC | |||
| CYP19A1 | F:AATGCTGCCCCAGGTGTT | 60 | 379 |
| R:CGGCGTCTCTGCGATTTT |
| 一抗名称Name of primary antibody | 品牌及货号Brand and coale | 稀释度Proportion of dilution | 分子量Molecular weight/kD |
|---|---|---|---|
| Caspase-3 | Abcam ab13847 | 1∶1 000 | 34 |
| StAR | Abcam ab133657 | 1∶1 000 | 32 |
| 3β-HSD | Abcam ab55268 | 1∶1 000 | 42 |
| Bax | Abcam ab32503 | 1∶1 000 | 21 |
| Bcl-2 | Abcam ab182858 | 1∶1 500 | 26 |
| CCNB1 | Abcam ab181593 | 1∶1 500 | 55 |
| CCND1 | Abcam ab16663 | 1∶200 | 33 |
| CYP11A1 | Abcam ab272494 | 1∶1 000 | 60 |
| CYP19A1 | Abcam ab18995 | 1∶800 | 55 |
| PCNA | Abcam ab29 | 1∶800 | 30 |
| β-actin(C4)(内参) | Santa Cruz SC-47778 | 1∶1 500 | 43 |
Table 2 Primary antibody information
| 一抗名称Name of primary antibody | 品牌及货号Brand and coale | 稀释度Proportion of dilution | 分子量Molecular weight/kD |
|---|---|---|---|
| Caspase-3 | Abcam ab13847 | 1∶1 000 | 34 |
| StAR | Abcam ab133657 | 1∶1 000 | 32 |
| 3β-HSD | Abcam ab55268 | 1∶1 000 | 42 |
| Bax | Abcam ab32503 | 1∶1 000 | 21 |
| Bcl-2 | Abcam ab182858 | 1∶1 500 | 26 |
| CCNB1 | Abcam ab181593 | 1∶1 500 | 55 |
| CCND1 | Abcam ab16663 | 1∶200 | 33 |
| CYP11A1 | Abcam ab272494 | 1∶1 000 | 60 |
| CYP19A1 | Abcam ab18995 | 1∶800 | 55 |
| PCNA | Abcam ab29 | 1∶800 | 30 |
| β-actin(C4)(内参) | Santa Cruz SC-47778 | 1∶1 500 | 43 |

Fig. 1 Identification of ovine ovarian granulosa cells(400×) A: FSHR staining of ovine ovarian granulosa cells. B: DAPI staining of ovine ovarian granulosa cell nucleus. C: Superimposed dyeing effect

Fig. 2 Effects of different concentrations of GABA on proliferation in ovine granulosa cells The different letters indicate the significant difference(P<0.05), the same below
