








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (6): 23-33.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1164
Previous Articles Next Articles
WANG Di ZHANG Xiao-yu SONG Yu-xin ZHENG Dong-ran TIAN Jing LI Yu-hua WANG Yu WU Hao1,2( )
)
Received:2023-12-10
Online:2024-06-26
Published:2024-06-24
WANG Di ZHANG Xiao-yu SONG Yu-xin ZHENG Dong-ran TIAN Jing LI Yu-hua WANG Yu WU Hao. Advances in the Molecular Mechanisms of Plant Tissue Culture and Regeneration Regulated by Totipotency-related Transcription Factors[J]. Biotechnology Bulletin, 2024, 40(6): 23-33.
| 转录因子名称Name of transcription factor | 结构域示意图Schematic diagram of domain | 三级结构预测图Tertiary structure prediction diagram |
|---|---|---|
| Homeobox |  | 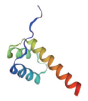 |
| AP2/ERF |  | 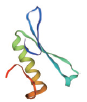 |
| LOB |  | 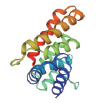 |
| LEC |  | 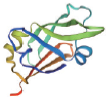 |
Table 1 Tertiary structure prediction diagram of major transcription factor domains
| 转录因子名称Name of transcription factor | 结构域示意图Schematic diagram of domain | 三级结构预测图Tertiary structure prediction diagram |
|---|---|---|
| Homeobox |  |  |
| AP2/ERF |  |  |
| LOB |  |  |
| LEC |  |  |
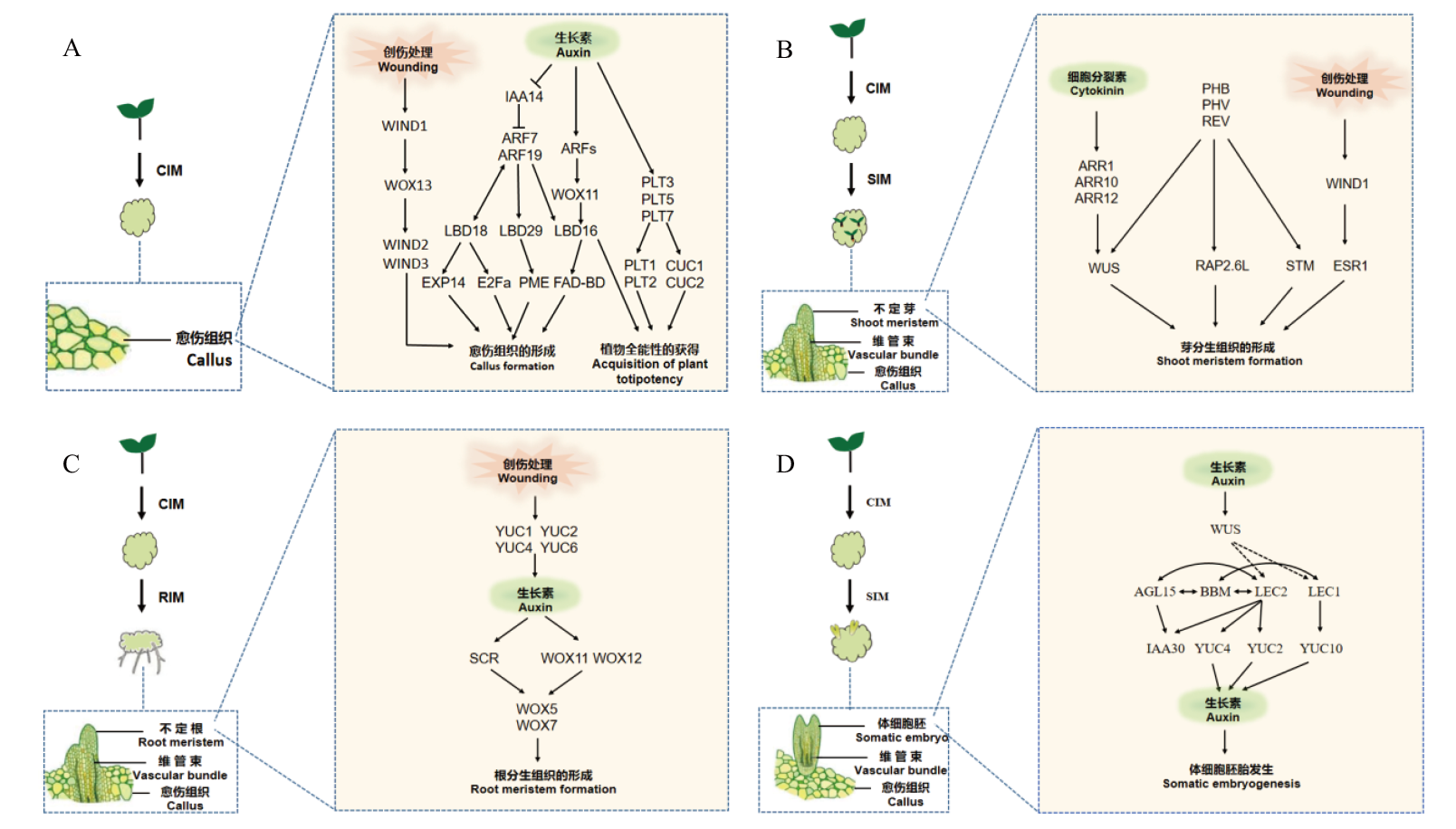
Fig. 2 Regulation mechanism of related transcription factors in plant tissue culture regeneration A: Regulation mechanism of related transcription factors during callus formation; B: regulation mechanism of related transcription factors in shoot regeneration; C: regulation mechanism of related transcription factors during root meristem formation; D: regulation mechanism of related transcription factors in somatic embryogenesis
