








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (5): 290-299.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0979
Previous Articles Next Articles
WANG Zhou1( ), YU Jie1, WANG Jin-hua1,2,3, WANG Yong-ze1,2,3, ZHAO Xiao1,2,3(
), YU Jie1, WANG Jin-hua1,2,3, WANG Yong-ze1,2,3, ZHAO Xiao1,2,3( )
)
Received:2023-10-19
Online:2024-05-26
Published:2024-05-23
Contact:
ZHAO Xiao
E-mail:2797807952@qq.com;zhaoxiao1@hbut.edu.cn
WANG Zhou, YU Jie, WANG Jin-hua, WANG Yong-ze, ZHAO Xiao. Anaerobic Expression of Lactate Dehydrogenase to Improve the D-lactic Acid Optical Purity Procluced by Escherichia coli[J]. Biotechnology Bulletin, 2024, 40(5): 290-299.
| 菌株及质粒 Strain and plasmid | 特征 Characteristic | 来源 Source |
|---|---|---|
| HBUT-D | W ∆frdBC ∆pta ∆adhE ∆pflB ∆aldA | 本实验室保藏 |
| HBUT-DP | HBUT-D carrying pUC19 | 本研究 |
| HBUT-D3 | HBUT-D carrying pUC19-PLD | 本研究 |
| HBUT-D5 | HBUT-D carrying pUC19-PNLD | 本研究 |
| HBUT-D7 | HBUT-D carrying pUC19-NLD | 本研究 |
| pUC19-PLD | pUC19::PpflBp6-lldD, Apr | 本研究 |
| pUC19-NLD | pUC19::PnirB-lldD, Apr | 本研究 |
| pUC19-PNLD | pUC19::PpflBp6-PnirB-lldD, Apr | 本研究 |
Table 1 Strains and plasmids in this study
| 菌株及质粒 Strain and plasmid | 特征 Characteristic | 来源 Source |
|---|---|---|
| HBUT-D | W ∆frdBC ∆pta ∆adhE ∆pflB ∆aldA | 本实验室保藏 |
| HBUT-DP | HBUT-D carrying pUC19 | 本研究 |
| HBUT-D3 | HBUT-D carrying pUC19-PLD | 本研究 |
| HBUT-D5 | HBUT-D carrying pUC19-PNLD | 本研究 |
| HBUT-D7 | HBUT-D carrying pUC19-NLD | 本研究 |
| pUC19-PLD | pUC19::PpflBp6-lldD, Apr | 本研究 |
| pUC19-NLD | pUC19::PnirB-lldD, Apr | 本研究 |
| pUC19-PNLD | pUC19::PpflBp6-PnirB-lldD, Apr | 本研究 |
| 引物名称 Primer name | 序列 Sequence(5'-3') |
|---|---|
| P1 | CCGTGACTTAAGAAAATTTATAC |
| P2 | CTGGCTGCGGAAATAATCATTTTTGCCTCGATTTCTTTTC |
| P3 | GAAAAGAAATCGAGGCAAAAATGTCTTCCATGACAACAACTG |
| P4 | ACATACAGCGCCGAACGGTC |
| P5 | CAGCAATATACCCATTAAGGAGTAT |