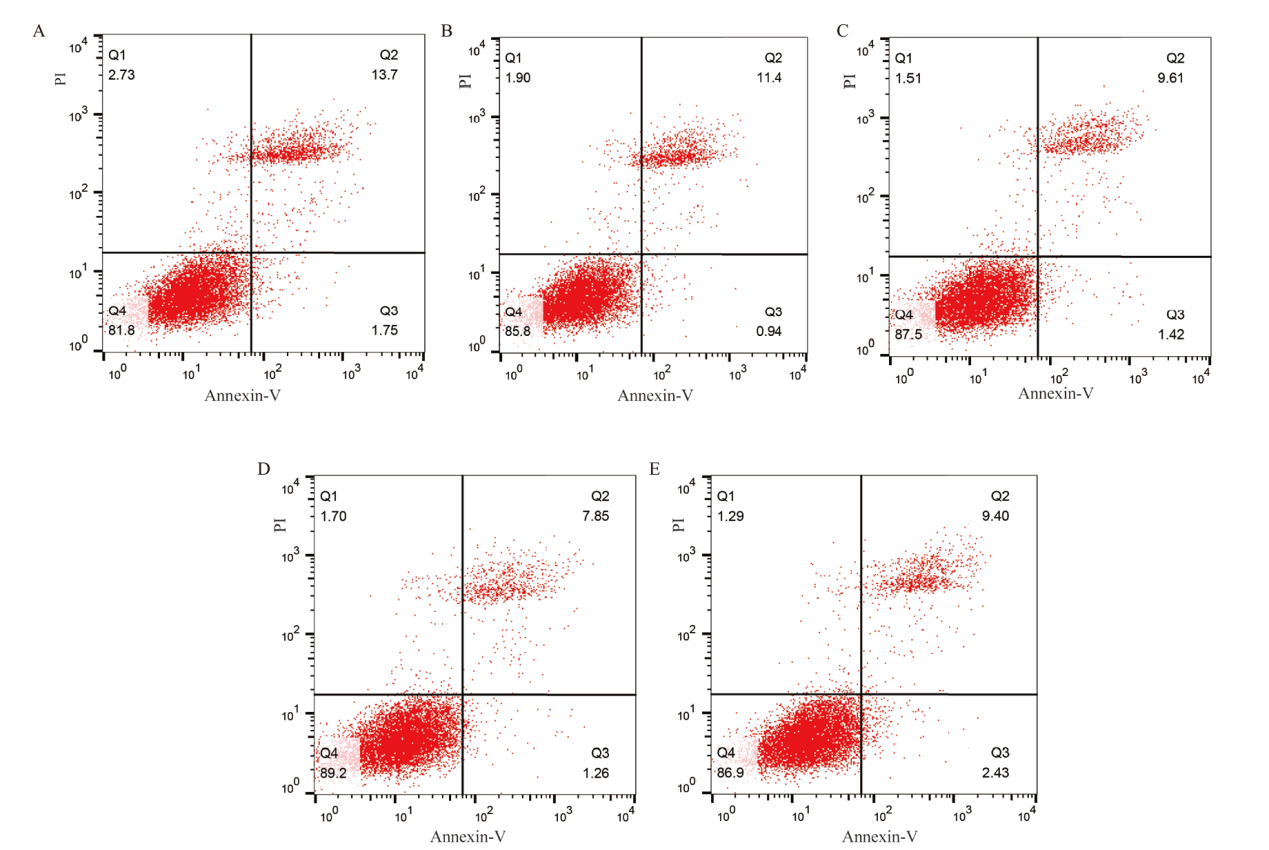
Fig. 3 Results of apoptosis in ovine granulosa cells treated with GABA A、B、C、D、E is the effects of 0, 10-8, 10-7, 10-6, 10-5 mol/L GABA on the apoptosis of ovine granulosa cells. Q1 was dead cells caused by mechanical injury; Q2 was late apoptosis; Q3 was early apoptosis; Q4 was living cells
| 项目Item | 正常细胞率Normal cell rate/% | 早期凋亡率Early apoptosis rate/% | 晚期凋亡率Late apoptosis rate/% | 总凋亡率Total apoptosis rate/% |
|---|---|---|---|---|
| Control | 81.97±0.61a | 1.58±0.22ab | 13.43±0.15a | 15.02±0.26a |
| 10-8 | 84.97±0.49b | 1.42±0.27a | 11.43±0.38b | 12.85±0.56b |
| 10-7 | 87.63±0.24c | 1.63±0.21ab | 9.16±0.52c | 10.78±0.31c |
| 10-6 | 89.27±0.03d | 1.21±0.09a | 7.76±0.24d | 8.97±0.16d |
| 10-5 | 86.80±0.58c | 2.12±0.16b | 9.70±0.17c | 11.82±0.04e |
Table 3 Effect of different concentrations of GABA on apoptosis rate in ovine granulosa cells
| 项目Item | 正常细胞率Normal cell rate/% | 早期凋亡率Early apoptosis rate/% | 晚期凋亡率Late apoptosis rate/% | 总凋亡率Total apoptosis rate/% |
|---|---|---|---|---|
| Control | 81.97±0.61a | 1.58±0.22ab | 13.43±0.15a | 15.02±0.26a |
| 10-8 | 84.97±0.49b | 1.42±0.27a | 11.43±0.38b | 12.85±0.56b |
| 10-7 | 87.63±0.24c | 1.63±0.21ab | 9.16±0.52c | 10.78±0.31c |
| 10-6 | 89.27±0.03d | 1.21±0.09a | 7.76±0.24d | 8.97±0.16d |
| 10-5 | 86.80±0.58c | 2.12±0.16b | 9.70±0.17c | 11.82±0.04e |
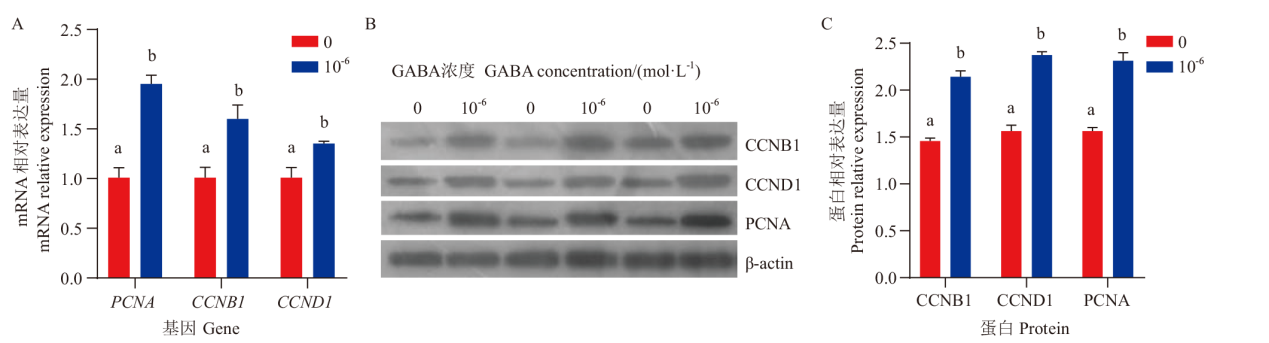
