








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (10): 6-19.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0548
Previous Articles Next Articles
LUO Chun-mei1( ), LI Yan-jun1, CHEN Gen-yun2, QU Ming-nan1,2(
), LI Yan-jun1, CHEN Gen-yun2, QU Ming-nan1,2( )
)
Received:2025-05-30
Online:2025-10-26
Published:2025-10-28
Contact:
QU Ming-nan
E-mail:18605861790@163.com;qmn@yzu.edu.cn
LUO Chun-mei, LI Yan-jun, CHEN Gen-yun, QU Ming-nan. Analysis of Photosynthetic Traits of High Heritability in Crops and Mining of High Light-efficiency Regulatory Genes[J]. Biotechnology Bulletin, 2025, 41(10): 6-19.
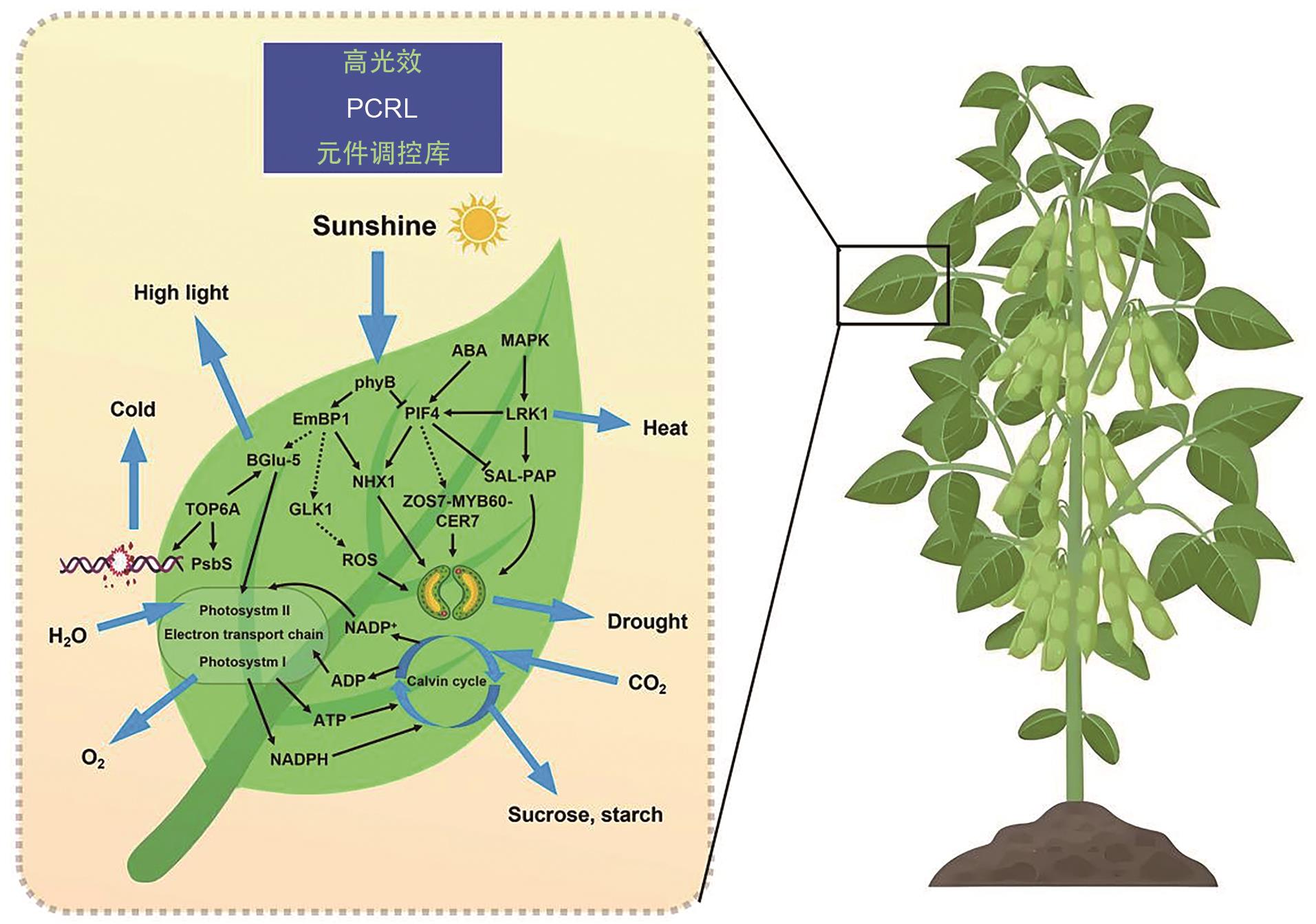
Fig. 1 Schematic diagram of regulatory network for crop high-light efficiency molecular elementsPCRL refers to photosynthetic component regulator library. The black solid arrow indicates the direct promoting effect, and the black dashed arrow indicates the indirect promoting effect. The cutoff line indicates the inhibitory effect, and the blue arrows indicate the interactive effect with the environment
物种 Species | 基因名称 Gene name | 遗传学手段 Approaches of genetics | 方法 Method | 光合表型 Photosynthetic phenotype | 参考文献 References |
|---|---|---|---|---|---|
| 水稻 | OsNHX1 | 正向 | 通过GWAS发现OsNHX1的遗传变异与气孔关闭时间常数τcl的变化密切相关 | 干旱下提高生物量和产量 | [ |
| 水稻 | OsBGlu-5 | 正向 | 通过GWAS挖掘到Bglu-5基因,该基因调控叶绿素荧光Fv/Fm | 提高光系统II的最大量子产率 | [ |
| 水稻 | OsLRK1 | 正向 | 调查叶片暗呼吸(Rd)的自然变异进行GWAS,发现OsLRK1调节叶片暗呼吸通量 | 提高高温下光合效率 | [ |
| 水稻 | OsACP2 | 正向 | 通过GWAS和DEG分析发现ACP2基因介导低磷下丝氨酸合成,调控光合效率 | 提高光合-磷利用效率67% | [ |
| 大豆 | GmFtsH25 | 正向 | 利用GWAS和QTL关联研究,挖掘到GmFtsH25参与大豆光合作用过程 | 提升光合效率和淀粉含量 | [ |
| 玉米 | ZmRAF1 | 反向 | 通过改造Rubisco,创制玉米Rubisco及其亚基组装因子ZmRAF过表达材料 | 增加Rubisco的催化效率和生物量 | [ |
| 小麦 | TaFBA | 反向 | 根据基因家族注释,发现多个TaFBA基因受逆境胁迫诱导 | 提高抗非生物胁迫能力和生物量 | [ |
| 水稻 | OsGATA8 | 反向 | OsGATA8基因位于Saltol QTL中,受到盐、干旱和ABA诱导 | 提高光合效率和生物量 | [ |
| 玉米 | ZmGLK1 | 反向 | 利用GWAS和QTL鉴定到ZmGLK1基因,该基因影响叶绿体发育 | 提升光能利用效率 | [ |
| 水稻 | OsRBCS2 | 反向 | 利用RBCS-sense创制Rubisco过表达水稻材料 | 提高产量和氮利用效率 | [ |
| 水稻 | OsRCA | 反向 | 过表达RCA(Rubisco activase) | 光合速率常温下不提高,高温下提高21% | [ |
| 小麦 | TaSBPase | 反向 | 在小麦中过表达Brachypodium distachyon的SBPase | 提高生物量和产量 | [ |
| 水稻 | OsMGT3 | 反向 | 过表达OsMGT3,提高光合速率 | 提高生物量和产量 | [ |
| 大豆 | OsictB | 反向 | 在大豆中导入蓝细菌的无机碳转运蛋白(inorganic carbon transporter B,ictB) | 提高生物量和产量 | [ |
| 大豆 | GmictB | 反向 | 在水稻中导入蓝藻的ictB | 提高生物量 | [ |
| 玉米 | ZmictB | 反向 | 在玉米中导入蓝藻的ictB | 提高产量 | [ |
| 水稻 | OsNF-YB4 | 反向 | 创制水稻过表达OsNF-YB4基因材料 | 减少株高和叶片数,产量不变 | [ |
| 水稻 | OsOSA1 | 反向 | 过表达水稻质子ATP酶(Oryza sativa plasma membrane(PM)H+ -ATPase 1,OSA1) | 提高生物量和产量 | [ |
| 水稻 | OsPIP1;2 | 反向 | 过表达OsPIP1;2,提高叶肉导度 | 提高生物量和产量 | [ |
| 水稻 | OsHXK1 | 反向 | 敲除OsHXK1,提高气孔导度 | 提高产量 | [ |
| 水稻 | Osslac1 | 反向 | 敲除slac1,导致弱光到高光过程光合诱导延迟 | 气孔导度提高,有利于光合效率 | [ |
| 水稻 | OsNRP1 | 反向 | 敲除光合作用负调节因子NRP1提升光合效率 | 提高生物量和产量 | [ |
| 水稻 | OsTOP6 | 反向 | 编辑TOP6介导光合基因修复损伤,调控低温下光合效率 | 调节碳同化速率 | [ |
| 水稻 | OsZOS7-MYB60 | 反向 | 过表达ZOS7-MYB60调控气孔密度,提高水稻抗旱性 | 提高生物量和产量 | [ |
| 水稻 | OsRAN1 | 反向 | 过表达OsRAN1维持细胞分裂和细胞周期进程 | 提高耐寒性 | [ |
| 水稻 | OsDREB1 | 反向 | 过表达OsDREB1,游离脯氨酸和可溶性糖的含量升高 | 提高干旱、高盐和冷胁迫耐受性 | [ |
| 水稻 | OsLOS5 | 反向 | 在水稻品种中导入LOS5抗旱基因 | 提高抗旱性和产量 | [ |
| 水稻 | ZmPEPC | 反向 | 将C4作物玉米中与光合作用相关的ZmPEPC基因导入水稻 | 提高抗旱性和产量 | [ |
Table 1 Research progress on genes related to high photosynthetic efficiency in crops
物种 Species | 基因名称 Gene name | 遗传学手段 Approaches of genetics | 方法 Method | 光合表型 Photosynthetic phenotype | 参考文献 References |
|---|---|---|---|---|---|
| 水稻 | OsNHX1 | 正向 | 通过GWAS发现OsNHX1的遗传变异与气孔关闭时间常数τcl的变化密切相关 | 干旱下提高生物量和产量 | [ |
| 水稻 | OsBGlu-5 | 正向 | 通过GWAS挖掘到Bglu-5基因,该基因调控叶绿素荧光Fv/Fm | 提高光系统II的最大量子产率 | [ |
| 水稻 | OsLRK1 | 正向 | 调查叶片暗呼吸(Rd)的自然变异进行GWAS,发现OsLRK1调节叶片暗呼吸通量 | 提高高温下光合效率 | [ |
| 水稻 | OsACP2 | 正向 | 通过GWAS和DEG分析发现ACP2基因介导低磷下丝氨酸合成,调控光合效率 | 提高光合-磷利用效率67% | [ |
| 大豆 | GmFtsH25 | 正向 | 利用GWAS和QTL关联研究,挖掘到GmFtsH25参与大豆光合作用过程 | 提升光合效率和淀粉含量 | [ |
| 玉米 | ZmRAF1 | 反向 | 通过改造Rubisco,创制玉米Rubisco及其亚基组装因子ZmRAF过表达材料 | 增加Rubisco的催化效率和生物量 | [ |
| 小麦 | TaFBA | 反向 | 根据基因家族注释,发现多个TaFBA基因受逆境胁迫诱导 | 提高抗非生物胁迫能力和生物量 | [ |
| 水稻 | OsGATA8 | 反向 | OsGATA8基因位于Saltol QTL中,受到盐、干旱和ABA诱导 | 提高光合效率和生物量 | [ |
| 玉米 | ZmGLK1 | 反向 | 利用GWAS和QTL鉴定到ZmGLK1基因,该基因影响叶绿体发育 | 提升光能利用效率 | [ |
| 水稻 | OsRBCS2 | 反向 | 利用RBCS-sense创制Rubisco过表达水稻材料 | 提高产量和氮利用效率 | [ |
| 水稻 | OsRCA | 反向 | 过表达RCA(Rubisco activase) | 光合速率常温下不提高,高温下提高21% | [ |
| 小麦 | TaSBPase | 反向 | 在小麦中过表达Brachypodium distachyon的SBPase | 提高生物量和产量 | [ |