| P6 | AACGACTATGCCGCATTC |
| P7 | TTCGCCTGATGCTCCC |
| P8 | CTGGCTGCGGAAATAATCATACTAACTCTCTCTTTATTAAGTCGGC |
| P9 | GCCGACTTAATAAAGAGAGAGTTAGTATGATTATTTCCGCAGCCAG |
| P10 | ATATGCGGTGTGAAATACC |
| P11 | GTTTTACAACGTCGTGACTTTGGATAATCAAATATTTACTCCGT |
| P12 | TAAGGAGAAAATACCGCATCTATGCCGCATTCCCTTT |
| P13 | AGTCACGACGTTGTAAAAC |
| P14 | ATGCGGTATTTTCTCCTTA |
| P15 | ATAGTTTAGCGGCCGCATTCTTATACAGATGCGTAAGGAGAAAA |
| P16 | ATAAGAATGCGGCCGCTAAACTATTTTTCTCCTTACGCATCTGT |
| P17 | GCTCATACCTGAATGCGCA |
| P18 | CGGAAGAGCGCCCAAT |
| P19 | CTTATCGCCACTGGCAGCC |
| P20 | TGTAGGTCGTTTCGCTCCAA |
Table 2 Primers in this study
| 引物名称 Primer name | 序列 Sequence(5'-3') |
|---|---|
| P1 | CCGTGACTTAAGAAAATTTATAC |
| P2 | CTGGCTGCGGAAATAATCATTTTTGCCTCGATTTCTTTTC |
| P3 | GAAAAGAAATCGAGGCAAAAATGTCTTCCATGACAACAACTG |
| P4 | ACATACAGCGCCGAACGGTC |
| P5 | CAGCAATATACCCATTAAGGAGTAT |
| P6 | AACGACTATGCCGCATTC |
| P7 | TTCGCCTGATGCTCCC |
| P8 | CTGGCTGCGGAAATAATCATACTAACTCTCTCTTTATTAAGTCGGC |
| P9 | GCCGACTTAATAAAGAGAGAGTTAGTATGATTATTTCCGCAGCCAG |
| P10 | ATATGCGGTGTGAAATACC |
| P11 | GTTTTACAACGTCGTGACTTTGGATAATCAAATATTTACTCCGT |
| P12 | TAAGGAGAAAATACCGCATCTATGCCGCATTCCCTTT |
| P13 | AGTCACGACGTTGTAAAAC |
| P14 | ATGCGGTATTTTCTCCTTA |
| P15 | ATAGTTTAGCGGCCGCATTCTTATACAGATGCGTAAGGAGAAAA |
| P16 | ATAAGAATGCGGCCGCTAAACTATTTTTCTCCTTACGCATCTGT |
| P17 | GCTCATACCTGAATGCGCA |
| P18 | CGGAAGAGCGCCCAAT |
| P19 | CTTATCGCCACTGGCAGCC |
| P20 | TGTAGGTCGTTTCGCTCCAA |
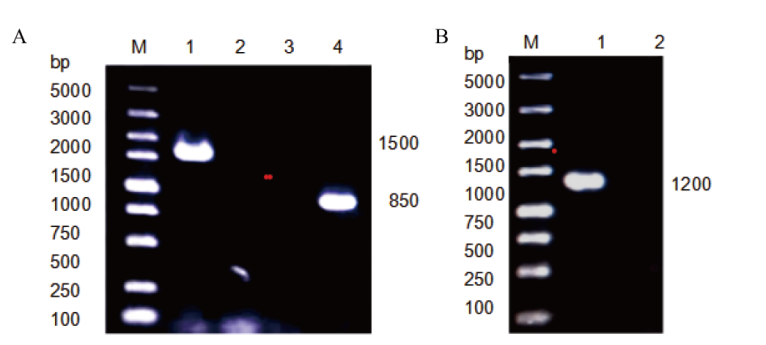
Fig. 1 Validation of different plasmids A: Validation of plasmid pUC19-PLD and plasmid pUC19-NLD(M:5000 marker;1:pUC19-PLD;2-3:pUC19;4:pUC19-PNLD). B: Validation of plasmid pUC19-PNLD(M:5000 marker;1:pUC19-NLD;2:pUC19)
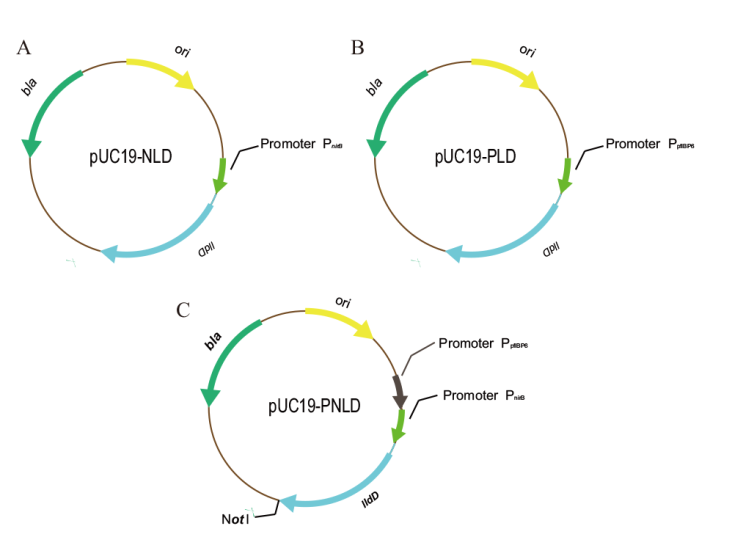
Fig. 2 Plasmids expressing lldD from different promoters A: PnirB promoter recombinant plasmid. B: PpflBP6 promoter recombinant plasmid. C: PpflBP6-PnirB tandem promoter plasmid