Fig. 4 Effects of GABA on the expressions of proliferation-related genes mRNA and proteins in ovine granulosa cells A: Statistics of mRNA expression results of proliferation-related genes after GABA treatment. B: Western blot results. C: Statistics of protein expression results of proliferation-related genes after GABA treatment
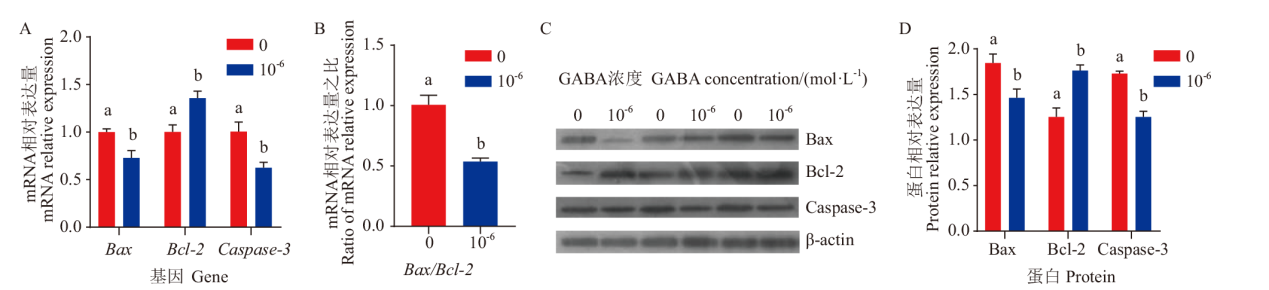
Fig. 5 Effects of GABA on the expressions of apoptosis-related genes mRNA and proteins in ovine granulosa cells A: Statistics of mRNA expression results of apoptosis-related genes after GABA treatment. B: Effect of GABA on ratio of mRNA expression of Bax/Bcl-2. C: Western blot results. D: Statistics of protein expression results of apoptosis-related genes after GABA treatment
| 项目 Item | 对照组 Control group | 试验组 Test group |
|---|---|---|
| 雌二醇(E2)/(pg·mL-1) | 77.25±1.79a | 61.30±0.74b |
| 孕酮(P4)/(pmol·L-1) | 344.45±7.03a | 400.66±14.78b |
Table 4 Effects of 10-6 mol/L GABA on steroid hormone secretion of GCs in ovine granulosa cells
| 项目 Item | 对照组 Control group | 试验组 Test group |
|---|---|---|
| 雌二醇(E2)/(pg·mL-1) | 77.25±1.79a | 61.30±0.74b |
| 孕酮(P4)/(pmol·L-1) | 344.45±7.03a | 400.66±14.78b |

Fig. 6 Effects of GABA on the expressions of steroid hormone synthesis-related genes and proteins in ovine granulosa cells A: Statistics of mRNA expression results of steroid hormone synthesis-related genes after GABA treatment. B: Western blot results. C: Statistics of protein expression results of steroid hormone synthesis-related genes after GABA treatment
| [1] |
Kim DH, Dasagrandhi C, Park SK, et al. Optimization of gamma-aminobutyric acid production using sea tangle extract by lactic acid bacterial fermentation[J]. LWT, 2018, 90: 636-642.