| 水稻 | OsMGT3 | 反向 | 过表达OsMGT3,提高光合速率 | 提高生物量和产量 | [ |
| 大豆 | OsictB | 反向 | 在大豆中导入蓝细菌的无机碳转运蛋白(inorganic carbon transporter B,ictB) | 提高生物量和产量 | [ |
| 大豆 | GmictB | 反向 | 在水稻中导入蓝藻的ictB | 提高生物量 | [ |
| 玉米 | ZmictB | 反向 | 在玉米中导入蓝藻的ictB | 提高产量 | [ |
| 水稻 | OsNF-YB4 | 反向 | 创制水稻过表达OsNF-YB4基因材料 | 减少株高和叶片数,产量不变 | [ |
| 水稻 | OsOSA1 | 反向 | 过表达水稻质子ATP酶(Oryza sativa plasma membrane(PM)H+ -ATPase 1,OSA1) | 提高生物量和产量 | [ |
| 水稻 | OsPIP1;2 | 反向 | 过表达OsPIP1;2,提高叶肉导度 | 提高生物量和产量 | [ |
| 水稻 | OsHXK1 | 反向 | 敲除OsHXK1,提高气孔导度 | 提高产量 | [ |
| 水稻 | Osslac1 | 反向 | 敲除slac1,导致弱光到高光过程光合诱导延迟 | 气孔导度提高,有利于光合效率 | [ |
| 水稻 | OsNRP1 | 反向 | 敲除光合作用负调节因子NRP1提升光合效率 | 提高生物量和产量 | [ |
| 水稻 | OsTOP6 | 反向 | 编辑TOP6介导光合基因修复损伤,调控低温下光合效率 | 调节碳同化速率 | [ |
| 水稻 | OsZOS7-MYB60 | 反向 | 过表达ZOS7-MYB60调控气孔密度,提高水稻抗旱性 | 提高生物量和产量 | [ |
| 水稻 | OsRAN1 | 反向 | 过表达OsRAN1维持细胞分裂和细胞周期进程 | 提高耐寒性 | [ |
| 水稻 | OsDREB1 | 反向 | 过表达OsDREB1,游离脯氨酸和可溶性糖的含量升高 | 提高干旱、高盐和冷胁迫耐受性 | [ |
| 水稻 | OsLOS5 | 反向 | 在水稻品种中导入LOS5抗旱基因 | 提高抗旱性和产量 | [ |
| 水稻 | ZmPEPC | 反向 | 将C4作物玉米中与光合作用相关的ZmPEPC基因导入水稻 | 提高抗旱性和产量 | [ |
| [1] | Anderson R, Bayer PE, Edwards D. Climate change and the need for agricultural adaptation [J]. Curr Opin Plant Biol, 2020, 56: 197-202. |
| [2] | Ray DK, Mueller ND, West PC, et al. Yield trends are insufficient to double global crop production by 2050 [J]. PLoS One, 2013, 8(6): e66428. |
| [3] | 程建峰, 沈允钢. 作物高光效之管见 [J]. 作物学报, 2010, 36(8): 1235-1247. |
| Cheng JF, Shen YG. My humble opinions on high photosynthetic efficiency of crop [J]. Acta Agron Sin, 2010, 36(8): 1235-1247. | |
| [4] | Zhu XG, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield [J]. Annu Rev Plant Biol, 2010, 61: 235-261. |
| [5] | 刘扶桑, 宋青峰, 于桂朝, 等. 光合作用系统模型与作物高光效改良 [J]. 生命科学, 2024, 36(9): 1123-1140. |
| Liu FS, Song QF, Yu GC, et al. Photosynthesis system model and high light efficiency improvement of crops [J]. Biological Sciences, 2024, 36(9): 1123-1140. | |
| [6] | Si FF, Fan FF, Wei X, et al. Quantitative trait locus mapping of high photosynthetic efficiency and biomass in Oryza longistaminata [J]. Rice Sci, 2022, 29(6): 569-576. |
| [7] | Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species [J]. Planta, 1980, 149(1): 78-90. |
| [8] | Rumeau D, Peltier G, Cournac L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response [J]. Plant Cell Environ, 2007, 30(9): 1041-1051. |
| [9] | Wang FB, Liu JC, Chen MX, et al. Involvement of abscisic acid in PSII photodamage and D1 protein turnover for light-induced premature senescence of rice flag leaves [J]. PLoS One, 2016, 11(8): e0161203. |
| [10] | Takagi D, Takumi S, Hashiguchi M, et al. Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition [J]. Plant Physiol, 2016, 171(3): 1626-1634. |
| [11] | McAusland L, Vialet-Chabrand S, Jauregui I, et al. Variation in key leaf photosynthetic traits across wheat wild relatives is accession dependent not species dependent [J]. New Phytol, 2020, 228(6): 1767-1780. |
| [12] | Liu D, Lin Q, Huang S, et al. Transcriptome and metabolome analysis reveals different photosynthetic characteristics of mulberry trees with different ploidy levels [J]. Sci Rep, 2025, 15(1): 15892. |
| [13] | Wang Y. Improving photosynthetic efficiency in fluctuating light to enhance yield of C3 and C4 crops [J]. Crop Environ, 2024, 3(4): 184-193. |
| [14] | Spreitzer RJ, Salvucci ME. Rubisco: structure, regulatory interactions, and possibilities for a better enzyme [J]. Annu Rev Plant Biol, 2002, 53: 449-475. |
| [15] | Balparda M, Bouzid M, Martinez MDP, et al. Regulation of plant carbon assimilation metabolism by post-translational modifications [J]. Plant J, 2023, 114(5): 1059-1079. |
| [16] | Lawson T, Blatt MR. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency[J]. Plant Physiol, 2014, 164(4): 1556-1570. |
| [17] | Xiong DL. Perspectives of improving rice photosynthesis for higher grain yield [J]. Crop Environ, 2024, 3(3): 123-137. |
| [18] | Nugent JH. Oxygenic photosynthesis. Electron transfer in photosystem I and photosystem II [J]. Eur J Biochem, 1996, 237(3): 519-531. |
| [19] | Pospíšil P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress [J]. Front Plant Sci, 2016, 7: 1950. |
| [20] | Jahns P, Holzwarth AR. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II [J]. Biochim Biophys Acta Bioenerg, 2012, 1817(1): 182-193. |
