








Biotechnology Bulletin ›› 2026, Vol. 42 ›› Issue (1): 338-351.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0600
Previous Articles Next Articles
KANG Kai1( ), YANG Wei2, LI Ying-chun1, XIE Wei-tian1, WU Hai-yan1, YOU Yu-pin1, CHEN Zhi-bao1,3(
), YANG Wei2, LI Ying-chun1, XIE Wei-tian1, WU Hai-yan1, YOU Yu-pin1, CHEN Zhi-bao1,3( )
)
Received:2025-06-11
Online:2026-01-26
Published:2026-02-04
Contact:
CHEN Zhi-bao
E-mail:kangk@gdou.edu.cn;chenzb@gdou.edu.cn
KANG Kai, YANG Wei, LI Ying-chun, XIE Wei-tian, WU Hai-yan, YOU Yu-pin, CHEN Zhi-bao. Inotodiol Prevention of Aflatoxin B1-induced Liver Injury by Activating Nrf2/PGC-1α/Mitophagy[J]. Biotechnology Bulletin, 2026, 42(1): 338-351.
| 基因 Gene | 引物信息 Primer sequence | 产物长度 Product length (bp) | 基因序号 Gene ID |
|---|---|---|---|
| Nrf2 | F: GTGCTGCCAGAGGTCCTTAATGC R:CAGGAACAGTGAGGTGCCAGTAAC | 113 | NM_013693.1 |
| TNF⁃α | F: CGCTCTTCTGTCTACTGAACTTCGG R: GTGGTTTGTGAGTGTGAGGGTCTG | 113 | NM_013693.1 |
| IL-6 | F: CTTCTTGGGACTGATGCTGGTGAC R: TCTGTTGGGAGTGGTATCCTCTGTG | 91 | NM_012589.2 |
| IL-1β | F: CACTACAGGCTCCGAGATGAACAA R: TGTCGTTGCTTGGTTCTCCTTGTAC | 145 | NM_008361.4 |
| HO-1 | F: GGAAATCATCCCTTGCACGC R: TGTTTGAACTTGGTGGGGCT | 91 | NM_012589.2 |
| NQO1 | F: GGTGAGCTGAAGGACTCGAA R: GCTCAGGCGTCCTTCCTTAT | 115 | NM_008361.4 |
| p62 | F: AGGAGGAGACGATGACTGGACAC R: TTGGTCTGTAGGAGCCTGGTGAG | 125 | NM_001411994.1 |
| LC3 | F: CTGTAAGGAGGTGCAGCAGAT R: TGCTTCTCACCCTTGTAGCGTA | 96 | NM_009741.5 |
| PGC-1α | F: CGCTCTTCTGTCTACTGAACTTCGG R: GTGGTTTGTGAGTGTGAGGGTCTG | 135 | NM_001330751.2 |
| PINK1 | F: CACTACAGGCTCCGAGATGAACAC R: TGTCGTTGCTTGGTTCTCCTTGTAC | 141 | NM_032409.3 |
| Parkin | F: CGTG AGCGGCTGCTTGTCTG R: ATGGTGAGCGAGGCGGTGAG | 124 | NM_168884.2 |
| CYP450 | F: TTTGCCACCTTCTGTCTCTTGTCAC R: AGTGTCGCCAGTGTTCTTAACCAT | 136 | NM_001327275.2 |
| β⁃actin | F: TATGCTCTCCCTCACGCCATCC R: GTCACGCACGATTTCCCTCTCAG | 129 | NM_011577.2 |
Table 1 Sequences of primers used for RT-qPCR