doi: 10.1016/j.lwt.2018.01.011 URL |
| [2] |
Zhou HL, Chen HY, Bao DP, et al. Recent advances of γ-aminobutyric acid: physiological and immunity function, enrichment, and metabolic pathway[J]. Front Nutr, 2022, 9: 1076223.
doi: 10.3389/fnut.2022.1076223 URL |
| [3] |
Chiu CQ, Barberis A, Higley MJ. Preserving the balance: diverse forms of long-term GABAergic synaptic plasticity[J]. Nat Rev Neurosci, 2019, 20(5): 272-281.
doi: 10.1038/s41583-019-0141-5 pmid: 30837689 |
| [4] | Kleppner SR, Tobin AJ. GABA signalling: therapeutic targets for epilepsy, Parkinson's disease and Huntington's disease[J]. Expert Opin Ther Targets, 2001, 5(2): 219-239. |
| [5] |
Oketch-Rabah HA, Madden EF, Roe AL, et al. United states pharmacopeia(USP)safety review of Gamma-aminobutyric acid(GABA)[J]. Nutrients, 2021, 13(8): 2742.
doi: 10.3390/nu13082742 URL |
| [6] | 包华琼, 王新庄. γ-氨基丁酸(GABA)的生殖生理作用[J]. 动物医学进展, 2002, 23(3): 39-41. |
| Bao HQ, Wang XZ. Effect of gamma-amino-butyric acid on reproduction and physiology[J]. Prog Vet Med, 2002, 23(3): 39-41. | |
| [7] | 许吉怡, 王志鹏, 邹宇川, 等. 食源性GABA对人体各系统的保护作用和机制的研究进展[J]. 世界科学技术-中医药现代化, 2022, 24(10): 3835-3843. |
| Xu JY, Wang ZP, Zou YC, et al. Research progress on the protective effect and mechanism of oral-intake GABA on human systems[J]. Mod Tradit Chin Med Mater Med World Sci Technol, 2022, 24(10): 3835-3843. | |
| [8] |
de Bie TH, Balvers MGJ, de Vos RCH, et al. The influence of a tomato food matrix on the bioavailability and plasma kinetics of oral gamma-aminobutyric acid(GABA)and its precursor glutamate in healthy men[J]. Food Funct, 2022, 13(16): 8399-8410.
doi: 10.1039/D2FO01358D URL |
| [9] |
Hepsomali P, Groeger JA, Nishihira J, et al. Effects of oral gamma-aminobutyric acid(GABA)administration on stress and sleep in humans: a systematic review[J]. Front Neurosci, 2020, 14: 923.
doi: 10.3389/fnins.2020.00923 URL |
| [10] |
Zhong G, Shao D, Wang Q, et al. Effects of dietary supplemented of γ-amino butyric acid on growth performance, blood biochemical indices and intestinal morphology of yellow-feathered broilers exposed to a high temperature environment[J]. Ital J Anim Sci, 2020, 19(1): 431-438.
doi: 10.1080/1828051X.2020.1747953 URL |
| [11] |
Guo K, Cao HB, Zhu YJ, et al. Improving effects of dietary rumen protected γ-aminobutyric acid additive on apparent nutrient digestibility, growth performance and health status in heat-stressed beef cattle[J]. Anim Sci J, 2018, 89(9): 1280-1286.
doi: 10.1111/asj.13053 pmid: 29923358 |
| [12] |
Chen J, Guo K, Song XZ, et al. The anti-heat stress effects of Chinese herbal medicine prescriptions and rumen-protected γ-aminobutyric acid on growth performance, apparent nutrient digestibility, and health status in beef cattle[J]. Anim Sci J, 2020, 91(1): e13361.