| [1] | 胡彦, 赵艳. 植物组织培养技术的应用以及在培养过程中存在的问题[J]. 陕西师范大学学报: 自然科学版, 2004, 32(S1): 130-134. |
| Hu Y, Zhao Y. Application of plant tissue culture technology and problems existing in the process of culture[J]. J Shaanxi Norm Univ Nat Sci Ed, 2004, 32(S1): 130-134. | |
| [2] | 许智宏, 张宪省, 苏英华, 等. 植物细胞全能性和再生[J]. 中国科学: 生命科学, 2019, 49(10): 1282-1300. |
| Xu ZH, Zhang XS, Su YH, et al. Plant cell totipotency and regeneration[J]. Sci Sin Vitae, 2019, 49(10): 1282-1300. | |
| [3] |
Ikeuchi M, Favero DS, Sakamoto Y, et al. Molecular mechanisms of plant regeneration[J]. Annu Rev Plant Biol, 2019, 70: 377-406.
doi: 10.1146/annurev-arplant-050718-100434 pmid: 30786238 |
| [4] |
Birnbaum KD, Alvarado AS. Slicing across Kingdoms: regeneration in plants and animals[J]. Cell, 2008, 132(4): 697-710.
doi: 10.1016/j.cell.2008.01.040 pmid: 18295584 |
| [5] |
Méndez-Hernández HA, Ledezma-Rodríguez M, Avilez-Montalvo RN, et al. Signaling overview of plant somatic embryogenesis[J]. Front Plant Sci, 2019, 10: 77.
doi: 10.3389/fpls.2019.00077 pmid: 30792725 |
| [6] |
Su YH, Tang LP, Zhao XY, et al. Plant cell totipotency: insights into cellular reprogramming[J]. J Integr Plant Biol, 2021, 63(1): 228-243.
doi: 10.1111/jipb.12972 |
| [7] |
Xu J, Hofhuis H, Heidstra R, et al. A molecular framework for plant regeneration[J]. Science, 2006, 311(5759): 385-388.