| 基因 Gene | 引物信息 Primer sequence | 产物长度 Product length (bp) | 基因序号 Gene ID |
|---|---|---|---|
| Nrf2 | F: GTGCTGCCAGAGGTCCTTAATGC R:CAGGAACAGTGAGGTGCCAGTAAC | 113 | NM_013693.1 |
| TNF⁃α | F: CGCTCTTCTGTCTACTGAACTTCGG R: GTGGTTTGTGAGTGTGAGGGTCTG | 113 | NM_013693.1 |
| IL-6 | F: CTTCTTGGGACTGATGCTGGTGAC R: TCTGTTGGGAGTGGTATCCTCTGTG | 91 | NM_012589.2 |
| IL-1β | F: CACTACAGGCTCCGAGATGAACAA R: TGTCGTTGCTTGGTTCTCCTTGTAC | 145 | NM_008361.4 |
| HO-1 | F: GGAAATCATCCCTTGCACGC R: TGTTTGAACTTGGTGGGGCT | 91 | NM_012589.2 |
| NQO1 | F: GGTGAGCTGAAGGACTCGAA R: GCTCAGGCGTCCTTCCTTAT | 115 | NM_008361.4 |
| p62 | F: AGGAGGAGACGATGACTGGACAC R: TTGGTCTGTAGGAGCCTGGTGAG | 125 | NM_001411994.1 |
| LC3 | F: CTGTAAGGAGGTGCAGCAGAT R: TGCTTCTCACCCTTGTAGCGTA | 96 | NM_009741.5 |
| PGC-1α | F: CGCTCTTCTGTCTACTGAACTTCGG R: GTGGTTTGTGAGTGTGAGGGTCTG | 135 | NM_001330751.2 |
| PINK1 | F: CACTACAGGCTCCGAGATGAACAC R: TGTCGTTGCTTGGTTCTCCTTGTAC | 141 | NM_032409.3 |
| Parkin | F: CGTG AGCGGCTGCTTGTCTG R: ATGGTGAGCGAGGCGGTGAG | 124 | NM_168884.2 |
| CYP450 | F: TTTGCCACCTTCTGTCTCTTGTCAC R: AGTGTCGCCAGTGTTCTTAACCAT | 136 | NM_001327275.2 |
| β⁃actin | F: TATGCTCTCCCTCACGCCATCC R: GTCACGCACGATTTCCCTCTCAG | 129 | NM_011577.2 |
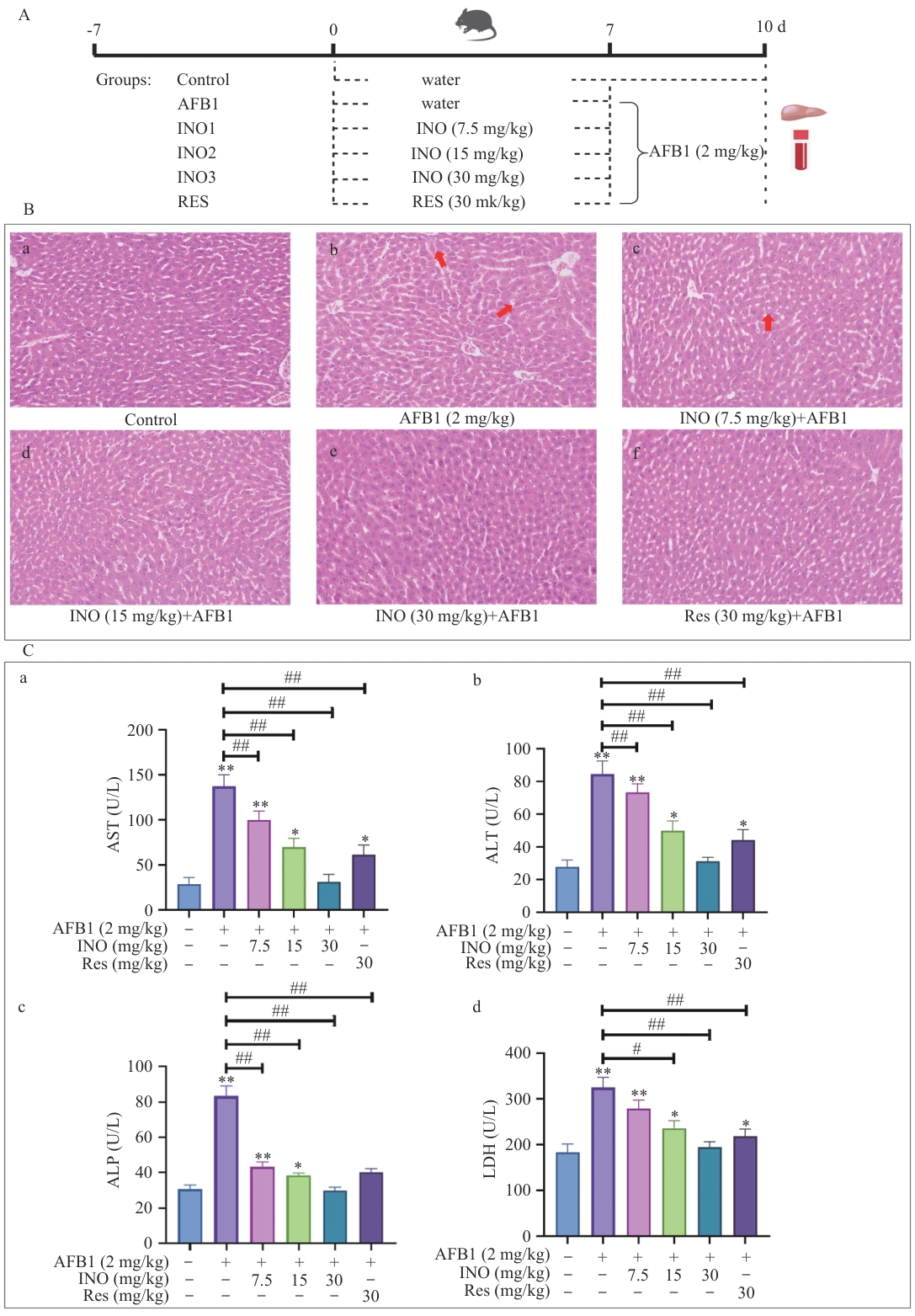
Fig. 1 INO protects liver of mice from AFB1-induced injuryA: Protocol of the animal experiments. B: Histological sectioning and H&E staining of mouse livers. C: Enzymatic activity of AST, ALT, ALP, and LDH in mouse serum was detected using a commercial kit. The red arrow indicates the hepatic sinusoidal dilatation. The bar in the B is 50 μm. Compared with the control group: *P<0.05; **P<0.01. Compared with the AFB1 group: #, P<0.05; ##, P<0.01. The same below
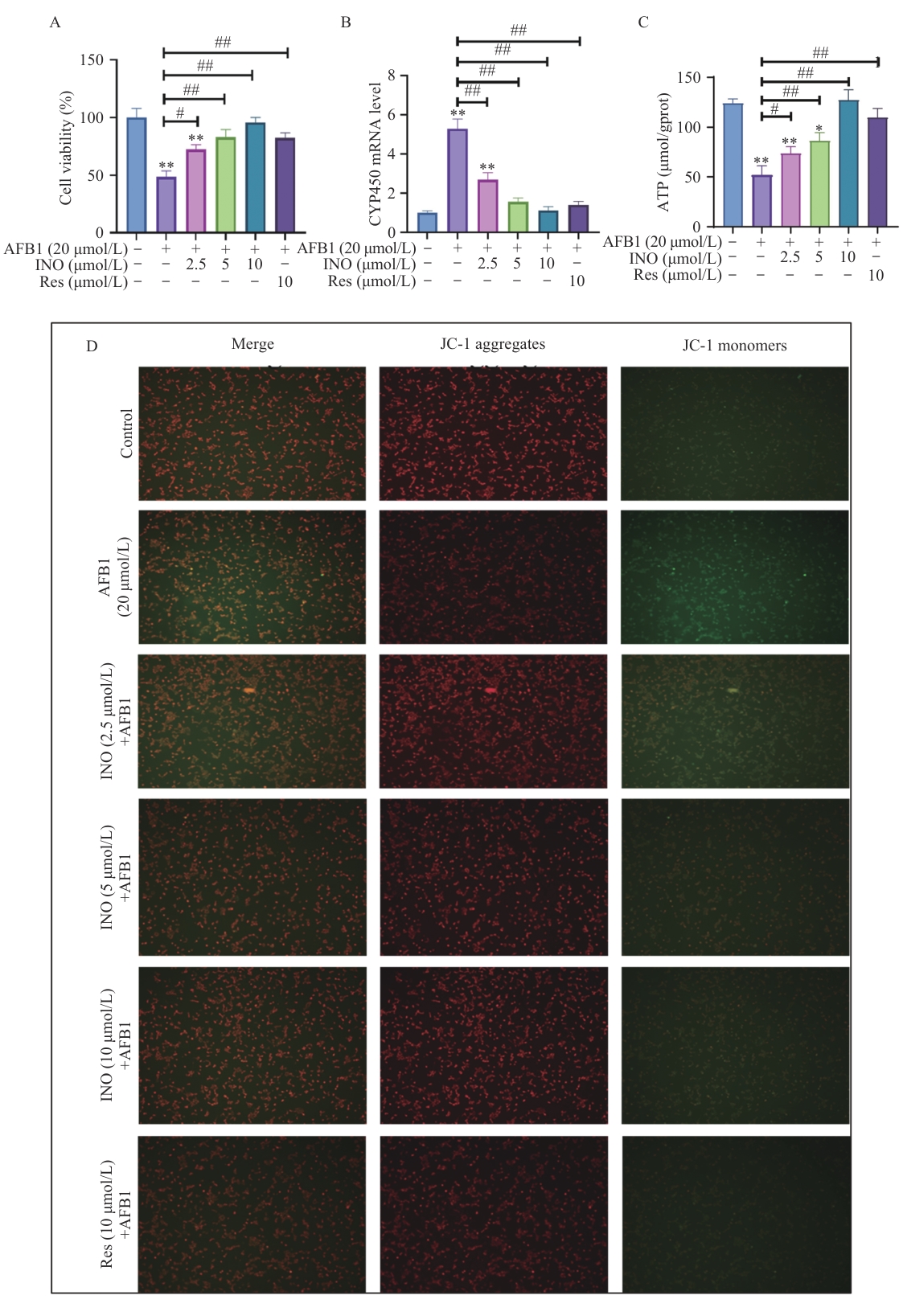
Fig. 3 INO prevents AFB1-induced AML12 cell viability decreases and mitochondrial function impairmentA: AML12 cell viability detected by CCK8. B: CYP450 gene expression detected using qPCR. C: ATP content. D: Mitochondrial membrane potential