doi: 10.1111/asj.v91.1 URL |
| [13] |
Yuan XK, Zhang XY, Wu YJ, et al. Maternal amino acid mixtures supplementation during late gestation and lactation improved growth performance of piglets through improving colostrum composition and antioxidant capacity[J]. Antioxidants, 2022, 11(11): 2144.
doi: 10.3390/antiox11112144 URL |
| [14] |
Turathum B, Gao EM, Chian RC. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization[J]. Cells, 2021, 10(9): 2292.
doi: 10.3390/cells10092292 URL |
| [15] |
Fontana J, Martínková S, Petr J, et al. Metabolic cooperation in the ovarian follicle[J]. Physiol Res, 2020, 69(1): 33-48.
doi: 10.33549/physiolres.934233 pmid: 31854191 |
| [16] |
Dompe C, Kulus M, Stefańska K, et al. Human granulosa cells-stemness properties, molecular cross-talk and follicular angiogenesis[J]. Cells, 2021, 10(6): 1396.
doi: 10.3390/cells10061396 URL |
| [17] |
Krawczyk K, Marynowicz W, Pich K, et al. Persistent organic pollutants affect steroidogenic and apoptotic activities in granulosa cells and reactive oxygen species concentrations in oocytes in the mouse[J]. Reprod Fertil Dev, 2023, 35(3): 294-305.
doi: 10.1071/RD21326 URL |
| [18] |
Worku T, Rehman ZU, Talpur HS, et al. microRNAs: new insight in modulating follicular atresia: a review[J]. Int J Mol Sci, 2017, 18(2): 333.
doi: 10.3390/ijms18020333 URL |
| [19] |
Ullah A, Jahan S, Razak S, et al. Protective effects of GABA against metabolic and reproductive disturbances in letrozole induced polycystic ovarian syndrome in rats[J]. J Ovarian Res, 2017, 10(1): 62.
doi: 10.1186/s13048-017-0359-7 pmid: 28915843 |
| [20] | 姜杰, 倪江, 李庆雷, 等. γ-氨基丁酸对离体大鼠黄体化颗粒细胞孕酮分泌的影响[J]. 基础医学与临床, 2000, 20(6): 547-548. |
| Jiang J, Ni J, Li QL, et al. Effect of GABA on progesterone secretion in rat luteinising granulosa cells in vitro[J]. Basic Med Sci Clin, 2000, 20(6): 547-548. | |
| [21] | 杨美琼. γ-氨基丁酸对人卵巢颗粒细胞雌二醇和孕酮分泌及凋亡的影响[D]. 广州: 南方医科大学, 2009. |
| Yang MQ. Effects of GABA on E2 and P secretions and apoptosis by human ovarian granulosa cells in vitro[D]. Guangzhou: Southern Medical University, 2009. | |
| [22] | 姜杰, 倪江, 朱辉, 等. γ-GABA对大鼠离体培养颗粒细胞生成雌二醇的影响及作用机制[J]. 中国应用生理学杂志, 2000, 16(3): 272-274. |
| Jiang J, Ni J, Zhu H, et al. Effect of γ-gaba on estradiol production and its mechanism by rat granulosa cells cultured in vitro[J]. Chin J Appl Physiol, 2000, 16(3): 272-274. | |
| [23] |
Xu DJ, Jiang XH, Wang YK, et al. Liver receptor homolog-1 regulates apoptosis of bovine ovarian granulosa cells by progestogen receptor signaling pathway[J]. Animals, 2022, 12(9): 1213.
doi: 10.3390/ani12091213 URL |
| [24] |
Zhu ZH, Shi ZG, Xie CL, et al. A novel mechanism of Gamma-aminobutyric acid(GABA)protecting human umbilical vein endothelial cells(HUVECs)against H2O2-induced oxidative injury[J]. Comp Biochem Physiol C Toxicol Pharmacol, 2019, 217: 68-75.