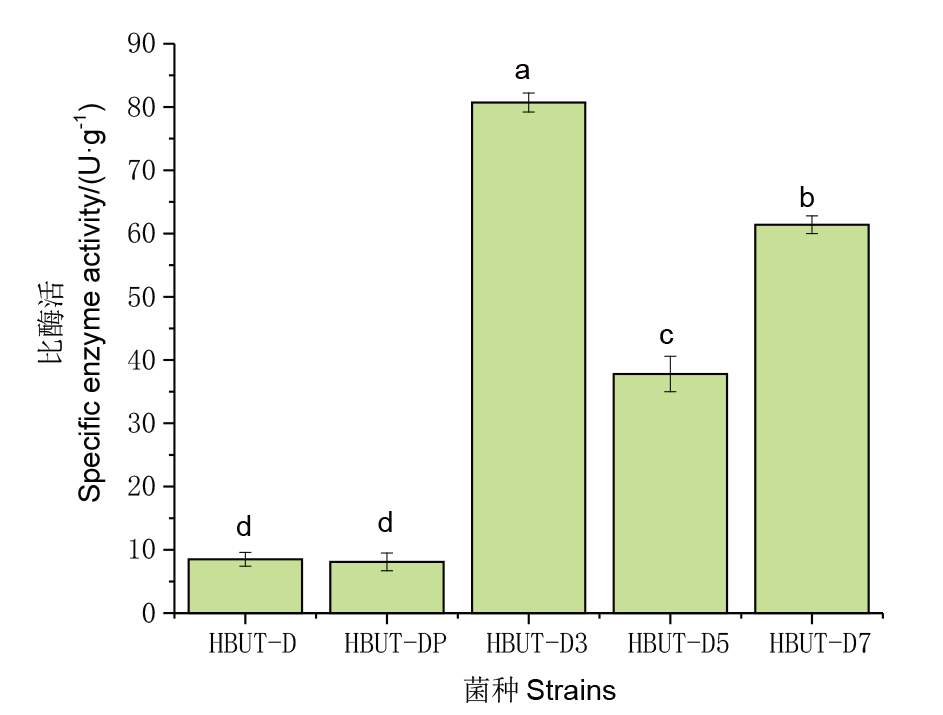
Fig. 3 LldD specific enzyme activities from different strains Strain HBUT-D is the blank control, strain HBUT-DP was transferred to the plasmid pUC19 by HBUT-D, strain HBUT-D3 to plasmid pUC19-PLD, strain HBUT-D5 to plasmid pUC19-NLD by HBUT-D, and strain HBUT-D7 to plasmid pUC19-PNLD. Different lowercase letters indicate significant differences in LldD specific enzyme activities of different strains(P<0.05)
| 菌株 Strain | 酶活Enzyme activity/U | 蛋白量Protein content/mg | 比酶活Specific enzyme activity/(U·g-1) |
|---|---|---|---|
| HBUT-DP | 20±6 | 2 489 | 8.5±1.1 |
| HBUT-D | 20±4 | 2 596 | 8.1±1.4 |
| HBUT-D3 | 632±12 | 7 893 | 80.7±1.5 |
| HBUT-D5 | 275 ±10 | 7 274 | 37.8±2.8 |
| HBUT-D7 | 483±11 | 7 863 | 61.4±1.4 |
Table 3 LldD enzyme activity results for different strains
| 菌株 Strain | 酶活Enzyme activity/U | 蛋白量Protein content/mg | 比酶活Specific enzyme activity/(U·g-1) |
|---|---|---|---|
| HBUT-DP | 20±6 | 2 489 | 8.5±1.1 |
| HBUT-D | 20±4 | 2 596 | 8.1±1.4 |
| HBUT-D3 | 632±12 | 7 893 | 80.7±1.5 |
| HBUT-D5 | 275 ±10 | 7 274 | 37.8±2.8 |
| HBUT-D7 | 483±11 | 7 863 | 61.4±1.4 |

Fig. 4 Fermentation of different strains in inorganic salt medium A: L-lactate consumption curves of different strains. B: Acid production curves of D-lactic acid of different strains. C: Glucose consumption curves of different strains. D: Growth curves of different strains
| 菌株 Strain | L-乳酸消耗速率Rate of L-lactate depletion/(mg·L-1·h-1) | L-乳酸消耗量L-lactate depletion concentration/(g·L-1) | D-乳酸生产强度D-lactate productivity/(g·L-1) | 葡萄糖消耗速率Rate of glucose consumption/(g·L-1·h-1) | OD600 nm | D-乳酸糖酸转化率D-lactate yield/% | 初始L-乳酸浓度Initial L -lactate concentration/(g·L-1) |
|---|---|---|---|---|---|---|---|
| HBUT-D | 8±0.13d | 0.21±0.01d | 4.09±0.11a | 4.16±0.04a | 3.22±0.01a | 98.31±0.21 | 1.01±0.11 |