| [21] | Yamori W. Photosynthetic response to fluctuating environments and photoprotective strategies under abiotic stress [J]. J Plant Res, 2016, 129(3): 379-395. |
| [22] | Long SP, Taylor SH, Burgess SJ, et al. Into the shadows and back into sunlight: photosynthesis in fluctuating light [J]. Annu Rev Plant Biol, 2022, 73: 617-648. |
| [23] | Croce R, Carmo-Silva E, Cho YB, et al. Perspectives on improving photosynthesis to increase crop yield [J]. Plant Cell, 2024, 36(10): 3944-3973. |
| [24] | Moore RL. Using the green alga, Chlamydomonas reinhardtii, as a chassis to express non-green Rubisco variants[D]. Newcastle University, 2024. |
| [25] | Niyogi KK, Grossman AR, Björkman O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion [J]. Plant Cell, 1998, 10(7): 1121-1134. |
| [26] | Iwai M, Patel-Tupper D, Niyogi KK. Structural diversity in eukaryotic photosynthetic light harvesting [J]. Annu Rev Plant Biol, 2024, 75(1): 119-152. |
| [27] | Schiphorst C, Koeman C, Caracciolo L, et al. The effects of different daily irradiance profiles on Arabidopsis growth, with special attention to the role of PsbS [J]. Front Plant Sci, 2023, 14: 1070218. |
| [28] | Demmig-Adams B, Cohu CM, Muller O, et al. Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons [J]. Photosynth Res, 2012, 113(1-3): 75-88. |
| [29] | Xu ZZ, Jiang YL, Zhou GS. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants [J]. Front Plant Sci, 2015, 6: 701. |
| [30] | Li XG, Li JY, Zhao JP, et al. Xanthophyll cycle and inactivation of photosystem II reaction centers alleviating reducing pressure to photosystem I in morning glory leaves under short-term high irradiance [J]. J Integr Plant Biol, 2007, 49(7): 1047-1053. |
| [31] | Wang YF, Lei B, Deng HB, et al. Exogenous abscisic acid affects the heat tolerance of rice seedlings by influencing the accumulation of ROS [J]. Antioxidants, 2023, 12(7): 1404. |
| [32] | Lu FF, Jiao GA, Qiu JH, et al. A molecular module improves rice grain quality and yield at high temperatures [J]. Natl Sci Rev, 2024, 12(2): nwae416. |
| [33] | 孙克香, 杨莎, 郭峰, 等. 高温强光胁迫下外源钙对甜椒(Capsicum fructescens L.)幼苗光合生理特性的影响 [J]. 植物生理学报, 2015, 51(3): 280-286. |
| Sun KX, Yang S, Guo F, et al. Effects of exogenous calcium on photosynthetic chracteristics of sweet pepper (Capsicum fructescens L.) seedlings [J]. Plant Physiol J, 2015, 51(3): 280-286. | |
| [34] | Sharma A, Kumar V, Shahzad B, et al. Photosynthetic response of plants under different abiotic stresses: a review [J]. J Plant Growth Regul, 2020, 39(2): 509-531. |
| [35] | Wang FH, Yang F, Zhu DF, et al. MYB44 plays key roles in regulating plant responses to abiotic and biotic stress, metabolism, and development [J]. J Plant Biochem Biotechnol, 2024, 33(4): 462-473. |
| [36] | Hao YH, Zeng ZX, Yuan MH, et al. The blue-light receptor CRY1 serves as a switch to balance photosynthesis and plant defense [J]. Cell Host Microbe, 2025, 33(1): 137-150.e6. |
| [37] | Yamauchi S, Mano S, Oikawa K, et al. Autophagy controls reactive oxygen species homeostasis in guard cells that is essential for stomatal opening [J]. Proc Natl Acad Sci USA, 2019, 116(38): 19187-19192. |
| [38] | Aycan M, Nahar L, Baslam M, et al. Transgenerational plasticity in salinity tolerance of rice: unraveling non-genetic phenotypic modifications and environmental influences [J]. J Exp Bot, 2024, 75(16): 5037-5053. |
| [39] | Wu GZ, Bock R. GUN control in retrograde signaling: How GENOMES UNCOUPLED proteins adjust nuclear gene expression to plastid biogenesis [J]. Plant Cell, 2021, 33(3): 457-474. |
| [40] | Li YH, Cao TJ, Guo YL, et al. Regulatory and retrograde signaling networks in the chlorophyll biosynthetic pathway [J]. J Integr Plant Biol, 2025, 67(4): 887-911. |
| [41] | Dong HX, Liu J, He GH, et al. Photoexcited phytochrome B interacts with brassinazole resistant 1 to repress brassinosteroid signaling in Arabidopsis [J]. J Integr Plant Biol, 2020, 62(5): 652-667. |
| [42] | Lee KP, Kim C. Photosynthetic ROS and retrograde signaling pathways [J]. New Phytol, 2024, 244(4): 1183-1198. |
| [43] | Khan I, Sohail, Zaman S, et al. Adaptive responses of plants to light stress: mechanisms of photoprotection and acclimation. A review [J]. Front Plant Sci, 2025, 16: 1550125. |
| [44] | Grabsztunowicz M, Koskela MM, Mulo P. Post-translational modifications in regulation of chloroplast function: recent advances [J]. Front Plant Sci, 2017, 8: 240. |