doi: 10.1016/j.cbpc.2018.11.018 URL |
| [25] |
温静, 余哲琪, 田佳迎, 等. γ-氨基丁酸对热应激雏鸡胰腺组织结构、抗氧化能力、消化酶活性及细胞凋亡的影响[J]. 动物营养学报, 2021, 33(5): 2927-2938.
doi: 10.3969/j.issn.1006-267x.2021.05.050 |
| Wen J, Yu ZQ, Tian JY, et al. Effects of γ-aminobutyric acid on pancreatic tissue structure, antioxidant capacity, digestive enzyme activities and cell apoptosis of heat-stressed chicks[J]. Chin J Anim Nutr, 2021, 33(5): 2927-2938. | |
| [26] | 孙士平. GABA对LPS诱导MAC-T细胞增殖和凋亡的影响[D]. 郑州: 河南农业大学, 2018. |
| Sun SP. Effect of GABA on the proliferation and apoptosis induced by LPS in MAC-T cell line[D]. Zhengzhou: Henan Agricultural University, 2018. | |
| [27] | 赵海. GABA信号在小鼠胎盘形成期的表达及功能研究[D]. 重庆: 重庆医科大学, 2014. |
| Zhao H. The expressions of GABA signal and its potential role in the mouse placenta formation in mice[D]. Chongqing: Chongqing Medical University, 2014. | |
| [28] | 徐静文, 刘勇, 张德普, 等. γ-氨基丁酸能系统对肺上皮细胞增殖的影响[J]. 山东大学学报: 医学版, 2011, 49(5): 34-37. |
| Xu JW, Liu Y, Zhang DP, et al. Effect of the GABAergic system on lung epithelial cell proliferation[J]. J Shandong Univ Heath Sci, 2011, 49(5): 34-37. | |
| [29] |
Peng A, Xu XY, Wang CL, et al. EZH2 promotes DNA replication by stabilizing interaction of POLδ and PCNA via methylation-mediated PCNA trimerization[J]. Epigenetics Chromatin, 2018, 11(1): 44.
doi: 10.1186/s13072-018-0213-1 pmid: 30071900 |
| [30] |
Hives M, Jurecekova J, Holeckova KH, et al. The driving power of the cell cycle: cyclin-dependent kinases, cyclins and their inhibitors[J]. Bratisl Lek Listy, 2023, 124(4): 261-266.
doi: 10.4149/BLL_2023_039 pmid: 36598318 |
| [31] |
Basu A. The interplay between apoptosis and cellular senescence: Bcl-2 family proteins as targets for cancer therapy[J]. Pharmacol Ther, 2022, 230: 107943.
doi: 10.1016/j.pharmthera.2021.107943 URL |
| [32] |
Czabotar PE, Lessene G, Strasser A, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy[J]. Nat Rev Mol Cell Biol, 2014, 15(1): 49-63.
doi: 10.1038/nrm3722 |
| [33] |
Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death[J]. Cell Death Differ, 2018, 25(1): 65-80.
doi: 10.1038/cdd.2017.186 pmid: 29149100 |
| [34] | Marquez RT, Xu L. Bcl-2: Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch[J]. Am J Cancer Res, 2012, 2(2): 214-221. |
| [35] |
Yuan Z, Dewson G, Czabotar PE, et al. VDAC2 and the BCL-2 family of proteins[J]. Biochem Soc Trans, 2021, 49(6): 2787-2795.
doi: 10.1042/BST20210753 URL |
| [36] |
McComb S, Chan PK, Guinot A, et al. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or-7[J]. Sci Adv, 2019, 5(7): eaau9433.
doi: 10.1126/sciadv.aau9433 URL |
| [37] |
Li M, Gao P, Zhang JP. Crosstalk between autophagy and apoptosis: potential and emerging therapeutic targets for cardiac diseases[J]. Int J Mol Sci, 2016, 17(3): 332.
doi: 10.3390/ijms17030332 pmid: 26950124 |
| [38] |
Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update[J]. Arch Toxicol, 2015, 89(3): 289-317.
doi: 10.1007/s00204-014-1448-7 pmid: 25618543 |
| [39] |
Brooks C, Cho SG, Wang CY, et al. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis[J]. Am J Physiol Cell Physiol, 2011, 300(3): C447-C455.