| HBUT-D3 | 40±0.32a | 0.98±0.02a | 2.45±0.04c | 2.52±0.06d | 2.75±0.02b | 97.21±0.11 | 1.02±0.02 |
| HBUT-D5 | 14±0.33c | 0.34±0.01c | 4.09±0.04a | 4.15±0.08b | 3.12±0.01a | 98.55±0.33 | 1.04±0.02 |
| HBUT-D7 | 34±0.23b | 0.82±0.01b | 3.92±0.03b | 3.98±0.01c | 2.97±0.01a | 98.49±0.31 | 1.02±0.05 |
Table 4 Data on the fermentation of different strains in inorganic salt medium
| 菌株 Strain | L-乳酸消耗速率Rate of L-lactate depletion/(mg·L-1·h-1) | L-乳酸消耗量L-lactate depletion concentration/(g·L-1) | D-乳酸生产强度D-lactate productivity/(g·L-1) | 葡萄糖消耗速率Rate of glucose consumption/(g·L-1·h-1) | OD600 nm | D-乳酸糖酸转化率D-lactate yield/% | 初始L-乳酸浓度Initial L -lactate concentration/(g·L-1) |
|---|---|---|---|---|---|---|---|
| HBUT-D | 8±0.13d | 0.21±0.01d | 4.09±0.11a | 4.16±0.04a | 3.22±0.01a | 98.31±0.21 | 1.01±0.11 |
| HBUT-D3 | 40±0.32a | 0.98±0.02a | 2.45±0.04c | 2.52±0.06d | 2.75±0.02b | 97.21±0.11 | 1.02±0.02 |
| HBUT-D5 | 14±0.33c | 0.34±0.01c | 4.09±0.04a | 4.15±0.08b | 3.12±0.01a | 98.55±0.33 | 1.04±0.02 |
| HBUT-D7 | 34±0.23b | 0.82±0.01b | 3.92±0.03b | 3.98±0.01c | 2.97±0.01a | 98.49±0.31 | 1.02±0.05 |
| 菌株 Strain | D+ | D- | C- | 排序Sort |
|---|---|---|---|---|
| HBUT-D | 0.334 3 | 0.139 7 | 0.294 8 | 4 |
| HBUT-D3 | 0.141 2 | 0.334 3 | 0.702 9 | 2 |
| HBUT-D5 | 0.271 6 | 0.154 2 | 0.362 2 | 3 |
| HBUT-D7 | 0.070 0 | 0.296 3 | 0.808 8 | 1 |
Table 5 Euclidean distance D+ and D- between each target value and the ideal point and the relative closeness C- of each target
| 菌株 Strain | D+ | D- | C- | 排序Sort |
|---|---|---|---|---|
| HBUT-D | 0.334 3 | 0.139 7 | 0.294 8 | 4 |
| HBUT-D3 | 0.141 2 | 0.334 3 | 0.702 9 | 2 |
| HBUT-D5 | 0.271 6 | 0.154 2 | 0.362 2 | 3 |
| HBUT-D7 | 0.070 0 | 0.296 3 | 0.808 8 | 1 |