pmid: 16424342 |
| [8] | Junker A, Mönke G, Rutten T, et al. Elongation-related functions of LEAFY COTYLEDON1 during the development of Arabidopsis thaliana[J]. Plant J, 2012, 71(3): 427-442. |
| [9] |
Braybrook SA, Stone SL, Park S, et al. Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis[J]. Proc Natl Acad Sci USA, 2006, 103(9): 3468-3473.
doi: 10.1073/pnas.0511331103 pmid: 16492731 |
| [10] |
Lotan T, Ohto M, Yee KM, et al. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells[J]. Cell, 1998, 93(7): 1195-1205.
doi: 10.1016/s0092-8674(00)81463-4 pmid: 9657152 |
| [11] | Ren C, Zhang Z, Wang Y, et al. Genome-wide identification and characterization of the NF-Y gene family in grape(Vitis vinifera L.)[J]. BMC Genomics, 2016, 17(1): 605. |
| [12] |
Horstman A, Li MF, Heidmann I, et al. The BABY BOOM transcription factor activates the LEC1-ABI3-FUS3-LEC2 network to induce somatic embryogenesis[J]. Plant Physiol, 2017, 175(2): 848-857.
doi: 10.1104/pp.17.00232 pmid: 28830937 |
| [13] | Ledwoń A, Gaj MD. LEAFY COTYLEDON2 gene expression and auxin treatment in relation to embryogenic capacity of Arabidopsis somatic cells[J]. Plant Cell Rep, 2009, 28(11): 1677-1688. |
| [14] | Li K, Wang J, Liu CL, et al. Expression of AtLEC2 and AtIPTs promotes embryogenic callus formation and shoot regeneration in tobacco[J]. BMC Plant Biol, 2019, 19(1): 314. |
| [15] | Gallois JL, Nora FR, Mizukami Y, et al. WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem[J]. Genes Dev, 2004, 18(4): 375-380. |
| [16] | Nair S, Bahn JH, Lee G, et al. A homeobox transcription factor scarecrow(SCRO)negatively regulates pdf neuropeptide expression through binding an identified cis-acting element in Drosophila melanogaster[J]. Mol Neurobiol, 2020, 57(4): 2115-2130. |
| [17] |
Hu XM, Xu L. Transcription factors WOX11/12 directly activate WOX5/7 to promote root primordia initiation and organogenesis[J]. Plant Physiol, 2016, 172(4): 2363-2373.
pmid: 27784768 |
| [18] | Liu JC, Sheng LH, Xu YQ, et al. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis[J]. Plant Cell, 2014, 26(3): 1081-1093. |
| [19] | Meng WJ, Cheng ZJ, Sang YL, et al. Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL[J]. Plant Cell, 2017, 29(6): 1357-1372. |
| [20] | Su YH, Zhao XY, Liu YB, et al. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis[J]. Plant J, 2009, 59(3): 448-460. |
| [21] | Zhang TQ, Lian H, Zhou CM, et al. A two-step model for de novo activation of WUSCHEL during plant shoot regeneration[J]. Plant Cell, 2017, 29(5): 1073-1087. |
| [22] | Kim JY, Yang W, Forner J, et al. Epigenetic reprogramming by histone acetyltransferase HAG1/AtGCN5 is required for pluripotency acquisition in Arabidopsis[J]. EMBO J, 2018, 37(20): e98726. |
| [23] |
Zhang X, Zong J, Liu JH, et al. Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar[J]. J Integr Plant Biol, 2010, 52(11): 1016-1026.
