








生物技术通报 ›› 2021, Vol. 37 ›› Issue (8): 221-232.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1326
孙宝婷( ), 邱萌霞, 王子辰, 王梓源, 崔建东(
), 邱萌霞, 王子辰, 王梓源, 崔建东( ), 贾士儒
), 贾士儒
收稿日期:2020-10-28
出版日期:2021-08-26
发布日期:2021-09-10
作者简介:孙宝婷,女,博士研究生,研究方向:发酵工程;E-mail: 基金资助:
SUN Bao-ting( ), QIU Meng-xia, WANG Zi-chen, WANG Zi-yuan, CUI Jian-dong(
), QIU Meng-xia, WANG Zi-chen, WANG Zi-yuan, CUI Jian-dong( ), JIA Shi-ru
), JIA Shi-ru
Received:2020-10-28
Published:2021-08-26
Online:2021-09-10
摘要:
以共沉淀包埋的策略将酶固定在ZIF-8金属有机骨架中能显著提高酶的催化稳定性,但是,所制备的酶@ZIF-8固定化酶的固定化效率和酶活回收率却很低。为了解决这一问题,本研究以半胱氨酸(cysteine,Cys)为辅助剂,采用共沉淀包埋的策略将苯丙氨酸解氨酶(PAL)固定在ZIF-8载体中,制备出PAL@ZIF-8固定化酶。利用单因素实验及响应面实验优化了PAL@ZIF-8制备条件,并对其pH耐受性、温度耐受性和重复使用性等催化性能进行研究。响应面优化结果表明,2-甲基咪唑浓度、醋酸锌浓度和Cys浓度对酶活回收率有显著影响,醋酸锌浓度和Cys浓度对酶活回收率存在交互影响,在200 mmol/L 2-甲基咪唑、60 mmol/L醋酸锌和4 mg Cys,2 mg聚乙烯吡咯烷酮(polyvinylpyrrolidone,PVP)的优化条件下,PAL@ZIF-8最大酶活回收率为(56.15±0.94)%。与不添加Cys制备的PAL@ZIF-8相比,酶活回收率提高了近40%,而且PAL@ZIF-8比游离PAL表现出更好的温度耐受性和pH耐受性。重复使用7次后,PAL@ZIF-8仍能保持初始酶活的60%左右,表现出较好的重复使用稳定性。以上结果表明,这种以Cys为辅助剂将酶共沉淀包埋在ZIF-8中的方法是一种高效的酶固定化策略。
孙宝婷, 邱萌霞, 王子辰, 王梓源, 崔建东, 贾士儒. 半胱氨酸辅助的酶@ZIF-8固定化酶制备及其特性研究[J]. 生物技术通报, 2021, 37(8): 221-232.
SUN Bao-ting, QIU Meng-xia, WANG Zi-chen, WANG Zi-yuan, CUI Jian-dong, JIA Shi-ru. Preparation of @ZIF-8 Immobilized Enzyme by Using Cysteine as Auxiliary Reagent and Its Characterization[J]. Biotechnology Bulletin, 2021, 37(8): 221-232.