Fig. 4 INO inhibits AFB1-induced oxidative stress in AML12 cellsA: Enzymatic activity of AST and ALT. B: Detection of oxidative-stress-related proteins(a: Statistical results of fluorescence intensity for ROS according to Fig.1-C. b and c: GSH and MDA content. d: Enzymatic activity of SOD). C: The ROS in AML12 cells. Compared with the control group: *, P<0.05; **, P<0.01. Compared with the AFB1 group: #, P<0.05; ##, P<0.01
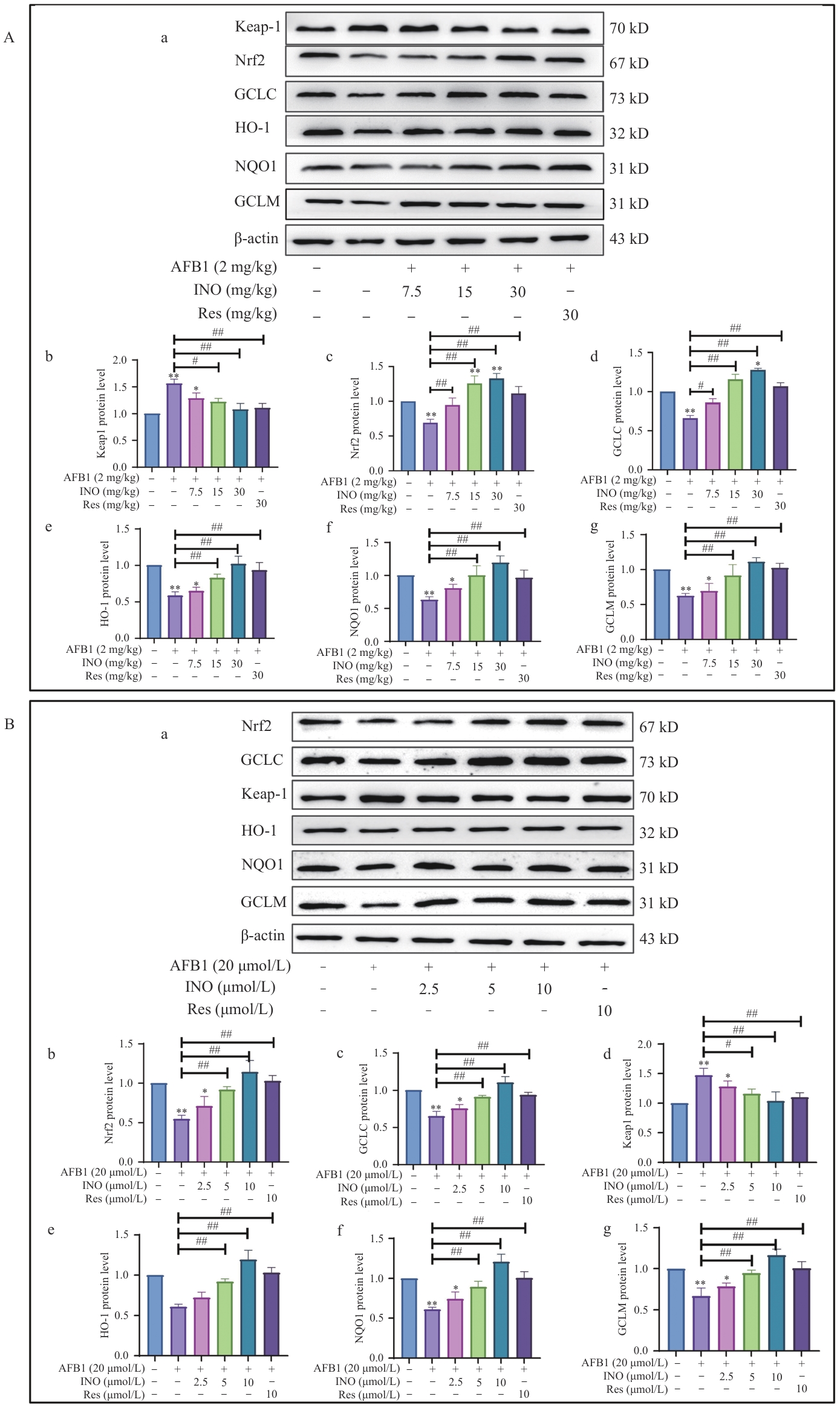
Fig. 5 INO activates the Keap1/Nrf2 antioxidant signal pathway both in vivo and in vitroA: INO activates the Keap1/Nrf2 antioxidant signal pathway in mouse livers. B: INO activates the Keap1/Nrf2 antioxidant signal pathway in AML12 cells
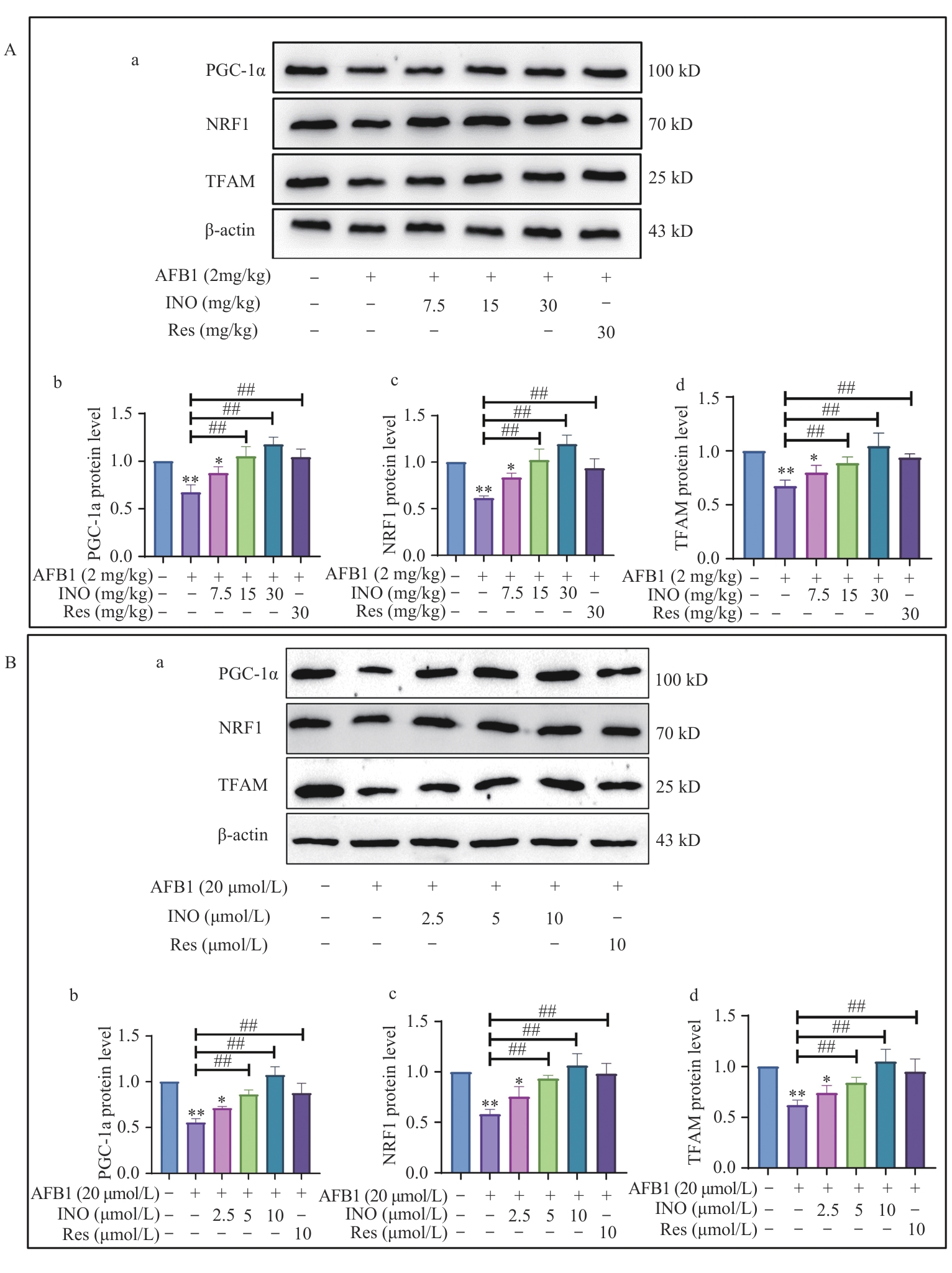
Fig. 6 INO activates the PGC-1α/NRF1 signaling pathway both in vivo and in vitroA: INO activates the PGC-1α/NRF1 signaling pathway in mouse livers. B: INO activates the PGC-1α/NRF1 antioxidant signal pathway in AML12 cells
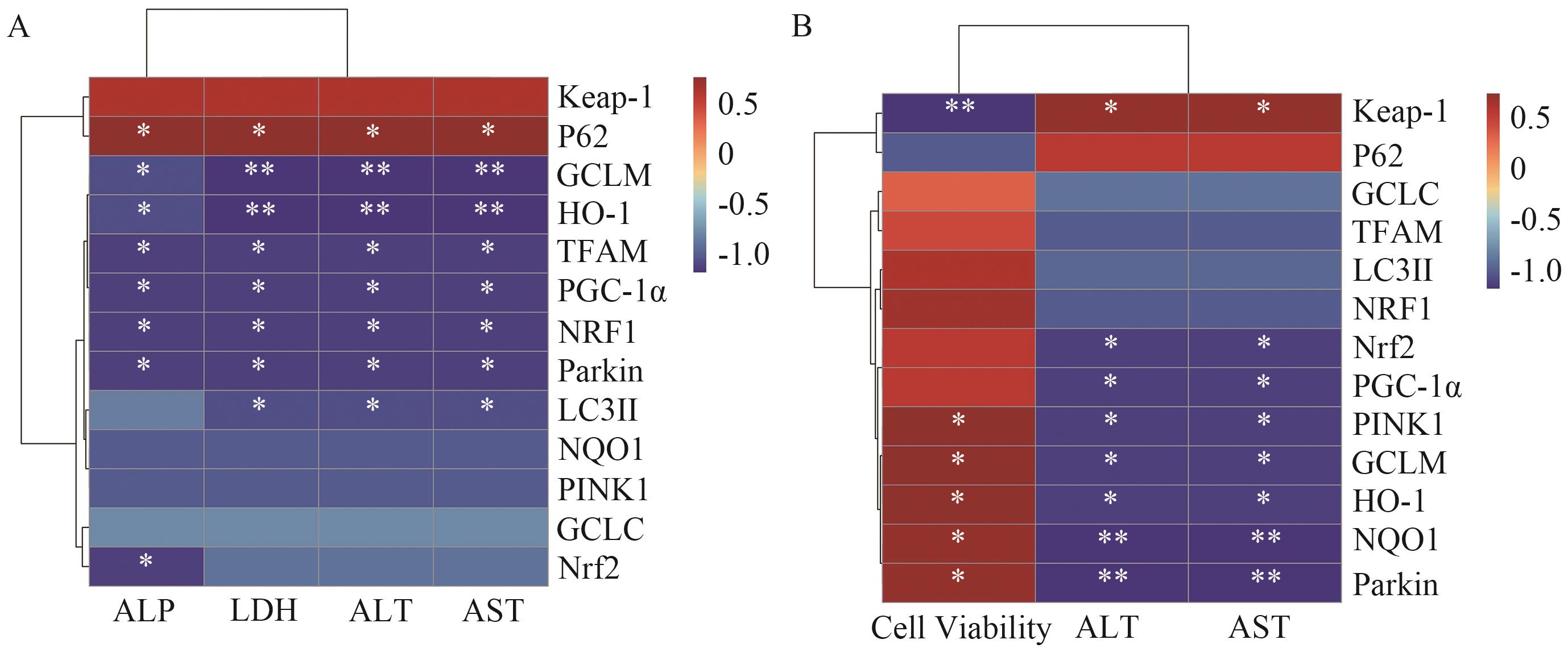
Fig. 8 Correlation analysis between liver function index and signaling pathway proteins in miceA: Correlation analysis of liver function indices and expressions of signaling pathway proteins in mice. B: Correlation analysis of liver function indices and signaling pathway proteins in AML12 cells of mice
| [1] | 黄年来. 俄罗斯神秘的民间药用真菌——桦褐孔菌 [J]. 中国食用菌, 2002, 21(4): 7-8. |
| Huang NL. A mysterious folk medicinal A mysterious folk medicinal fungus in Russia—Inonotus obliquus [J]. Edible Fungi China, 2002, 21(4): 7-8. | |
| [2] | Zhao FQ, Mai QQ, Ma JH, et al. Triterpenoids from Inonotus obliquus and their antitumor activities [J]. Fitoterapia, 2015, 101: 34-40. |
| [3] | Yu SC, Lai ZX, Xue HM, et al. Inonotus obliquus aqueous extract inhibits intestinal inflammation and insulin metabolism defects in Drosophila [J]. Toxicol Mech Meth, 2024, 34(9): 970-984. |