| [45] | Zhang X, Wen H, Shi XB. Lysine methylation: beyond histones [J]. Acta Biochim Biophys Sin, 2012, 44(1): 14-27. |
| [46] | Trievel RC, Flynn EM, Houtz RL, et al. Mechanism of multiple lysine methylation by the SET domain enzyme Rubisco LSMT [J]. Nat Struct Biol, 2003, 10(7): 545-552. |
| [47] | Houtz RL, Magnani R, Nayak NR, et al. Co- and post-translational modifications in rubisco: unanswered questions [J]. J Exp Bot, 2008, 59(7): 1635-1645. |
| [48] | Li PG, An ZH, Xu N, et al. Phenotypic plasticity and stability in plants: genetic mechanisms, environmental adaptation, evolutionary implications, and future directions [J]. Plant Cell Environ, 2025, 48(8): 5847-5860. |
| [49] | Acevedo-Siaca LG, Coe R, Quick WP, et al. Evaluating natural variation, heritability, and genetic advance of photosynthetic traits in rice (Oryza sativa) [J]. Plant Breed, 2021, 140(5): 745-757. |
| [50] | Visscher PM, Wray NR, Zhang Q, et al. 10 years of GWAS discovery: biology, function, and translation [J]. Am J Hum Genet, 2017, 101(1): 5-22. |
| [51] | Scott MF, Ladejobi O, Amer S, et al. Multi-parent populations in crops: a toolbox integrating genomics and genetic mapping with breeding [J]. Heredity, 2020, 125(6): 396-416. |
| [52] | Visscher PM, Hill WG, Wray NR. Heritability in the genomics era—concepts and misconceptions [J]. Nat Rev Genet, 2008, 9(4): 255-266. |
| [53] | Liu SS, Xu Z, Essemine J, et al. GWAS unravels acid phosphatase ACP2 as a photosynthesis regulator under phosphate starvation conditions through modulating serine metabolism in rice [J]. Plant Commun, 2024, 5(7): 100885. |
| [54] | Qu MN, Zheng GY, Hamdani S, et al. Leaf photosynthetic parameters related to biomass accumulation in a global rice diversity survey [J]. Plant Physiol, 2017, 175(1): 248-258. |
| [55] | Qu MN, Essemine J, Xu JL, et al. Alterations in stomatal response to fluctuating light increase biomass and yield of rice under drought conditions [J]. Plant J, 2020, 104(5): 1334-1347. |
| [56] | Qu MN, Essemine J, Li M, et al. Genome-wide association study unravels LRK1 as a dark respiration regulator in rice (Oryza sativa L.) [J]. Int J Mol Sci, 2020, 21(14): 4930. |
| [57] | Fu P, Montes CM, Siebers MH, et al. Advances in field-based high-throughput photosynthetic phenotyping [J]. J Exp Bot, 2022, 73(10): 3157-3172. |
| [58] | Singh A, Ganapathysubramanian B, Singh AK, et al. Machine learning for high-throughput stress phenotyping in plants [J]. Trends Plant Sci, 2016, 21(2): 110-124. |
| [59] | Wu TA, Zhang W, Wu SY, et al. Retrieving rice (Oryza sativa L.) net photosynthetic rate from UAV multispectral images based on machine learning methods [J]. Front Plant Sci, 2023, 13: 1088499. |
| [60] | Yu GR, Wang QF, Zhuang J. Modeling the water use efficiency of soybean and maize plants under environmental stresses: application of a synthetic model of photosynthesis-transpiration based on stomatal behavior [J]. J Plant Physiol, 2004, 161(3): 303-318. |
| [61] | Deng YX, Xin N, Zhao LG, et al. Precision detection of salt stress in soybean seedlings based on deep learning and chlorophyll fluorescence imaging [J]. Plants, 2024, 13(15): 2089. |
| [62] | Chandel NS, Rajwade YA, Dubey K, et al. Water stress identification of winter wheat crop with state-of-the-art AI techniques and high-resolution thermal-RGB imagery [J]. Plants, 2022, 11(23): 3344. |
| [63] | Sangphukieo A, Laomettachit T, Ruengjitchatchawalya M. Photosynthetic protein classification using genome neighborhood-based machine learning feature [J]. Sci Rep, 2020, 10(1): 7108. |
| [64] | Farooq MA, Gao S, Hassan MA, et al. Artificial intelligence in plant breeding [J]. Trends Genet, 2024, 40(10): 891-908. |
| [65] | Gibbs JA, Burgess AJ. Application of deep learning for the analysis of stomata: a review of current methods and future directions [J]. J Exp Bot, 2024, 75(21): 6704-6718. |
| [66] | Altabaji WIAE, Umair M, Tan WH, et al. Comparative analysis of transfer learning, LeafNet, and modified LeafNet models for accurate rice leaf diseases classification [J]. IEEE Access, 2024, 12: 36622-36635. |
| [67] | Wei KF, Li YX. Functional genomics of the protein kinase superfamily from wheat [J]. Mol Breed, 2019, 39(10): 141. |