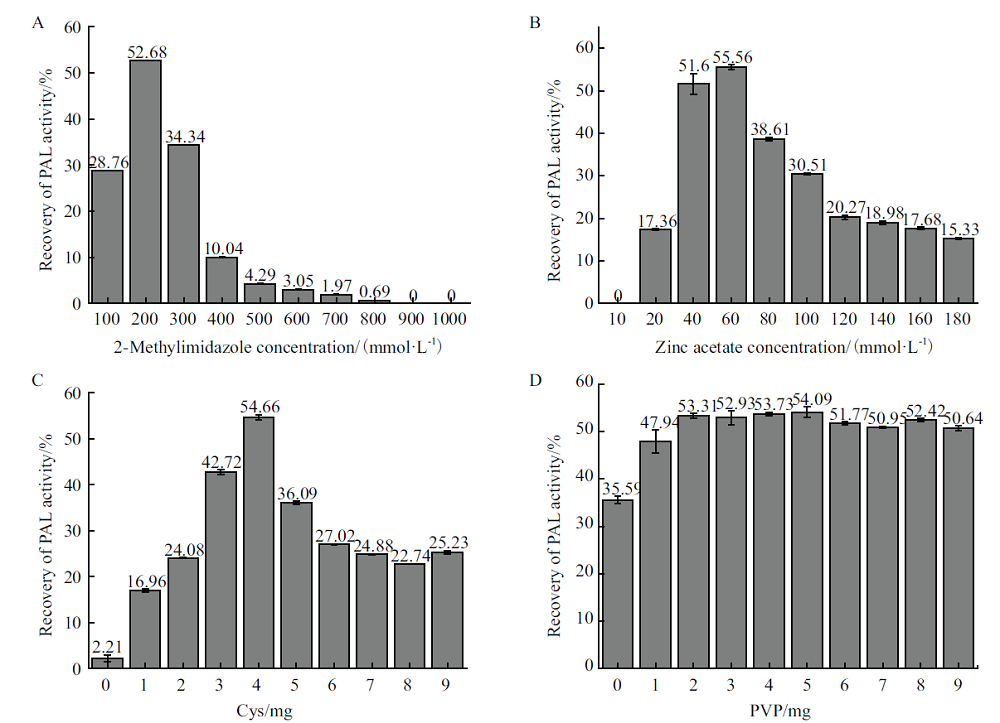
图3 各因素对PAL@ZIF-8酶活回收率的影响 A:2-甲基咪唑浓度对PAL@ZIF-8酶活回收率的影响;B:醋酸锌浓度对PAL@ZIF-8酶活回收率的影响;C:Cys对PAL@ZIF-8酶活回收率的影响;D:PVP对PAL@ZIF-8酶活回收率的影响
Fig.3 Influence of various factors on the recovery of PAL@ZIF-8 activity A: Effect of 2-methylimidazole concentration on the recovery of PAL@ZIF-8 activity. B: Effect of zinc acetate concentration on the recovery of PAL@ZIF-8 activity. C: Effect of addition of Cys on the recovery of PAL@ZIF-8 activity. D: Effect of addition of PVP on the recovery of PAL@ZIF-8 activity
| 编号 No. | 因素 Factor/(mmol·L-1) | 水平 Level | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| A | 2-甲基咪唑 | 100 | 200 | 300 |
| B | 醋酸锌 | 40 | 60 | 80 |
| C | Cys | 3 | 4 | 5 |
表1 响应面实验因素及水平表
Table 1 Test factors and level table of response surface
| 编号 No. | 因素 Factor/(mmol·L-1) | 水平 Level | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| A | 2-甲基咪唑 | 100 | 200 | 300 |
| B | 醋酸锌 | 40 | 60 | 80 |
| C | Cys | 3 | 4 | 5 |
| 编号 No. | A | B | C | 酶活回收率 Recovery of PAL activity /% |
|---|---|---|---|---|
| 1 | 200 | 60 | 4 | 55.21 |
| 2 | 300 | 80 | 4 | 23.86 |
| 3 | 200 | 40 | 5 | 34.83 |
| 4 | 200 | 60 | 4 | 55.47 |
| 5 | 200 | 80 | 3 | 30.15 |
| 6 | 100 | 80 | 4 | 19.98 |
| 7 | 300 | 60 | 3 | 26.83 |
| 8 | 100 | 60 | 5 | 18.52 |
| 9 | 100 | 40 | 4 | 26.71 |
| 10 | 200 | 80 | 5 | 25.49 |
| 11 | 200 | 60 | 4 | 55.81 |
| 12 | 200 | 60 | 4 | 55.97 |
| 13 | 300 | 60 | 5 | 22.67 |
| 14 | 200 | 60 | 4 | 55.62 |
| 15 | 200 | 40 | 3 | 40.32 |
| 16 | 300 | 40 | 4 | 31.89 |
| 17 | 100 | 60 | 3 | 20.47 |
表2 Box-Behnken实验设计及响应值
Table 2 Box-Behnken experimental design and response values
| 编号 No. | A | B | C | 酶活回收率 Recovery of PAL activity /% |
|---|---|---|---|---|
| 1 | 200 | 60 | 4 | 55.21 |
| 2 | 300 | 80 | 4 | 23.86 |
| 3 | 200 | 40 | 5 | 34.83 |
| 4 | 200 | 60 | 4 | 55.47 |
| 5 | 200 | 80 | 3 | 30.15 |
| 6 | 100 | 80 | 4 | 19.98 |
| 7 | 300 | 60 | 3 | 26.83 |
| 8 | 100 | 60 | 5 | 18.52 |
| 9 | 100 | 40 | 4 | 26.71 |
| 10 | 200 | 80 | 5 | 25.49 |