| [4] | Endo T, Nakagomi Y, Kawaguchi E, et al. Anti-malarial activity in a Chinese herbal supplement containing Inonotus obliquus and Panax notoginseng [J]. Parasitol Int, 2022, 87: 102532. |
| [5] | 朱彦彬. 桦褐孔菌醇介导NF-κB/Nrf2通路促进自噬抑制LPS诱导小鼠肠损伤 [D]. 湛江: 广东海洋大学, 2023. |
| Zhu YB. Inotodiol promotes autophagy and inhibits LPS-induced intestinal injury in mice by regulating NF-κB/Nrf2 signal pathway [D]. Zhanjiang: Guangdong Ocean University, 2023. | |
| [6] | Marchese S, Polo A, Ariano A, et al. Aflatoxin B1 and M1: biological properties and their involvement in cancer development [J]. Toxins, 2018, 10(6): 214. |
| [7] | Gizachew D, Chang CH, Szonyi B, et al. Aflatoxin B1 (AFB1) production by Aspergillus flavus and Aspergillus parasiticus on ground Nyjer seeds: The effect of water activity and temperature [J]. Int J Food Microbiol, 2019, 296: 8-13. |
| [8] | Valencia-Quintana R, Milić M, Jakšić D, et al. Environment changes, aflatoxins, and health issues, a review [J]. Int J Environ Res Public Health, 2020, 17(21): 7850. |
| [9] | Qiao BX, He Y, Gao XL, et al. Curcumin attenuates AFB1-induced duck liver injury by inhibiting oxidative stress and lysosomal damage [J]. Food Chem Toxicol, 2023, 172: 113593. |
| [10] | Qiu Z, Wang HY, Li GQ, et al. Lactobacillus salivarius Ameliorates AFB1-induced hepatotoxicity via PINK1/Parkin-mediated mitophagy in Geese [J]. Ecotoxicol Environ Saf, 2024, 280: 116574. |
| [11] | Liu HY, He Y, Gao XL, et al. Curcumin alleviates AFB1-induced nephrotoxicity in ducks: regulating mitochondrial oxidative stress, ferritinophagy, and ferroptosis [J]. Mycotoxin Res, 2023, 39(4): 437-451. |
| [12] | Cook KL, Clarke PAG, Parmar J, et al. Knockdown of estrogen receptor-α induces autophagy and inhibits antiestrogen-mediated unfolded protein response activation, promoting ROS-induced breast cancer cell death [J]. FASEB J, 2014, 28(9): 3891-3905. |