| [68] | Fan BL, Chen LH, Chen LL, et al. Integrative multi-omics approaches for identifying and characterizing biological elements in crop traits: current progress and future prospects [J]. Int J Mol Sci, 2025, 26(4): 1466. |
| [69] | Sargent D, Amthor JS, Stinziano JR, et al. The importance of species-specific and temperature-sensitive parameterisation of A/Ci models: a case study using cotton (Gossypium hirsutum L.) and the automated ‘OptiFitACi’ R-package [J]. Plant Cell Environ, 2024, 47(5): 1701-1715. |
| [70] | 朱新广, 常天根, 宋青峰, 等. 数字植物:科学内涵、瓶颈及发展策略 [J]. 合成生物学, 2020, 1(3): 285-297. |
| Zhu XG, Chang TG, Song QF, et al. Digital plant: scientific connotation, bottlenecks and development strategies [J]. Synthetic Biology Journal, 2020, 1(3): 285-297. | |
| [71] | 宋青峰, 曲明南, 徐建龙, 等. 冠层光能利用效率改良的理论、分子途径及展望 [J]. 生命科学, 2018, 30(10): 1044-1050. |
| Song QF, Qu MN, Xu JL, et al. The canopy light use efficiency [J]. Chin Bull Life Sci, 2018, 30(10): 1044-1050. | |
| [72] | Schneeberger K, Weigel D. Fast-forward genetics enabled by new sequencing technologies [J]. Trends Plant Sci, 2011, 16(5): 282-288. |
| [73] | Aklilu E. Review on forward and reverse genetics in plant breeding [J]. Life, 2021, 14(1): 127-135. |
| [74] | Yeh SY, Lin HH, Chang YM, et al. Maize Golden2-like transcription factors boost rice chloroplast development, photosynthesis, and grain yield [J]. Plant Physiol, 2022, 188(1): 442-459. |
| [75] | Nutan KK, Singla-Pareek SL, Pareek A. The Saltol QTL-localized transcription factor OsGATA8 plays an important role in stress tolerance and seed development in Arabidopsis and rice [J]. J Exp Bot, 2020, 71(2): 684-698. |
| [76] | Lv GY, Guo XG, Xie LP, et al. Molecular characterization, gene evolution, and expression analysis of the fructose-1,6-bisphosphate aldolase (FBA) gene family in wheat (Triticum aestivum L.) [J]. Front Plant Sci, 2017, 8: 1030. |
| [77] | Soyk S, Müller NA, Park SJ, et al. Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato [J]. Nat Genet, 2017, 49(1): 162-168. |
| [78] | Wang L, Yang YM, Yang ZY, et al. GmFtsH25 overexpression increases soybean seed yield by enhancing photosynthesis and photosynthates [J]. J Integr Plant Biol, 2023, 65(4): 1026-1040. |
| [79] | Hu Z, Guo YT, Ying SP, et al. OsCBL1 modulates rice nitrogen use efficiency via negative regulation of OsNRT2.2 by OsCCA1 [J]. BMC Plant Biol, 2023, 23(1): 502. |
| [80] | Hubbart S, Ajigboye OO, Horton P, et al. The photoprotective protein PsbS exerts control over CO2 assimilation rate in fluctuating light in rice [J]. Plant J, 2012, 71(3): 402-412. |
| [81] | Zhou YJ, Shi LF, Li X, et al. Genetic engineering of RuBisCO by multiplex CRISPR editing small subunits in rice [J]. Plant Biotechnol J, 2025, 23(3): 731-749. |
| [82] | Perveen S, Qu MN, Chen FM, et al. Overexpression of maize transcription factor mEmBP-1 increases photosynthesis, biomass, and yield in rice [J]. J Exp Bot, 2020, 71(16): 4944-4957. |
| [83] | Wei SB, Li X, Lu ZF, et al. A transcriptional regulator that boosts grain yields and shortens the growth duration of rice [J]. Science, 2022, 377(6604): eabi8455. |
| [84] | Bezrutczyk M, Zöllner NR, Kruse CPS, et al. Evidence for phloem loading via the abaxial bundle sheath cells in maize leaves [J]. Plant Cell, 2021, 33(3): 531-547. |
| [85] | Long BM, Hee WY, Sharwood RE, et al. Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts [J]. Nat Commun, 2018, 9(1): 3570. |
| [86] | 李霞, 周文彬, 钱前. 作物高光效育种研究进展和展望 [J]. 中国基础科学, 2022, 24(5): 1-6, 14. |
| Li X, Zhou WB, Qian Q. Research progress and prospects in high-efficiency photosynthesis breeding of crops [J]. China Basic Science, 2022, 24(5): 1-6, 14. | |
| [87] | Ermakova M, Arrivault S, Giuliani R, et al. Installation of C4 photosynthetic pathway enzymes in rice using a single construct [J]. Plant Biotechnol J, 2021, 19(3): 575-588. |
| [88] | Li X, Wang P, Li J, et al. Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition [J]. Commun Biol, 2020, 3(1): 151. |
| [89] | Hamdani S, Wang HR, Zheng GY, et al. Genome-wide association study identifies variation of glucosidase being linked to natural variation of the maximal quantum yield of photosystem II [J]. Physiol Plant, 2019, 166(1): 105-119. |
| [90] | Salesse-Smith CE, Sharwood RE, Busch FA, et al. Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize [J]. Nat Plants, 2018, 4(10): 802-810. |