| 11 | 200 | 60 | 4 | 55.81 |
| 12 | 200 | 60 | 4 | 55.97 |
| 13 | 300 | 60 | 5 | 22.67 |
| 14 | 200 | 60 | 4 | 55.62 |
| 15 | 200 | 40 | 3 | 40.32 |
| 16 | 300 | 40 | 4 | 31.89 |
| 17 | 100 | 60 | 3 | 20.47 |
| 方差来源 Source | 平方和 Sum of squares | 自由度 Degree of freedom | 均方差 Mean square | F | P | 显著性 Significance |
|---|---|---|---|---|---|---|
| Model | 3983.91 | 9 | 442.66 | 161.95 | < 0.0001 | ** |
| A | 47.78 | 1 | 47.78 | 17.48 | 0.0041 | ** |
| B | 107.24 | 1 | 107.24 | 39.23 | 0.0004 | ** |
| C | 21.58 | 1 | 21.58 | 7.90 | 0.0261 | * |
| AB | 4.02 | 1 | 4.02 | 1.47 | 0.2646 | |
| AC | 14.59 | 1 | 14.59 | 5.34 | 0.0541 | |
| BC | 23.23 | 1 | 23.23 | 8.50 | 0.0225 | * |
| A2 | 2247.70 | 1 | 2247.70 | 822.32 | < 0.0001 | ** |
| B2 | 352.11 | 1 | 352.11 | 128.82 | < 0.0001 | ** |
| C2 | 832.62 | 1 | 832.62 | 304.61 | < 0.0001 | ** |
| 残差误差 | 19.13 | 7 | 2.73 | |||
| 失拟 | 15.65 | 3 | 5.22 | 6.00 | 0.0581 | |
| 纯误差 | 3.48 | 4 | 0.8702 | |||
| 合计 | 4003.04 | 16 |
表3 Box-Behnken实验设计方差分析表
Table 3 Analysis for regression equation of Box-Behnken design
| 方差来源 Source | 平方和 Sum of squares | 自由度 Degree of freedom | 均方差 Mean square | F | P | 显著性 Significance |
|---|---|---|---|---|---|---|
| Model | 3983.91 | 9 | 442.66 | 161.95 | < 0.0001 | ** |
| A | 47.78 | 1 | 47.78 | 17.48 | 0.0041 | ** |
| B | 107.24 | 1 | 107.24 | 39.23 | 0.0004 | ** |
| C | 21.58 | 1 | 21.58 | 7.90 | 0.0261 | * |
| AB | 4.02 | 1 | 4.02 | 1.47 | 0.2646 | |
| AC | 14.59 | 1 | 14.59 | 5.34 | 0.0541 | |
| BC | 23.23 | 1 | 23.23 | 8.50 | 0.0225 | * |
| A2 | 2247.70 | 1 | 2247.70 | 822.32 | < 0.0001 | ** |
| B2 | 352.11 | 1 | 352.11 | 128.82 | < 0.0001 | ** |
| C2 | 832.62 | 1 | 832.62 | 304.61 | < 0.0001 | ** |
| 残差误差 | 19.13 | 7 | 2.73 | |||
| 失拟 | 15.65 | 3 | 5.22 | 6.00 | 0.0581 | |
| 纯误差 | 3.48 | 4 | 0.8702 | |||
| 合计 | 4003.04 | 16 |
| Mean | 34.74 |
|---|---|
| R-square | 99.52% |
| Adj.R-square | 98.91% |
| Std. Dev. | 1.65 |
| CV | 4.76% |
表4 模型的可信度分析
Table 4 Reliability analysis of the model
| Mean | 34.74 |