doi: 10.1152/ajpcell.00402.2010 URL |
| [40] |
Shi SJ, Zhou XG, Li JJ, et al. miR-214-3p promotes proliferation and inhibits estradiol synthesis in porcine granulosa cells[J]. J Anim Sci Biotechnol, 2020, 11: 94.
doi: 10.1186/s40104-020-00500-y |
| [41] |
Xiao CY, Wang J, Zhang CP. Synthesis, regulatory factors, and signaling pathways of estrogen in the ovary[J]. Reprod Sci, 2023, 30(2): 350-360.
doi: 10.1007/s43032-022-00932-z |
| [42] |
Lee EB, Chakravarthi VP, Wolfe MW, et al. ERβ regulation of gonadotropin responses during folliculogenesis[J]. Int J Mol Sci, 2021, 22(19): 10348.
doi: 10.3390/ijms221910348 URL |
| [43] |
Kolatorova L, Vitku J, Suchopar J, et al. Progesterone: a steroid with wide range of effects in physiology as well as human medicine[J]. Int J Mol Sci, 2022, 23(14): 7989.
doi: 10.3390/ijms23147989 URL |
| [44] | van der Linden M, Buckingham K, Farquhar C, et al. Luteal phase support for assisted reproduction cycles[J]. Cochrane Database Syst Rev, 2015, 2015(7): CD009154. |
| [45] |
Song TL, Chen JY, Yang SZ, et al. Resveratrol stimulates StAR expression and progesterone production by GPER-mediated downregulation of Snail expression in human granulosa cells[J]. J Food Drug Anal, 2023, 31(2): 315-325.
doi: 10.38212/2224-6614.3460 pmid: 37335164 |
| [46] |
Fang LL, Li YR, Wang SJ, et al. Melatonin induces progesterone production in human granulosa-lutein cells through upregulation of StAR expression[J]. Aging, 2019, 11(20): 9013-9024.
doi: 10.18632/aging.v11i20 URL |
| [47] |
Mosa A, Neunzig J, Gerber A, et al. 2β- and 16β-hydroxylase activity of CYP11A1 and direct stimulatory effect of estrogens on pregnenolone formation[J]. J Steroid Biochem Mol Biol, 2015, 150: 1-10.
doi: 10.1016/j.jsbmb.2015.02.014 URL |
| [48] |
Johnson AL, Woods DC. Dynamics of avian ovarian follicle development: cellular mechanisms of granulosa cell differentiation[J]. Gen Comp Endocrinol, 2009, 163(1-2): 12-17.
doi: 10.1016/j.ygcen.2008.11.012 URL |
| [49] |
Ding ZQ, Duan HW, Ge WB, et al. Regulation of progesterone during follicular development by FSH and LH in sheep[J]. Anim Reprod, 2022, 19(2): e20220027.
doi: 10.1590/1984-3143-ar2022-0027 URL |
| [50] |
An LP, Maeda T, Sakaue T, et al. Purification, molecular cloning and functional characterization of swine phosphatidylethanolamine-binding protein 4 from seminal plasma[J]. Biochem Biophys Res Commun, 2012, 423(4): 690-696.
doi: 10.1016/j.bbrc.2012.06.016 URL |
| [51] |
Ghosh D, Egbuta C, Kanyo JE, et al. Phosphorylation of human placental aromatase CYP19A1[J]. Biochem J, 2019, 476(21): 3313-3331.