| [91] | Yoon DK, Ishiyama K, Suganami M, et al. Transgenic rice overproducing Rubisco exhibits increased yields with improved nitrogen-use efficiency in an experimental paddy field [J]. Nat Food, 2020, 1(2): 134-139. |
| [92] | Suganami M, Suzuki Y, Tazoe Y, et al. Co-overproducing Rubisco and Rubisco activase enhances photosynthesis in the optimal temperature range in rice [J]. Plant Physiol, 2021, 185(1): 108-119. |
| [93] | Driever SM, Simkin AJ, Alotaibi S, et al. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions [J]. Philos Trans R Soc Lond B Biol Sci, 2017, 372(1730): 20160384. |
| [94] | Li J, Yokosho K, Liu S, et al. Diel magnesium fluctuations in chloroplasts contribute to photosynthesis in rice [J]. Nat Plants, 2020, 6(7): 848-859. |
| [95] | Hay WT, Bihmidine S, Mutlu N, et al. Enhancing soybean photosynthetic CO2 assimilation using a cyanobacterial membrane protein, ictB [J]. J Plant Physiol, 2017, 212: 58-68. |
| [96] | Yang SM, Chang CY, Yanagisawa M, et al. Transgenic rice expressing cyanobacterial bicarbonate transporter exhibited enhanced photosynthesis, growth and grain yield [M]//Photosynthesis. Energy from the Sun. Dordrecht: Springer Netherlands, 2008: 1243-1246. |
| [97] | Koester RP, Pignon CP, Kesler DC, et al. Transgenic insertion of the cyanobacterial membrane protein ictB increases grain yield in Zea mays through increased photosynthesis and carbohydrate production [J]. PLoS One, 2021, 16(2): e0246359. |
| [98] | Peng MF, Gan F, Lin XM, et al. Overexpression of OsNF-YB4 leads to flowering early, improving photosynthesis and better grain yield in hybrid rice [J]. Plant Sci, 2023, 331: 111661. |
| [99] | Zhang MX, Wang Y, Chen X, et al. Plasma membrane H+-ATPase overexpression increases rice yield via simultaneous enhancement of nutrient uptake and photosynthesis [J]. Nat Commun, 2021, 12(1): 735. |
| [100] | Xu FY, Wang K, Yuan W, et al. Overexpression of rice aquaporin OsPIP1;2 improves yield by enhancing mesophyll CO2 conductance and phloem sucrose transport [J]. J Exp Bot, 2019, 70(2): 671-681. |
| [101] | Zheng SY, Ye CJ, Lu JQ, et al. Improving the rice photosynthetic efficiency and yield by editing OsHXK1 via CRISPR/Cas9 system [J]. Int J Mol Sci, 2021, 22(17): 9554. |
| [102] | Yamori W, Kusumi K, Iba K, et al. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice [J]. Plant Cell Environ, 2020, 43(5): 1230-1240. |
| [103] | Chen FM, Zheng GY, Qu MN, et al. Knocking out negative regulator of photosynthesis 1 increases rice leaf photosynthesis and biomass production in the field [J]. J Exp Bot, 2021, 72(5): 1836-1849. |
| [104] | Xu Z, Zhang JX, Wang X, et al. Cold-induced inhibition of photosynthesis-related genes integrated by a TOP6 complex in rice mesophyll cells [J]. Nucleic Acids Res, 2023, 51(4): 1823-1842. |
| [105] | Zhou SB, He LH, Iqbal Z, et al. The ZOS7-MYB60 module confers drought-stress tolerance in rice [J]. Crop J, 2024, 12(5): 1369-1378. |
| [106] | Xu PP, Cai WM. RAN1 is involved in plant cold resistance and development in rice (Oryza sativa) [J]. J Exp Bot, 2014, 65(12): 3277-3287. |
| [107] | Ito Y, Katsura K, Maruyama K, et al. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice [J]. Plant Cell Physiol, 2006, 47(1): 141-153. |
| [108] | Xiao BZ, Chen X, Xiang CB, et al. Evaluation of seven function-known candidate genes for their effects on improving drought resistance of transgenic rice under field conditions [J]. Mol Plant, 2009, 2(1): 73-83. |
| [109] | Zhang C, Li X, He YF, et al. Physiological investigation of C4-phosphoenolpyruvate-carboxylase-introduced rice line shows that sucrose metabolism is involved in the improved drought tolerance [J]. Plant Physiol Biochem, 2017, 115: 328-342. |
| [110] | Yang WN, Duan LF, Chen GX, et al. Plant phenomics and high-throughput phenotyping: accelerating rice functional genomics using multidisciplinary technologies [J]. Curr Opin Plant Biol, 2013, 16(2): 180-187. |
| [111] | Yang WN, Feng H, Zhang XH, et al. Crop phenomics and high-throughput phenotyping: past decades, current challenges, and future perspectives [J]. Mol Plant, 2020, 13(2): 187-214. |
| [112] | Riches M, Lee D, Farmer DK. Simultaneous leaf-level measurement of trace gas emissions and photosynthesis with a portable photosynthesis system [J]. Atmos Meas Tech, 2020, 13(8): 4123-4139. |