|---|---|
| R-square | 99.52% |
| Adj.R-square | 98.91% |
| Std. Dev. | 1.65 |
| CV | 4.76% |

图4 各因素交互作用对苯丙氨酸解氨酶酶活回收率影响的响应面图和等高线图 a:Cys添加量对酶活回收率影响;b:醋酸锌浓度对酶活回收率影响;c:2-甲基咪唑浓度对酶活回收率影响
Fig.4 Response surface plot and contour plot of the effect of interaction of various factors on the recovery of PAL activity a:The effect of Cys addition on the recovery of PAL activity. b:Effect of zinc acetate concentration on the recovery of PAL activity. c:Effect of 2-methylimidazole concentration on the recovery of PAL activity

图5 PAL@ZIF-8扫描电镜图 A:传统ZIF-8扫描电镜图;B:Cys辅助合成PAL@ZIF-8扫描电镜图
Fig.5 Scanning electron microscope(SEM)of PAL@ZIF-8 A: SEM of traditional ZIF-8; B: SEM of cysteine-assisted PAL@ZIF-8
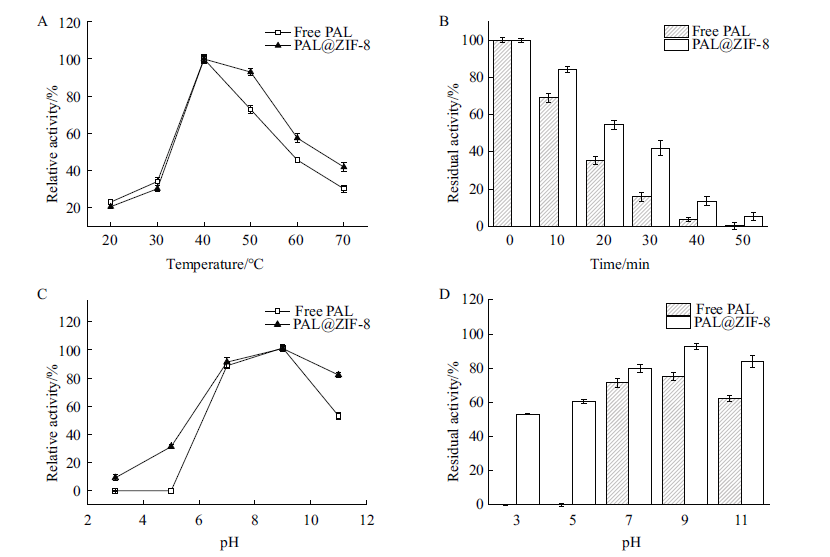
图8 PAL@ZIF-8的催化性能 A:最适催化温度;B:温度耐受性;C:最适催化pH;D:pH耐受性
Fig. 8 Catalytic performance of PAL@ZIF-8 A: Optimal temperature. B: Temperature tolerance. C: Optimal pH. D: pH tolerance
| Enzyme | Km | Vmax |
|---|---|---|
| PAL | 8.2756 ±0.67% | 0.0269 ±1.08% |
| PAL@ZIF-8 | 10.9602 ±0.58% | 0.0171±0.72% |
表5 PAL与PAL@ZIF-8的表观动力学常数
Table 5 Comparison of apparent kinetic parameters of free PAL and PAL@ZIF-8
| Enzyme | Km | Vmax |
|---|---|---|
| PAL | 8.2756 ±0.67% | 0.0269 ±1.08% |
| PAL@ZIF-8 | 10.9602 ±0.58% | 0.0171±0.72% |
| 分组 Group | 酶蛋白负载率 Enzyme load rate/% | 酶活回收率 Recovery of PAL activity/% |
|---|---|---|
| 传统PAL@ZIF-8 Traditional PAL@ZIF-8 | 82.11±0.27 | 13.38±0.12 |
| Cys辅助的PAL@ZIF-8 Cysteine-assisted PAL@ZIF-8 | 94.07±0.14 | 56.15±0.94 |
表6 负载率及酶活回收率对比
Table 6 Comparison of load rate and recovery of PAL activity
| 分组 Group | 酶蛋白负载率 Enzyme load rate/% | 酶活回收率 Recovery of PAL activity/% |
|---|---|---|
| 传统PAL@ZIF-8 Traditional PAL@ZIF-8 | 82.11±0.27 | 13.38±0.12 |
| Cys辅助的PAL@ZIF-8 Cysteine-assisted PAL@ZIF-8 | 94.07±0.14 | 56.15±0.94 |
| [1] |
Cui JD, Sun LM, Li LL. A simple technique of preparing stable CLEAs of phenylalanine ammonia lyase using co-aggregation with starch and bovine serum albumin[J]. Appl Biochem Biotechnol, 2013, 170(8):1827-1837.