| [13] | Jiang Y, Chen DK, Gong QM, et al. Elucidation of SIRT-1/PGC-1α-associated mitochondrial dysfunction and autophagy in nonalcoholic fatty liver disease [J]. Lipds Health Dis, 2021, 20(1): 40. |
| [14] | Gan ZY, Callegari S, Cobbold SA, et al. Activation mechanism of PINK1 [J]. Nature, 2022, 602(7896): 328-335. |
| [15] | Dagda RK, Cherra SJ, Kulich SM, et al. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission [J]. J Biol Chem, 2009, 284(20): 13843-13855. |
| [16] | Chen XX, Che CP, Korolchuk VI, et al. Selenomethionine alleviates AFB1-induced damage in primary chicken hepatocytes by inhibiting CYP450 1A5 expression via upregulated SelW expression [J]. J Agric Food Chem, 2017, 65(12): 2495-2502. |
| [17] | Liu FJ, Wang YJ, Zhou X, et al. Resveratrol relieved acute liver damage in ducks (Anas platyrhynchos) induced by AFB1 via modulation of apoptosis and Nrf2 signaling pathways [J]. Animals, 2021, 11(12): 3516. |
| [18] | 魏艳梅, 陈惠琴, 杨理, 等. 桦褐孔菌化学成分的胆碱酯酶抑制和细胞毒活性研究 [J]. 天然产物研究与开发, 2020, 32(7): 1156-1163. |
| Wei YM, Chen HQ, Yang L, et al. Cholinesterase inhibitory and cytotoxic activity of chemical constituents from Inonotus obliquus [J]. Nat Prod Res Dev, 2020, 32(7): 1156-1163. | |
| [19] | Rotimi OA, Rotimi SO, Goodrich JM, et al. Time-course effects of acute aflatoxin B1 exposure on hepatic mitochondrial lipids and oxidative stress in rats [J]. Front Pharmacol, 2019, 10: 467. |
| [20] | Jiang XX, Liu HY, You YL, et al. Multi-omics reveals the protective effects of curcumin against AFB1-induced oxidative stress and inflammatory damage in duckling intestines [J]. Comp Biochem Physiol Part C Toxicol Pharmacol, 2024, 276: 109815. |
| [21] | He F, Ru XL, Wen T. NRF2, a transcription factor for stress response and beyond [J]. Int J Mol Sci, 2020, 21(13): 4777. |
| [22] | Bellezza I, Giambanco I, Minelli A, et al. Nrf2-Keap1 signaling in oxidative and reductive stress [J]. Biochim Biophys Acta Mol Cell Res, 2018, 1865(5): 721-733. |