| [113] | Schreiber U, Klughammer C, Kolbowski J. Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer [J]. Photosynth Res, 2012, 113(1-3): 127-144. |
| [114] | Guadagno CR, Pugliese M, Bonanno S, et al. Gas exchange and chlorophyll a fluorescence measurements as proxies of X-ray resistance in Phaseolus vulgaris L [J]. Radiat Environ Biophys, 2019, 58(4): 575-583. |
| [115] | Zhang JC, Hu YC, Li F, et al. Meta-analysis assessing potential of drone remote sensing in estimating plant traits related to nitrogen use efficiency [J]. Remote Sens, 2024, 16(5): 838. |
| [116] | Lu Z, Yao WB, Pei SK, et al. Inversion of soybean net photosynthetic rate based on UAV multi-source remote sensing and machine learning [J]. Agronomy, 2024, 14(7): 1493. |
| [117] | Wang JZ, Zhang Y, Gu RR. Research status and prospects on plant canopy structure measurement using visual sensors based on three-dimensional reconstruction [J]. Agriculture, 2020, 10(10): 462. |
| [118] | Rivera G, Porras R, Florencia R, et al. LiDAR applications in precision agriculture for cultivating crops: a review of recent advances [J]. Comput Electron Agric, 2023, 207: 107737. |
| [119] | Špundová M, Kučerová Z, Nožková V, et al. What to choose for estimating leaf water status—spectral reflectance or in vivo chlorophyll fluorescence? [J]. Plant Phenomics, 2024, 6: 243. |
| [120] | 刘晶,刘文清,赵南京,等. 基于光诱导荧光技术的浮游植物光合作用活性原位测量方法[J]. 光谱学与光谱分析, 2013, 33(9): 2443-2447. |
| Liu J, Liu WQ, Zhao NJ, et al. The method of phytoplankton photosynthesis activity in situ measurement based on light induced fluorescence [J]. Spectroscopy and Spectral Analysis, 2013, 33(9): 2443-2447. | |
| [121] | Xiao QL, Wu N, Tang WT, et al. Visible and near-infrared spectroscopy and deep learning application for the qualitative and quantitative investigation of nitrogen status in cotton leaves [J]. Front Plant Sci, 2022, 13: 1080745. |
| [122] | Su M, Zhou D, Yun YZ, et al. Design and implementation of a high-throughput field phenotyping robot for acquiring multisensor data in wheat [J]. Plant Phenomics, 2025, 7(2): 100014. |
| [123] | Wu J, Wu Q, Pagès L, et al. RhizoChamber-Monitor: a robotic platform and software enabling characterization of root growth [J]. Plant Methods, 2018, 14: 44. |
| [124] | Xu BH, Zhang JF, Tang ZX, et al. Nighttime environment enables robust field-based high-throughput plant phenotyping: a system platform and a case study on rice [J]. Comput Electron Agric, 2025, 235: 110337. |
| [125] | He SY, Li XN, Chen MG, et al. Excellent canopy structure in soybeans can improve their photosynthetic performance and increase yield [J]. Agriculture, 2024, 14(10): 1783. |
| [126] | Melis A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency [J]. Plant Sci, 2009, 177(4): 272-280. |
| [127] | Deng CH, Naithani S, Kumari S, et al. Genotype and phenotype data standardization, utilization and integration in the big data era for agricultural sciences [J]. Database, 2023, 2023: baad088. |
| [128] | Pommier C, Michotey C, Cornut G, et al. Applying FAIR principles to plant phenotypic data management in GnpIS [J]. Plant Phenomics, 2019, 2019: 1671403. |
| [129] | Li X, Xie C, Cheng L, et al. The next Green Revolution: integrating crop architectype and physiotype [J]. Trends Biotechnol, 2025. DOI: 10.1016/j.tibtech.2025.04.002 . |
| [130] | Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors [J]. Nat Biotechnol, 2020, 38(7): 824-844. |
| [131] | Xu YB, Zhang XP, Li HH, et al. Smart breeding driven by big data, artificial intelligence, and integrated genomic-enviromic prediction [J]. Mol Plant, 2022, 15(11): 1664-1695. |
| [132] | Lyu HM, Yim WC, Yu QY. Genomic and transcriptomic insights into the evolution of C4 photosynthesis in grasses [J]. Genome Biol Evol, 2024, 16(8): evae163. |
| [133] | Liu B, Liu FL, Hogy P, et al. High-throughput phenotyping: The latest research tool for sustainable crop production under global climate change scenarios [M]. Sustainable Crop Productivity and Quality Under Climate Change. Academic Press, 2022: 313-381. |
| [134] | Zhang R, Guo R, Zhi H, et al. De novo creation of narrowed plant architecture via CRISPR/Cas9-mediated mutagenesis of SiLGs in foxtail millet [J]. Plant Biotechnol J, 2025, 23(6): 2400-2402. |
| [135] | Korenblum E, Massalha H, Aharoni A. Plant-microbe interactions in the rhizosphere via a circular metabolic economy [J]. Plant Cell, 2022, 34(9): 3168-3182. |