doi: 10.1007/s12010-013-0317-9 URL |
| [2] |
Christensen RD, Henry E, Wiedmeier SE, et al. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease[J]. J Perinatol, 2007, 27(5):284-290.
pmid: 17344923 |
| [3] |
Westrup B. Newborn individualized developmental care and assessment program(NIDCAP)-family-centered developmentally supportive care[J]. Early Hum Dev, 2007, 83(7):443-449.
pmid: 17459617 |
| [4] |
Blau N, van Spronsen FJ, Levy HL. Phenylketonuria[J]. Lancet, 2010, 376(9750):1417-1427.
doi: 10.1016/S0140-6736(10)60961-0 URL |
| [5] |
Zhou HC, Kitagawa S. Metal-organic frameworks(MOFs)[J]. Chem Soc Rev, 2014, 43(16):5415-5418.
doi: 10.1039/C4CS90059F URL |
| [6] |
Meng J, Niu C, Xu L, et al. General oriented formation of carbon nanotubes from metal-organic frameworks[J]. J Am Chem Soc, 2017, 139(24):8212-8221.
doi: 10.1021/jacs.7b01942 URL |
| [7] |
Lin ZJ, Lü J, Hong MC, et al. Metal-organic frameworks based on flexible ligands(FL-MOFs):structures and applications[J]. Chem Soc Rev, 2014, 43(16):5867-5895.
doi: 10.1039/C3CS60483G URL |
| [8] |
Saliba D, Ammar M, Rammal M, et al. Crystal growth of ZIF-8, ZIF-67, and their mixed-metal derivatives[J]. J Am Chem Soc, 2018, 140(5):1812-1823.
doi: 10.1021/jacs.7b11589 URL |
| [9] | Yang X, Chen W, Bian H, et al. Synjournal of mesoporous ZIF-8 nanoribbons and their conversion into carbon nanoribbons for high-performance supercapacitors[J]. Chemistry, 2018, 24(43):11185-11192. |
| [10] |
Sun Q, Fu CW, Aguila B, et al. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks[J]. J Am Chem Soc, 2018, 140(3):984-992.
doi: 10.1021/jacs.7b10642 pmid: 29275637 |
| [11] |
Lei ZL, Liu JT, Lei L, et al. UiO-66-NH2@PMAA:A Hybrid Polymer-MOFs architecture for pectinase immobilization[J]. Industrial & Engineering Chemistry Research, 2018, 57(2):559-567.
doi: 10.1021/acs.iecr.7b03398 URL |
| [12] |
Li T, Qiu H, Liu N, et al. Construction of self-activated cascade metal-organic framework/enzyme hybrid nanoreactors as antibacterial agents[J]. Colloids Surf B Biointerfaces, 2020, 191:111001.
doi: 10.1016/j.colsurfb.2020.111001 URL |
| [13] |
Jeong GY, Ricco R, Liang K, et al. Bioactive MIL-88A framework hollow spheres via interfacial reaction in-droplet microfluidics for enzyme and nanoparticle encapsulation[J]. Chem Mater, 2015, 27(23):7903-7909.
doi: 10.1021/acs.chemmater.5b02847 URL |
| [14] |
Li P, Moon SY, Guelta MA, et al. Encapsulation of a nerve agent detoxifying enzyme by a mesoporous zirconium metal-organic framework engenders thermal and long-term stability[J]. J Am Chem Soc, 2016, 138(26):8052-8055.
doi: 10.1021/jacs.6b03673 URL |
| [15] |
Liang K, Coghlan CJ, Bell SG, et al. Enzyme encapsulation in zeolitic imidazolate frameworks:a comparison between controlled co-precipitation and biomimetic mineralisation[J]. Chem Commun, 2016, 52(3):473-476.
doi: 10.1039/C5CC07577G URL |
| [16] |
Lin KYA, Chen YC, Phattarapattamawong S. Efficient demulsification of oil-in-water emulsions using a zeolitic imidazolate framework:Adsorptive removal of oil droplets from water[J]. J Colloid Interface Sci, 2016, 478:97-106.