doi: 10.1111/j.1744-7909.2010.00982.x |
| [24] | Luo HM, Xu ZC, Song JY, et al. Research and perspectives on AP2/ERF transcription factors in medicinal plants[J]. Chin Sci Bull, 2015, 60(14): 1272-1284. |
| [25] | Campbell SSB. Studies of the role of Baby Boom(BBM)in embryogenesis in Arabidopsis thaliana and Brassica napus[D]. University of Guelph(Canada). 2009. |
| [26] | Jha P, Kumar V. BABY BOOM(BBM): a candidate transcription factor gene in plant biotechnology[J]. Biotechnol Lett, 2018, 40(11/12): 1467-1475. |
| [27] | Li MF, Wrobel-Marek J, Heidmann I, et al. Auxin biosynthesis maintains embryo identity and growth during BABY BOOM-induced somatic embryogenesis[J]. Plant Physiol, 2022, 188(2): 1095-1110. |
| [28] | Srinivasan C, Liu ZR, Heidmann I, et al. Heterologous expression of the baby boom ap2/erf transcription factor enhances the regeneration capacity of tobacco(Nicotiana tabacum L.)[J]. Planta, 2007, 225(2): 341-351. |
| [29] | Zhou RN, Zhao YJ, Cheng P, et al. GmBBM7 promotes callus and root growth during somatic embryogenesis of soybean(Glycine max)[J]. Biotechnol Biotechnol Equip, 2023, 37(1). |
| [30] | Iwase A, Harashima H, Ikeuchi M, et al. WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis[J]. Plant Cell, 2017, 29(1): 54-69. |
| [31] | Iwase A, Mita K, Nonaka S, et al. WIND1-based acquisition of regeneration competency in Arabidopsis and rapeseed[J]. J Plant Res, 2015, 128(3): 389-397. |
| [32] | Iwase A, Mitsuda N, Koyama T, et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis[J]. Curr Biol, 2011, 21(6): 508-514. |
| [33] | Iwase A, Mitsuda N, Ikeuchi M, et al. Arabidopsis WIND1 induces callus formation in rapeseed, tomato, and tobacco[J]. Plant Signal Behav, 2013, 8(12): e27432. |
| [34] |
Kareem A, Durgaprasad K, Sugimoto K, et al. PLETHORA genes control regeneration by a two-step mechanism[J]. Curr Biol, 2015, 25(8): 1017-1030.
doi: 10.1016/j.cub.2015.02.022 pmid: 25819565 |
| [35] |
Bustillo-Avendaño E, Ibáñez S, Sanz O, et al. Regulation of hormonal control, cell reprogramming, and patterning during de novo root organogenesis[J]. Plant Physiol, 2018, 176(2): 1709-1727.
doi: 10.1104/pp.17.00980 pmid: 29233938 |
| [36] |
Ikeuchi M, Iwase A, Rymen B, et al. Wounding triggers callus formation via dynamic hormonal and transcriptional changes[J]. Plant Physiol, 2017, 175(3): 1158-1174.
doi: 10.1104/pp.17.01035 pmid: 28904073 |
| [37] |
Tsuwamoto R, Yokoi S, Takahata Y. Arabidopsis EMBRYOMAKER encoding an AP2 domain transcription factor plays a key role in developmental change from vegetative to embryonic phase[J]. Plant Mol Biol, 2010, 73(4-5): 481-492.
doi: 10.1007/s11103-010-9634-3 pmid: 20405311 |
| [38] | Lee K, Park OS, Seo PJ. Arabidopsis ATXR2 deposits H3K36me3 at the promoters of LBD genes to facilitate cellular dedifferentiation[J]. Sci Signal, 2017, 10(507): eaan0316. |
| [39] | Xu CY, Cao HF, Zhang QQ, et al. Control of auxin-induced callus formation by bZIP59-LBD complex in Arabidopsis regeneration[J]. Nat Plants, 2018, 4(2): 108-115. |
| [40] | Xu CY, Cao HF, Xu EJ, et al. Genome-wide identification of Arabidopsis LBD29 target genes reveals the molecular events behind auxin-induced cell reprogramming during callus formation[J]. Plant Cell Physiol, 2018, 59(4): 744-755. |
| [41] |
Okushima Y, Fukaki H, Onoda M, et al. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis[J]. Plant Cell, 2007, 19(1): 118-130.