doi: 10.1042/BCJ20190633 pmid: 31652308 |
| [1] | MA Yu-jing, DUAN Chun-hui, HE Ming-yang, ZHANG Ying-jie, YANG Ruo-chen, WANG Yong, LIU Yue-qin. Effects of Knockout of G0S2 Gene in Ovarian Granulosa Cell Proliferation, Steroids Hormones and Related Gene Expression [J]. Biotechnology Bulletin, 2023, 39(6): 325-334. |
| [2] | YIN Xiao-meng, CAO Xue-wei, WANG Fu-jun, ZHAO Jian, ZHANG Hui-zhan. Celastrol and Apoptin Mutant Exert Synergistic Anti-tumor Effects by Enhancing Nur77-induced Apoptosis Pathway [J]. Biotechnology Bulletin, 2020, 36(7): 119-129. |
| [3] | ZOU Kun, LU Li-li, Collins Asiamah Amponsah, XUE Yuan, ZHANG Shao-wei, SU Ying, ZHAO Zhi-hui. Research Progress on Mechanism of Poultry Follicular Atresia [J]. Biotechnology Bulletin, 2020, 36(4): 185-191. |
| [4] | LIU Shu-jun, CHEN Miao, WANG Feng-zhong, BAO Yu-ming, XIN Feng-jiao, WEN Bo-ting. In Vitro Fermentation of Monosodium Glutamate with Human Gut Microbes [J]. Biotechnology Bulletin, 2020, 36(12): 104-112. |
| [5] | HUANG Yuan-xia, PENG Chuan-hai, DING Ning, QIU Zhong-ping, LI Xing, ZOU Mei-hui. Study on the Cholesterol-lowering and Antioxidant Abilities of a Tri-lactobacillus In Vitro [J]. Biotechnology Bulletin, 2020, 36(12): 113-120. |
| [6] | ZHU Ping, DU Li-jie, MENG Kun, XUE Juan, YANG Jin, LI Shan. Research Progress on the Effects of T3SS Effectors on Apoptosis and Pyroptosis of Host Cells [J]. Biotechnology Bulletin, 2019, 35(4): 178-187. |
| [7] | HU Jian-ran, LI Ping, TIE Jun, JIN Shan. Study on Antioxidant and Antitumor Activity of Essential Oil from Flowers of Syringa oblata [J]. Biotechnology Bulletin, 2019, 35(12): 16-23. |
| [8] | LI Yan-wei, SONG Xing-hui, WANG Jia-jia, LIU Li, HUANG Ying-ying, GUO Chun. Establishment of the Real-time and Label-free Screening System for Tumor Cell Apoptosis [J]. Biotechnology Bulletin, 2019, 35(10): 220-226. |
| [9] | ZHAI Yi-zhou ,LU Mei-ya ,ZHAO Jian ,WANG Fu-jun. Screening of a Gelonin Fusion Protein with High Cell-penetrating Efficiency and Its Anti-tumor Activity and Apoptosis Pathway [J]. Biotechnology Bulletin, 2018, 34(6): 204-212. |
| [10] | GUO Hong-yan, GAO Han, WU Qi, SUN Xiao-jie, LIU Xiu-cai, ZHAO Li-qun. Construction of SGK3 Gene Lentiviral RNA Interference Vector and Effects on Cell proliferation and Apoptosis of Breast Cancer Cell Line MB-474 [J]. Biotechnology Bulletin, 2018, 34(1): 247-252. |
| [11] | AN Zhi-yuan ,SU Jian-rong. Expression and Purification of Outer Membrane Protein 34 of Acinetobacter baumannii and Analysis of Its Bioactivity [J]. Biotechnology Bulletin, 2017, 33(7): 185-194. |
| [12] | DENG Yu-qing, LI Ping, ZHOU Yan, XIONG Ke-cai, LI Zhong-an. Progress on Detection Technology of Programmed Cell Death in Plant [J]. Biotechnology Bulletin, 2017, 33(3): 52-57. |
| [13] | QI Ren-li, WANG Qi, WU Yong-jiang, WANG Jing, HUANG Jin-xiu, YANG Fei-yun. Over-expression of MicroRNA-199a Intensifies the TNFα-induced Apoptosis of Adipocytes [J]. Biotechnology Bulletin, 2017, 33(3): 180-185. |
| [14] | HOU Huan-huan, LU Jia, ZHANG Wei-jing, HUANG Fang, REN Wen-hua. Cloning,Expression and Anticancer Mechanism of Scolopin 2,an Antimicrobial Peptide from Centipede Venoms(Scolopendra subspinipes mutilans) [J]. Biotechnology Bulletin, 2016, 32(9): 172-178. |
| [15] | Han Yawei, Wang Xihua, Chen Liping, Shi Guiqin, Sun Liping, Zhou Wenshan. Toxic Effects of NNK on NCTC 1469 Cells [J]. Biotechnology Bulletin, 2015, 31(9): 218-223. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||