| 菌株 Strain | D-乳酸糖酸转化率D-lactate yield/% | D-乳酸光纯D-lactate optical purty/% | D-乳酸生产强度D-lactate productivity/(g·L-1·h-1) | L-乳酸消耗速率Rate of L-lac consumption/(mg·L-1·h-1) | 初始L-乳酸浓度Initial L-lac concentration/(g·L-1) |
|---|---|---|---|---|---|
| HBUT-D | 97.64±0.01 | 99.22±0.02 | 4.24±0.04 | 10.41±0.01*** | 1.0±0.02 |
| HBUT-D7 | 96.89±0.01 | 99.93±0.01 | 3.87±0.02 | 34.75±0.01*** | 1.0±0.02 |
Table 6 Data on fermentation of strain HBUT-D7 and HBUT-D in corn syrup medium
| 菌株 Strain | D-乳酸糖酸转化率D-lactate yield/% | D-乳酸光纯D-lactate optical purty/% | D-乳酸生产强度D-lactate productivity/(g·L-1·h-1) | L-乳酸消耗速率Rate of L-lac consumption/(mg·L-1·h-1) | 初始L-乳酸浓度Initial L-lac concentration/(g·L-1) |
|---|---|---|---|---|---|
| HBUT-D | 97.64±0.01 | 99.22±0.02 | 4.24±0.04 | 10.41±0.01*** | 1.0±0.02 |
| HBUT-D7 | 96.89±0.01 | 99.93±0.01 | 3.87±0.02 | 34.75±0.01*** | 1.0±0.02 |
| 菌株 Strain | D-乳酸糖酸转化率D-lactate yield/% | D-乳酸光纯 D-lactate optical purty/% | L-乳酸消耗速率Rate of L-lactate consumption/(mg·L-1·h-1) | D-乳酸生产强度D-lactate productivity/(g·L-1·h-1) |
|---|---|---|---|---|
| HBUT-D | 61.82±0.01 | 99.01±0.01 | 6.87±0.02*** | 1.93 |
| HBUT-D7 | 60.43±0.01 | 99.99±0.01 | 17.18±0.01*** | 1.88 |
Table 7 Data on the fermentation of strain HBUT-D and HBUT-D7 in molasses medium
| 菌株 Strain | D-乳酸糖酸转化率D-lactate yield/% | D-乳酸光纯 D-lactate optical purty/% | L-乳酸消耗速率Rate of L-lactate consumption/(mg·L-1·h-1) | D-乳酸生产强度D-lactate productivity/(g·L-1·h-1) |
|---|---|---|---|---|
| HBUT-D | 61.82±0.01 | 99.01±0.01 | 6.87±0.02*** | 1.93 |
| HBUT-D7 | 60.43±0.01 | 99.99±0.01 | 17.18±0.01*** | 1.88 |
| [1] | 吕汉强, 赵文花, 李含婷, 等. 聚乳酸可降解地膜对绿洲灌区玉米产量和农田水热特性的影响[J]. 甘肃农业大学学报, 2022, 57(5): 72-79, 88. |
| Lyu HQ, Zhao WH, Li HT, et al. Effects of polylactic acid degradable film on maize yield and soil hydrothermal characteristics in oasis irrigation area[J]. J Gansu Agric Univ, 2022, 57(5): 72-79, 88. | |
| [2] |
王健群, 张斌. 聚乳酸-羟基乙酸共聚物微球在骨组织工程中的应用[J]. 口腔医学研究, 2020, 36(9): 817-820.