doi: 10.1105/tpc.106.047761 pmid: 17259263 |
| [42] | Atta R, Laurens L, Boucheron-Dubuisson E, et al. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro[J]. Plant J, 2009, 57(4): 626-644. |
| [43] | Van Tuong Huan L, Takamura T, Tanaka M. Callus formation and plant regeneration from callus through somatic embryo structures in Cymbidium orchid[J]. Plant Sci, 2004, 166(6): 1443-1449. |
| [44] | Heyman J, Cools T, Canher B, et al. The heterodimeric transcription factor complex ERF115-PAT1 grants regeneration competence[J]. Nat Plants, 2016, 2(11): 16165. |
| [45] | Liu J, Hu XM, Qin P, et al. The WOX11-LBD16 pathway promotes pluripotency acquisition in callus cells during de novo shoot regeneration in tissue culture[J]. Plant Cell Physiol, 2018, 59(4): 734-743. |
| [46] | Fukaki H, Nakao Y, Okushima Y, et al. Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis[J]. Plant J, 2005, 44(3): 382-395. |
| [47] | Fan MZ, Xu CY, Xu K, et al. Lateral organ boundaries domain transcription factors direct callus formation in Arabidopsis regeneration[J]. Cell Res, 2012, 22(7): 1169-1180. |
| [48] | Pandey SK, Lee HW, Kim MJ, et al. LBD18 uses a dual mode of a positive feedback loop to regulate ARF expression and transcriptional activity in Arabidopsis[J]. Plant J, 2018, 95(2): 233-251. |
| [49] | Berckmans B, Vassileva V, Schmid SPC, et al. Auxin-dependent cell cycle reactivation through transcriptional regulation of Arabidopsis E2Fa by lateral organ boundary proteins[J]. Plant Cell, 2011, 23(10): 3671-3683. |
| [50] | Lee HW, Kim MJ, Kim NY, et al. LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis[J]. Plant J, 2013, 73(2): 212-224. |
| [51] |
Gordon SP, Heisler MG, Reddy GV, et al. Pattern formation during de novo assembly of the Arabidopsis shoot meristem[J]. Development, 2007, 134(19): 3539-3548.
doi: 10.1242/dev.010298 pmid: 17827180 |
| [52] | Higuchi M, Pischke MS, Mähönen AP, et al. In planta functions of the Arabidopsis cytokinin receptor family[J]. Proc Natl Acad Sci USA, 2004, 101(23): 8821-8826. |
| [53] | Shi BH, Zhang C, Tian CH, et al. Two-step regulation of a meristematic cell population acting in shoot branching in Arabidopsis[J]. PLoS Genet, 2016, 12(7): e1006168. |
| [54] | Che P, Lall S, Nettleton D, et al. Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture[J]. Plant Physiol, 2006, 141(2): 620-637. |
| [55] |
Yang SQ, Poretska O, Sieberer T. Altered meristem program1 restricts shoot meristem proliferation and regeneration by limiting hd-zip iii-mediated expression of rap2.6l[J]. Plant Physiol, 2018, 177(4): 1580-1594.
doi: 10.1104/pp.18.00252 pmid: 29884678 |
| [56] | Chen LQ, Tong JH, Xiao LT, et al. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis[J]. J Exp Bot, 2016, 67(14): 4273-4284. |
| [57] | Sheng LH, Hu XM, Du YJ, et al. Non-canonical WOX11-mediated root branching contributes to plasticity in Arabidopsis root system architecture[J]. Development, 2017, 144(17): 3126-3133. |
| [58] |
Ulmasov T, Hagen G, Guilfoyle TJ. Activation and repression of transcription by auxin-response factors[J]. Proc Natl Acad Sci USA, 1999, 96(10): 5844-5849.
pmid: 10318972 |
| [59] |
Forzani C, Aichinger E, Sornay E, et al. WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche[J]. Curr Biol, 2014, 24(16):1939-1944.