doi: 10.1016/j.jcis.2016.05.057 URL |
| [17] | Xu LH, Zhao DY, Yang L, et al. Improvement of the electro-optical properties of nematic liquid crystals by doping with ZIF-8 materials[J]. Acta Phys Chimica Sin, 2016, 32(9):2377-2382. |
| [18] |
Dai XQ, Hang YH, Zhao X, et al. ZIF-8 as an adsorbent of aqueous phase for Eu and Tb ions[J]. Micro Nano Lett, 2017, 12(3):187-190.
doi: 10.1049/mna2.v12.3 URL |
| [19] | Wang Q, Xiong SS, Xiang ZH, et al. Dynamic separation of Xe and Kr by metal-organic framework and covalent-organic materials:a comparison with activated charcoal[J]. Science China Chem, 2016(5):643-650. |
| [20] |
Huang DD, Xin QP, Ni YZ, et al. Synergistic effects of zeolite imidazole framework@graphene oxide composites in humidified mixed matrix membranes on CO2 separation[J]. RSC Adv, 2018, 8(11):6099-6109.
doi: 10.1039/C7RA09794H URL |
| [21] |
Yurderi M, Bulut A, Zahmakiran M, et al. Ruthenium(0) nanoparticles stabilized by metal-organic framework(ZIF-8):Highly efficient catalyst for the dehydrogenation of dimethylamine-borane and transfer hydrogenation of unsaturated hydrocarbons using dimethylamine-borane as hydrogen source[J]. Appl Catal B:Environ, 2014, 160/161:534-541.
doi: 10.1016/j.apcatb.2014.06.009 URL |
| [22] |
Zhang T, Li B, Zhang XF, et al. Pd nanoparticles immobilized in a microporous/mesoporous composite ZIF-8/MSS:a multifunctional catalyst for the hydrogenation of alkenes[J]. Microporous Mesoporous Mater, 2014, 197:324-330.
doi: 10.1016/j.micromeso.2014.07.002 URL |
| [23] |
Thanh MT, Thien TV, Du PD, et al. Iron doped zeolitic imidazolate framework(Fe-ZIF-8):synjournal and photocatalytic degradation of RDB dye in Fe-ZIF-8[J]. J Porous Mater, 2018, 25(3):857-869.
doi: 10.1007/s10934-017-0498-7 URL |
| [24] |
Wang X, Miao D, Liang X, et al. Nanocapsules engineered from polyhedral ZIF-8 templates for bone-targeted hydrophobic drug delivery[J]. Biomater Sci, 2017, 5(4):658-662.
doi: 10.1039/C6BM00915H URL |
| [25] |
Zheng CC, Wang Y, Phua SZF, et al. ZnO-DOX@ZIF-8 core-shell nanoparticles for pH-responsive drug delivery[J]. ACS Biomater Sci Eng, 2017, 3(10):2223-2229.
doi: 10.1021/acsbiomaterials.7b00435 URL |
| [26] |
Xiao YS, Huang W, Zhu DM, et al. Cancer cell membrane-camouflaged MOF nanoparticles for a potent dihydroartemisinin-based hepatocellular carcinoma therapy[J]. RSC Adv, 2020, 10(12):7194-7205.
doi: 10.1039/C9RA09233A URL |
| [27] |
Hu PP, Liu N, Wu KY, et al. Successive and specific detection of Hg2+ and i- by a DNA@MOF biosensor:experimental and simulation studies[J]. Inorg Chem, 2018, 57(14):8382-8389.
doi: 10.1021/acs.inorgchem.8b01051 URL |
| [28] |
Lian X, Fang Y, Joseph E, et al. Enzyme-MOF(metal-organic framework)composites[J]. Chem Soc Rev, 2017, 46(11):3386-3401.
doi: 10.1039/C7CS00058H URL |
| [29] |
Ran JY, Wang C, Zhang JJ, et al. New insight into Polydopamine@ZIF-8 nanohybrids:a zinc-releasing container for potential anticancer activity[J]. Polymers, 2018, 10(5):476.