doi: 10.13701/j.cnki.kqyxyj.2020.09.005 |
| Wang JQ, Zhang B. Application of poly(lactic-co-glycolic acid)copolymer microspheres in bone tissue engineering[J]. J Oral Sci Res, 2020, 36(9): 817-820. | |
| [3] | 吴一帆. D-乳酸嵌段共聚物的合成设计及其对左旋聚乳酸结晶行为和性能的影响[D]. 成都: 西南交通大学, 2020. |
| Wu YF. Synthesis design of D-lactic acid block copolymer and investigation on crystallization behaviors and properties of block copolymer/poly(L-lactic acid)blends[D]. Chengdu: Southwest Jiaotong University, 2020. | |
| [4] |
Grabar TB, Zhou S, Shanmugam KT, et al. Methylglyoxal bypass identified as source of chiral contamination in l(+)and d(-)-lactate fermentations by recombinant Escherichia coli[J]. Biotechnol Lett, 2006, 28(19): 1527-1535.
doi: 10.1007/s10529-006-9122-7 pmid: 16868860 |
| [5] | 张媛, 王小艳, 陈博, 等. 基因工程改造植物乳杆菌生产光学纯D-乳酸[J]. 当代化工, 2019, 48(4): 674-678, 682. |
| Zhang Y, Wang XY, Chen B, et al. Genetically engineered Lactobacillus plantarum for production of optical pure D-lactic acid[J]. Contemp Chem Ind, 2019, 48(4): 674-678, 682. | |
| [6] | 李义, 周卫强, 刘海军, 等. 玉米浆及其制备方法和应用以及高光学纯度乳酸的生产方法: CN114181980A[P]. 2022-03-15. |
| Li Y, Zhou W, Liu H, et al. Corn steep liquor, preparation method and application thereof, and production method of high-optical-purity lactic acid: CN114181980A[P]. 2022-03-15. | |
| [7] | Alexandri M, Hübner D, Schneider R, et al. Towards efficient production of highly optically pure d-lactic acid from lignocellulosic hydrolysates using newly isolated lactic acid bacteria[J]. N Biotechnol, 2022, 72: 1-10. |
| [8] | Zhou SD, Iverson AG, Grayburn WS. Doubling the catabolic reducing power(NADH)output of Escherichia coli fermentation for production of reduced products[J]. Biotechnol Prog, 2010, 26(1): 45-51. |
| [9] | Nasr R, Akbari Eidgahi MR. Construction of a synthetically engineered nirB promoter for expression of recombinant protein in Escherichia coli[J]. Jundishapur J Microbiol, 2014, 7(7): e15942. |
| [10] | 田甜. 高效利用蔗糖产D-乳酸工程菌的构建及其发酵研究[D]. 武汉: 湖北工业大学, 2013. |
| Tian T. Construction and fermentation of engineering bacteria producing D- lactic acid from sucrose with high efficiency[D]. Wuhan: Hubei University of Technology, 2013. | |
| [11] |
Kimura H, Futai M. Effects of phospholipids on L-lactate dehydrogenase from membranes of Escherichia coli. Activation and stabilization of the enzyme with phospholipids[J]. J Biol Chem, 1978, 253(4): 1095-1110.
pmid: 342518 |
| [12] |
刘红雨, 刘友存, 孟丽红, 等. 熵权法在水资源与水环境评价中的研究进展[J]. 冰川冻土, 2022, 44(1): 299-306.
doi: 10.7522/j.issn.1000-0240.2021.0131 |
|
Liu HY, Liu YC, Meng LH, et al. Research progress of entropy weight method in water resources and water environment[J]. J Glaciol Geocryol, 2022, 44(1): 299-306.
doi: 10.7522/j.issn.1000-0240.2021.0131 |
|
| [13] |
Aguilera L, Campos E, Giménez R, et al. Dual role of LldR in regulation of the lldPRD operon, involved in L-lactate metabolism in Escherichia coli[J]. J Bacteriol, 2008, 190(8): 2997-3005.
doi: 10.1128/JB.02013-07 pmid: 18263722 |
| [14] |
Futai M, Kimura H. Inducible membrane-bound L-lactate dehydrogenase from Escherichia coli. Purification and properties[J]. J Biol Chem, 1977, 252(16): 5820-5827.