| [136] | Dakora FD, Matiru VN, Kanu AS. Rhizosphere ecology of lumichrome and riboflavin, two bacterial signal molecules eliciting developmental changes in plants [J]. Front Plant Sci, 2015, 6: 700. |
| [137] | Shen QP, Xie YJ, Qiu XZ, et al. The era of cultivating smart rice with high light efficiency and heat tolerance has come of age [J]. Front Plant Sci, 2022, 13: 1021203. |
| [138] | Li W, Yang K, Hu CF, et al. A natural gene on-off system confers field thermotolerance for grain quality and yield in rice [J]. Cell, 2025: S0092-8674(25)00413-1. |
| [139] | Morales F, Ancín M, Fakhet D, et al. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement [J]. Plants, 2020, 9(1): 88. |
| [140] | Chen R, Wang ZW, Liu WK, et al. Side lighting of red, blue and green spectral combinations altered the growth, yield and quality of lettuce (Lactuca sativa L. cv. yidali) in plant factory [J]. Plants, 2023, 12(24): 4147. |
| [1] | CHEN Cai-ding, SONG Yun-jie, TIAN Meng-qing. Advance on the Changes of Rhizosphere Microbial Communities in the Growth Stages of the Four Major Staple Crops [J]. Biotechnology Bulletin, 2025, 41(6): 49-60. |
| [2] | ZHAO Chang-yan, LIU Yan-tao, JIA Xiu-ping, LIU Sheng-li, LEI Zhong-hua, WANG Peng, ZHU Zhi-feng, DONG Hong-ye, LYU Zeng-shuai, DUAN Wei, WAN Su-mei. Research Progress in the Effect of Melatonin on Crop Physiological Mechanism under Saline-alkali Stress [J]. Biotechnology Bulletin, 2025, 41(2): 18-29. |
| [3] | GAO Bo-wen, DING Shun-hua, CHEN Xiao-jun, WEN Xiao-gang, TIAN Li-jin, LU Qing-tao. Strategies for Optimizing Photosynthesis to Enhance Agricultural Production Efficiency [J]. Biotechnology Bulletin, 2025, 41(10): 54-63. |
| [4] | SUN Jing, YANG Yun-long, LIU Rong-zhi, YU Hong, LU Tie-gang. Strengthening Fundamental Research on Photosynthesis to Support Crop Breeding for High Yield [J]. Biotechnology Bulletin, 2025, 41(10): 1-5. |
| [5] | LI Hao, WU Guo-qiang, WEI Ming, HAN Yue-xin. Genome-wide Identification of the BvBADH Gene Family in Sugar Beet(Beta vulgaris)and Their Expression Analysis Under High Salt Stress [J]. Biotechnology Bulletin, 2024, 40(2): 233-244. |
| [6] | MA Sai-mai, LI Tong-yuan, MA Yan-jun, HAN Fu-jun, PENG Hai, KONG Wei-bao. Research Progress in Chitinase Involving in the Biocontrol of Crop Diseases and Pests [J]. Biotechnology Bulletin, 2023, 39(10): 29-40. |
| [7] | XIE Wei, LIU Chun-ming. Commercialization of Biological Breeding in China: Opportunities and Policy Issues [J]. Biotechnology Bulletin, 2023, 39(1): 16-20. |
| [8] | LIN Ying, YANG Wen-li, ZHOU Ling-yan, JIANG Da-gang. Research Progress in Agricultural Genetically Modified Nucleic Acid Reference Materials [J]. Biotechnology Bulletin, 2022, 38(8): 52-59. |
| [9] | XU Miao-yun, XING Li-juan, YANG Ming-yu, ZHANG Ling-xuan, WANG Lei, LIU Yue-ping. Research Progress in Germplasm Innovation and Utilization of High Amylose Cereal Crops [J]. Biotechnology Bulletin, 2022, 38(4): 20-28. |
| [10] | YIPARE·Paerhati , ZULIHUMAER·Rouzi , TIAN Yong-zhi, ZHU Yan-lei, LI Yuan-ting, MA Xiao-lin. Research Progress in Diversity of Endophytes Microbial Communities Isolated from Desert Plants and Their Strengthening Effects on Drought and Salt Tolerance in Crops [J]. Biotechnology Bulletin, 2022, 38(12): 88-99. |
| [11] | WANG Ting, YANG Yang, LI Jin-ping, DU Kun. Research Progress in the Effects of Genetically Modified Crops on Soil Microbial Community [J]. Biotechnology Bulletin, 2021, 37(9): 255-265. |
| [12] | WU Qi-man, ZHANG Jin-mei, LI Yue-ying, ZHANG Ying. Recent Advances on the Mechanism of Beneficial Microbial Fertilizers in Crops [J]. Biotechnology Bulletin, 2021, 37(5): 221-230. |
| [13] | DENG Pu-rong, LIU Yong-bo. Review on the Synergistic Insect-resistant Application of RNAi and Bt-transgenic Technologies [J]. Biotechnology Bulletin, 2021, 37(10): 216-224. |
| [14] | SUN Yu, CHANG Jing-jing, TIAN Chun-jie. Technical Systems of Reorganization and Construction of Crop Rhizosphere Microbiome [J]. Biotechnology Bulletin, 2020, 36(9): 25-30. |
| [15] | LI Yu-hua, REN Yong-kang, ZHAO Xing-hua, LIU Jiang, HAN bin, WANG Chang-biao, TANG Zhao-hui. Research Progress on Chloroplast Genome of Major Gramineous Crops [J]. Biotechnology Bulletin, 2020, 36(11): 112-121. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||