doi: 10.1016/j.cub.2014.07.019 pmid: 25127220 |
| [60] |
Fehér A. Somatic embryogenesis - Stress-induced remodeling of plant cell fate[J]. Biochim Biophys Acta, 2015, 1849(4): 385-402.
doi: 10.1016/j.bbagrm.2014.07.005 pmid: 25038583 |
| [61] |
Harding EW, Tang WN, Nichols KW, et al. Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-Like 15[J]. Plant Physiol, 2003, 133(2): 653-663.
doi: 10.1104/pp.103.023499 pmid: 14512519 |
| [62] |
Luo GB, Palmgren M. GRF-GIF chimeras boost plant regeneration[J]. Trends Plant Sci, 2021, 26(3): 201-204.
doi: 10.1016/j.tplants.2020.12.001 pmid: 33349565 |
| [63] |
Debernardi JM, Tricoli DM, Ercoli MF, et al. A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants[J]. Nat Biotechnol, 2020, 38(11): 1274-1279.
doi: 10.1038/s41587-020-0703-0 pmid: 33046875 |
| [64] |
Liu XM, Bie XM, Lin XL, et al. Uncovering the transcriptional regulatory network involved in boosting wheat regeneration and transformation[J]. Nat Plants, 2023, 9(6): 908-925.
doi: 10.1038/s41477-023-01406-z pmid: 37142750 |
| [65] |
Wang K, Shi L, Liang XN, et al. The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation[J]. Nat Plants, 2022, 8(2): 110-117.
doi: 10.1038/s41477-021-01085-8 pmid: 35027699 |
| [66] |
Krakowsky MD, Lee M, Garay L, et al. Quantitative trait loci for callus initiation and totipotency in maize(Zea mays L.)[J]. Theor Appl Genet, 2006, 113(5): 821-830.
pmid: 16896717 |
| [67] | Kausch AP, Adams TR, Mangano M, et al. Effects of microprojectile bombardment on embryogenic suspension cell cultures of maize(Zea mays L.) used for genetic transformation[J]. Planta, 1995, 196(3): 501-509. |
| [68] |
McFarland FL, Collier R, Walter N, et al. A key to totipotency: Wuschel-like homeobox 2a unlocks embryogenic culture response in maize(Zea mays L.)[J]. Plant Biotechnol J, 2023, 21(9): 1860-1872.
doi: 10.1111/pbi.14098 pmid: 37357571 |
| [69] | Muppala S, Gudlavalleti PK, Pagidoju S, et al. Distinctive response of maize(Zea mays L.) genotypes in vitro with the acceleration of phytohormones[J]. J Plant Biotechnol, 2020, 47(1): 26-39. |
| [70] | Nelson-Vasilchik K, Hague JP, Tilelli M, et al. Rapid transformation and plant regeneration of sorghum(Sorghum bicolor L.) mediated by altruistic Baby boom and Wuschel2[J]. Vitro Cell Dev Biol Plant, 2022, 58(3): 331-342. |
| [71] | Aregawi K, Shen JQ, Pierroz G, et al. Morphogene-assisted transformation of Sorghum bicolor allows more efficient genome editing[J]. Plant Biotechnol J, 2022, 20(4): 748-760. |
| [72] | Aregawi K, Shen JQ, Pierroz G, et al. Increased engineering and editing efficiency of Sorghum bicolor using morphogene-assisted transformation[J]. In Vitro Cellular and Development Biology-Plant: Journal of the Tissue Culture Association, 2022, 58(4):682-683. |
| [73] | Mao JP, Niu CD, Li K, et al. Melatonin promotes adventitious root formation in apple by promoting the function of MdWOX11[J]. BMC Plant Biol, 2020, 20(1): 536. |