| [23] | Taguchi K, Takaku M, Egner PA, et al. Generation of a new model rat: Nrf2 knockout rats are sensitive to aflatoxin B1 toxicity [J]. Toxicol Sci, 2016, 152(1): 40-52. |
| [24] | Li HT, Sang R, Zhao X, et al. Research Note: Taraxasterol alleviates aflatoxin B1-induced oxidative stress in chicken primary hepatocytes [J]. Poult Sci, 2023, 102(1): 102286. |
| [25] | Zhao YX, Zheng WF. Deciphering the antitumoral potential of the bioactive metabolites from medicinal mushroom Inonotus obliquus [J]. J Ethnopharmacol, 2021, 265: 113321. |
| [26] | Kou RW, Xia B, Han R, et al. Neuroprotective effects of a new triterpenoid from edible mushroom on oxidative stress and apoptosis through the BDNF/TrkB/ERK/CREB and Nrf2 signaling pathway in vitro and in vivo [J]. Food Funct, 2022, 13(23): 12121-12134. |
| [27] | Deng J, Zhao L, Zhang NY, et al. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents [J]. Mutat Res, 2018, 778: 79-89. |
| [28] | Ghallab A, Hassan R, Myllys M, et al. Subcellular spatio-temporal intravital kinetics of aflatoxin B1 and ochratoxin A in liver and kidney [J]. Arch Toxicol, 2021, 95(6): 2163-2177. |
| [29] | Qian X, Li XJ, Shi ZM, et al. KDM3A senses oxygen availability to regulate PGC-1α-mediated mitochondrial biogenesis [J]. Mol Cell, 2019, 76(6): 885-895.e7. |
| [30] | Wang Y, Chen XP, Baker JS, et al. Astaxanthin promotes mitochondrial biogenesis and antioxidant capacity in chronic high-intensity interval training [J]. Eur J Nutr, 2023, 62(3): 1453-1466. |
| [31] | Li PA, Hou XL, Hao SC. Mitochondrial biogenesis in neurodegeneration [J]. J Neurosci Res, 2017, 95(10): 2025-2029. |
| [32] | Xu FB, Li YF, Cao Z, et al. AFB1-induced mice liver injury involves mitochondrial dysfunction mediated by mitochondrial biogenesis inhibition [J]. Ecotoxicol Environ Saf, 2021, 216: 112213. |
| [33] | Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs [J]. Cell Death Differ, 2015, 22(3): 377-388. |
| [34] | Bartolini D, Dallaglio K, Torquato P, et al. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma [J]. Transl Res, 2018, 193: 54-71. |