pmid: 18473 |
| [15] | Bekker M, Alexeeva S, Laan W, et al. The ArcBA two-component system of Escherichia coli is regulated by the redox state of both the ubiquinone and the menaquinone pool[J]. J Bacteriol, 2010, 192(3): 746-754. |
| [16] | 许琼丹. 基于糖转运及代谢相关基因敲除的产D-乳酸大肠杆菌工程菌的构建[D]. 武汉: 湖北工业大学, 2020. |
| Xu QD. Construction of D-lactic acid-producing Escherichia coli strains by knockout of sugar transport and metabolism genes[D]. Wuhan: Hubei University of Technology, 2020. | |
| [17] | 鲁春阳, 文枫, 杨庆媛, 等. 基于改进TOPSIS法的城市土地利用绩效评价及障碍因子诊断——以重庆市为例[J]. 资源科学, 2011, 33(3): 535-541. |
| Lu CY, Wen F, Yang QY, et al. An evaluation of urban land use performance based on the improved TOPSIS method and diagnosis of its obstacle indicators: a case study of Chongqing[J]. Resour Sci, 2011, 33(3): 535-541. | |
| [18] | 张帆, 陈梦茹, 邢英英, 等. 基于熵权法和TOPSIS对马铃薯施肥和滴灌量组合的优化[J]. 植物营养与肥料学报, 2023, 29(4): 732-744. |
| Zhang F, Chen MR, Xing YY, et al. Optimization of fertilizer and drip irrigation levels for efficient potato production based on entropy weight method and TOPSIS[J]. J Plant Nutr Fertil, 2023, 29(4): 732-744. | |
| [19] | Fantino JR, Py B, Fontecave M, et al. A genetic analysis of the response of Escherichia coli to cobalt stress[J]. Environ Microbiol, 2010, 12(10): 2846-2857. |
| [20] |
Zaslaver A, Mayo AE, Rosenberg R, et al. Just-in-time transcription program in metabolic pathways[J]. Nat Genet, 2004, 36(5): 486-491.
pmid: 15107854 |
| [21] |
张玲, 林荣, 宋祖坤, 等. 启动子串联及改造提高FAD为辅基的葡萄糖脱氢酶在Bacillus subtilis中的表达[J]. 食品与发酵工业, 2019, 45(8): 15-21.
doi: 10.13995/j.cnki.11-1802/ts.018685 |
| Zhang L, Lin R, Song ZK, et al. Promoter tandem and transformation for FAD-conjugated glucose dehydrogenase expression in Bacillus subtilis[J]. Food Ferment Ind, 2019, 45(8): 15-21. | |
| [22] | 葛春蕾, 刘中美, 崔文璟, 等. 通过串联启动子实现纳豆激酶在枯草芽孢杆菌中的高效表达[J]. 现代食品科技, 2016, 32(11): 72-77, 15. |
| Ge CL, Liu ZM, Cui WJ, et al. Efficient overexpression of recombinant nattokinase in Bacillus subtilis by tandem promoters[J]. Mod Food Sci Technol, 2016, 32(11): 72-77, 15. | |
| [23] | Chauhan V, Bahrudeen MNM, Palma CSD, et al. Analytical kinetic model of native tandem promoters in E. coli[J]. PLoS Comput Biol, 2022, 18(1): e1009824. |
| [24] | Kasho K, Ozaki S, Katayama T. IHF and fis as Escherichia coli cell cycle regulators: activation of the replication origin oriC and the regulatory cycle of the DnaA initiator[J]. Int J Mol Sci, 2023, 24(14): 11572. |
| [25] |
Kim D, Seo SW, Gao Y, et al. Systems assessment of transcriptional regulation on central carbon metabolism by Cra and CRP[J]. Nucleic Acids Res, 2018, 46(6): 2901-2917.