| [74] | Xu XZ, Che QQ, Cheng CX, et al. Genome-wide identification of WOX gene family in apple and a functional analysis of MdWOX4b during adventitious root formation[J]. J Integr Agric, 2022, 21(5): 1332-1345. |
| [75] | 肖旭. 苹果Baby Boom基因在遗传转化和体胚发生中的功能鉴定[D]. 泰安: 山东农业大学, 2023. |
| Xiao X. Functional identification of apple Baby Boom in genetic transformation and somatic embryogenesis[D]. Tai'an: Shandong Agricultural University, 2023. |
| [1] | ZHANG Chan, WU You-gen, YU Jing, YANG Dong-mei, YAO Guang-long, YANG Hua-geng, ZHANG Jun-feng, CHEN Ping. Molecular Mechanism of Terpenoids Synthesis Intermediated by Light and Jasmonates Signals [J]. Biotechnology Bulletin, 2022, 38(8): 32-40. |
| [2] | ZONG Mei, HAN Shuo, GUO Ning, DUAN Meng-meng, LIU Fan, WANG Gui-xiang. Production of Marker-free Mutants of Brassica campestris Mediated by CRISPR/Cas9 Through Vacuum Infiltration [J]. Biotechnology Bulletin, 2022, 38(10): 159-163. |
| [3] | WANG Xiao-he, GU Xi-rong, LI Jie, CUI Yao. Review on Vegetative Propagation Techniques of Torreya Arn. [J]. Biotechnology Bulletin, 2020, 36(12): 178-187. |
| [4] | DAI Feng-bin, LIU Li-ping, LI Ai-jia, RAO Shu-pei, CHEN Jin-huan. Establishment of Highly Efficient and Rapid Propagation System of Lycium ruthenicum for Multiple Genotypes [J]. Biotechnology Bulletin, 2019, 35(4): 201-207. |
| [5] | YANG Meng-li, GAO Xi-xi, LIU Yuan-qiu, HU Dong-nan, LI Jia-jun, ZHANG Zhi-jian, ZOU Gui-wu, HUANG Guo-xian. Preliminary Study on Inducing Axillary Buds of Different Pinus serotina Explants [J]. Biotechnology Bulletin, 2018, 34(3): 128-135. |
| [6] | TAN Yu-rong, WANG Dan, GAO Xuan, LIU Jin-ping. Research Advance on Plant Long Noncoding RNAs [J]. Biotechnology Bulletin, 2018, 34(10): 1-10. |
| [7] | PENG Xiang, ZHANG De-chun. Research Advances on Tissue Culture Technology of Sorghum [J]. Biotechnology Bulletin, 2018, 34(10): 41-48. |
| [8] | FENG Lu ,WANG Yu-guo, WEN Yin-yuan, ZHAO Juan, LIU Yuan, REN Jian-hong, HE Mei-lin. Effects of Nano Carbon on the Growth and Differentiation of Several Plants in Vitro Culture [J]. Biotechnology Bulletin, 2017, 33(4): 164-168. |
| [9] | Liu Xinxing, Yu Xianghua, Liu Xueduan. Research Progress of Cultivation Technology of Taxus and Its Distribution in China [J]. Biotechnology Bulletin, 2015, 31(7): 51-57. |
| [10] | Wang Xiaofei,Zhou Zhiguang,Wang Yuyi. Advances in Studies on Propagation Technology of Cardiocrinum(Endl.)Lindl. [J]. Biotechnology Bulletin, 2014, 30(9): 22-27. |
| [11] | Li Jun, Gao Guangchun, Li Bai, Zhu Zhiming. Advance on Virus-free Plant Tissue Culture and its Application on Crocus sativus L. [J]. Biotechnology Bulletin, 2014, 30(7): 44-48. |
| [12] | Zhou Xiaofu, Lü Jie, Miao Lu, Gao Feng, Xu Hongwei . Establishment of Alfalfa Tissue Culture System and Research on Agrobacterium-mediated Transformation System in Alfalfa [J]. Biotechnology Bulletin, 2013, 0(4): 63-68. |
| [13] | Gao Yiping, Dong Fushuang, Wang Haibo. Progress and Prospect of Azuki Bean Biotechnologies Research [J]. Biotechnology Bulletin, 2013, 0(3): 10-14. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||