| [35] | Ichimura Y, Waguri S, Sou YS, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy [J]. Mol Cell, 2013, 51(5): 618-631. |
| [36] | Connelly EM, Frankel KS, Shaw GS. Parkin and mitochondrial signalling [J]. Cell Signal, 2023, 106: 110631. |
| [37] | Yamada T, Dawson TM, Yanagawa T, et al. SQSTM1/p62 promotes mitochondrial ubiquitination independently of PINK1 and PRKN/parkin in mitophagy [J]. Autophagy, 2019, 15(11): 2012-2018. |
| [38] | Robertson I, Wai Hau T, Sami F, et al. The science of resveratrol, formulation, pharmacokinetic barriers and its chemotherapeutic potential [J]. Int J Pharm, 2022, 618: 121605. |
| [1] | LI Wei-hua, WU Jing, JIN Xue-qin, LEI Yan-li. Exploring the Relative Differential Protein Expression of Carbon Tetrachloride-induced Acute Liver Injury in Mice Based on the Proteomics Method [J]. Biotechnology Bulletin, 2025, 41(2): 331-342. |
| [2] | YANG Wei, GUAN Hai-feng, REN Xin-hui, PENG Jin-ju, CHEN Zhi-bao. Alleviating Effect of Astaxanthin on Liver Injury Induced by Aflatoxin B 1 and Its Mechanism [J]. Biotechnology Bulletin, 2025, 41(10): 334-342. |
| [3] | WU Shuang, LU Rui-lin, FENG Cheng-tian, YUAN Kun, WANG Zhen-hui, LIU Jin-ping, LIU Hui. Expression of HbTRXh5 Gene of Hevea brasiliensis in Yeast and Analysis on Its Resistance to Stress [J]. Biotechnology Bulletin, 2024, 40(12): 136-144. |
| [4] | KANG Ling-yun, HAN Lu-lu, HAN De-ping, CHEN Jian-sheng, GAN Han-ling, XING Kai, MA You-ji, CUI Kai. Effect of Melatonin on Protecting the Jejunum Mucosal Epithelial Cells from Oxidative Stress Damage [J]. Biotechnology Bulletin, 2023, 39(9): 291-299. |
| [5] | WANG Shuai, FENG Yu-mei, BAI Miao, DU Wei-jun, YUE Ai-qin. Functional Analysis of Soybean Gene GmHMGR Responding to Exogenous Hormones and Abiotic Stresses [J]. Biotechnology Bulletin, 2023, 39(7): 131-142. |