doi: 10.1093/nar/gky069 pmid: 29394395 |
| [1] | YANG Hong-yan, HAN Xiao, YANG Jian-jun. Scaling up Production of pDNA Plasmids in Disposable Bioreactors [J]. Biotechnology Bulletin, 2024, 40(1): 168-175. |
| [2] | CHEN Cai-ping, REN Hao, LONG Teng-fei, HE Bing, LU Zhao-xiang, SUN Jian. Research Advances in the Treatment of Inflammation Bowel Disease Using Escherichia coli Nissle 1917 [J]. Biotechnology Bulletin, 2023, 39(6): 109-118. |
| [3] | LI Yan-xia, WANG Jin-peng, FENG Fen, BAO Bin-wu, DONG Yi-wen, WANG Xing-ping, LUORENG Zhuo-ma. Effects of Escherichia coli Dairy Cow Mastitis on the Expressions of Milk-producing Trait Related Genes [J]. Biotechnology Bulletin, 2023, 39(2): 274-282. |
| [4] | WU Li-dan, RAN Xue-qin, NIU Xi, HUANG Shi-hui, LI Sheng, WANG Jia-fu. Genome Comparison and Virulence Factor Analysis of Pathogenic Escherichia coli from Porcine [J]. Biotechnology Bulletin, 2023, 39(12): 287-299. |
| [5] | LI Yi-ya, WU Yi-fan, DING Neng-shui, FAN Xiao-ping, CHEN Fan. Establishment of a Luciferase-assisted Quantitative Method for Measuring Ultrasonic Disruption of Escherichia coli Cells [J]. Biotechnology Bulletin, 2023, 39(12): 90-98. |
| [6] | TANG Rui-qi, ZHAO Xin-qing, ZHU Du, WANG Ya. Stress Tolerance of Escherichia coli to Inhibitors in Lignocellulosic Hydrolysates [J]. Biotechnology Bulletin, 2023, 39(11): 205-216. |
| [7] | LI Hai-li, LANG Li-min, ZHANG Qing-xian, YOU Yi, ZHU Wen-hao, WANG Zhi-fang, ZHANG Li-xian, WANG Ke-ling. Identification and Drug Resistance of Escherichia coli Simultaneously Producing Carbapenemase NDM-1 and NDM-5 [J]. Biotechnology Bulletin, 2022, 38(9): 106-115. |
| [8] | CHENG Shen-wei, ZHANG Ke-qiang, LIANG Jun-feng, LIU Fu-yuan, GAO Xing-liang, DU Lian-zhu. Establishment of a Triple Droplet Digital PCR Quantitative Detection Method for Typical Pathogenic Bacteria in Livestock and Poultry Manure [J]. Biotechnology Bulletin, 2022, 38(9): 271-280. |
| [9] | ZHAO Yan-kun, LIU Hui-min, MENG Lu, WANG Cheng, WANG Jia-qi, ZHENG Nan. Research Progress in Heteroresistance of Escherichia coli [J]. Biotechnology Bulletin, 2022, 38(9): 59-71. |
| [10] | GAO Wei-xin, HUANG Huo-qing, ZHAO Jing, ZHANG Xin, YANG Ning, YANG Hao-meng. Construction and Activity Verification of Ribonucleoprotein Complex for Gene Editing [J]. Biotechnology Bulletin, 2022, 38(8): 60-68. |
| [11] | SUN Man-luan, GE Sai, BU Jia, ZHU Zhuang-yan. Regulation Mechanism of Ribonucleases in Escherichia coli [J]. Biotechnology Bulletin, 2022, 38(3): 234-245. |
| [12] | WANG Kai-kai, WANG Xiao-lu, SU Xiao-yun, ZHANG Jie. Optimization and Application of Double-plasmid CRISPR-Cas9 System in Escherichia coli [J]. Biotechnology Bulletin, 2021, 37(12): 252-264. |
| [13] | FENG Zhi-mei, ZHAO Ya-tong, LIU Ye-xue, LU Fu-ping, LI Yu. Recombinant Expression of NADPH-Dependent Mannitol Dehydrogenase and Transformation Conditions of Mannitol [J]. Biotechnology Bulletin, 2017, 33(8): 186-191. |
| [14] | YU Ying, XU Mei-xue ,LIU Jin-lei, FAN Rong ,FENG Hui-yong, LI Tian-ming. Metabolic Engineering for Modifying Corynebacterium glutamicum to Produce More Pyruvate [J]. Biotechnology Bulletin, 2016, 32(8): 226-232. |
| [15] | Ding Xiaoyun, Gu Jianjian, Wang Yongze, Zhao Jinfang, Wang Jinhua, Zhao Xiao. The Knockout of Gene ptsG of Recombinant Escherichia coli Producing D-lactic Acid and the Simultaneous Fermentation of Mixed Sugars [J]. Biotechnology Bulletin, 2015, 31(12): 221-226. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||