| [6] | WANG Qi, HU Zhe, FU Wei, LI Guang-zhe, HAO Lin. Regulation of Burkholderia sp. GD17 on the Drought Tolerance of Cucumber Seedlings [J]. Biotechnology Bulletin, 2023, 39(3): 163-175. |
| [7] | ZHU Ye-sheng, WU Guo-qiang, WEI Ming. Roles of Plasma Membrane Na+/H+ Antiporter SOS1 in Maintaining Ionic Homeostasis of Plants [J]. Biotechnology Bulletin, 2023, 39(12): 16-32. |
| [8] | YAN Xiong-ying, WANG Zhen, WANG Xia, YANG Shi-hui. Microbial Sulfur Metabolism and Stress Resistance [J]. Biotechnology Bulletin, 2023, 39(11): 150-167. |
| [9] | ZHOU Heng, XIE Yan-jie. Recent Progress in Oxidative Stress Signaling and Response in Plants [J]. Biotechnology Bulletin, 2023, 39(11): 36-43. |
| [10] | GAO Xiao-rong, DING Yao, LV Jun. Effects of Pseudomonas sp. PR3,a Pyrene-degrading Bacterium with Plant Growth-promoting Properties,on Rice Growth Under Pyrene Stress [J]. Biotechnology Bulletin, 2022, 38(9): 226-236. |
| [11] | XUE Xian-li, WANG Jing-ran, BI Hang-hang, WANG De-pei. Effect of Spt7 Overexpression of on the Growth and Stress Resistance of Aspergillus niger [J]. Biotechnology Bulletin, 2022, 38(5): 112-122. |
| [12] | ZU Guo-qiang, HU Zhe, WANG Qi, LI Guang-zhe, HAO Lin. Regulatory Role of Burkholderia sp. GD17 in Rice Seedling’s Responses to Cadmium Stress [J]. Biotechnology Bulletin, 2022, 38(4): 153-162. |
| [13] | ZHANG Xiao-ni, WENG Yi-chun, FAN Yi-hao, WANG Xiao-juan, ZHAO Jia-yu, ZHANG Yun-long. Mito-OS-Timer:A Targeted Fluorescent Stopwatch for Monitoring Mitochondrial Oxidative Stress [J]. Biotechnology Bulletin, 2022, 38(10): 97-105. |
| [14] | YANG Li, WANG Bo, LI Wen-jiao, WANG Xing-jun, ZHAO Shu-zhen. Research Progress on Production,Scavenging and Signal Transduction of ROS Under Drought Stress [J]. Biotechnology Bulletin, 2021, 37(4): 194-203. |
| [15] | PENG Wen-chao, LIU Jian-xin, WANG Di-ming. Research Progress on Metabolic Causes for Hypoxic Stress in Mammalian Animals [J]. Biotechnology Bulletin, 2021, 37(1): 262-271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||