doi: 10.3390/polym10050476 URL |
| [30] |
Chen GS, Huang SM, Kou XX, et al. A convenient and versatile amino-acid-boosted biomimetic strategy for the nondestructive encapsulation of biomacromolecules within metal-organic frameworks[J]. Angew Chem Int Ed, 2019, 58(5):1463-1467.
doi: 10.1002/anie.v58.5 URL |
| [31] |
Mohammad M, Razmjou A, Liang K, et al. Metal-organic-framework-based enzymatic microfluidic biosensor via surface patterning and biomineralization[J]. ACS Appl Mater Interfaces, 2019, 11(2):1807-1820.
doi: 10.1021/acsami.8b16837 URL |
| [32] |
Liang WB, Ricco R, Maddigan NK, et al. Control of structure topology and spatial distribution of biomacromolecules in Protein@ZIF-8 biocomposites[J]. Chem Mater, 2018, 30(3):1069-1077.
doi: 10.1021/acs.chemmater.7b04977 URL |
| [33] | Cui JD, Liu RL, Li LL. Imprinted cross-linked enzyme aggregate(iCLEA)of phenylalanine ammonia lyase:a new stable biocatalyst[M]// Advances in Applied Biotechnology. Heidelberg:Springer, 2015:223-231. |
| [34] |
Cui JD, Li LL, Bian HJ. Immobilization of cross-linked phenylalanine ammonia lyase aggregates in microporous silica gel[J]. PLoS One, 2013, 8(11):e80581.
doi: 10.1371/journal.pone.0080581 URL |
| [35] |
Chen WH, Luo GF, Vázquez-González M, et al. Glucose-responsive metal-organic-framework nanoparticles act as “smart” sense-and-treat carriers[J]. ACS Nano, 2018, 12(8):7538-7545.
doi: 10.1021/acsnano.8b03417 URL |
| [36] |
Feng Y, Zhong L, Hou Y, et al. Acid-resistant enzyme@MOF nanocomposites with mesoporous silica shells for enzymatic applications in acidic environments[J]. J Biotechnol, 2019, 306:54-61.
doi: 10.1016/j.jbiotec.2019.09.010 URL |
| [37] |
Peroza EA, Schmucki R, Güntert P, et al. The βE-domain of wheat ec-1 metallothionein:a metal-binding domain with a distinctive structure[J]. J Mol Biol, 2009, 387(1):207-218.
doi: 10.1016/j.jmb.2009.01.035 URL |
| [1] | 杜清洁, 周璐瑶, 杨思震, 张嘉欣, 陈春林, 李娟起, 李猛, 赵士文, 肖怀娟, 王吉庆. 过表达CaCP1提高转基因烟草对盐胁迫的敏感性[J]. 生物技术通报, 2023, 39(2): 172-182. |
| [2] | 尹国英, 刘畅, 常永春, 羽王洁, 王兵, 张盼, 郭玉双. 烟草半胱氨酸蛋白酶家族和相应miRNAs的鉴定及其对PVY的响应[J]. 生物技术通报, 2023, 39(10): 184-196. |
| [3] | 郝向阳, 刘范, 武欢, 王斌, 孙雪丽, 项蕾蕾, 王天池, 赖钟雄, 程春振. 非洲菊GjPAL的克隆及表达分析[J]. 生物技术通报, 2021, 37(6): 13-23. |
| [4] | 苏雨, 李宗芸, 韩永华. 植物液泡加工酶研究进展[J]. 生物技术通报, 2021, 37(6): 181-191. |
| [5] | 莫黎杰, 刘夏瞳, 李慧, 陆海. 植物半胱氨酸蛋白酶在植物生长发育中的功能研究[J]. 生物技术通报, 2021, 37(6): 202-212. |
| [6] | 邓寒梅, 邵可, 梁家豪, 陈烨同, 阎光绪. 漆酶的来源及固定化漆酶载体研究进展[J]. 生物技术通报, 2017, 33(6): 10-15. |
| [7] | 张礼, 孙堆, 王晓, 郑春丽. 半胱氨酸参与生物体重金属抗性的研究进展[J]. 生物技术通报, 2017, 33(5): 26-33. |
| [8] | 周文菲, 白娟, 龚春梅. 活性氧介导的植物蛋白质氧化修饰研究进展[J]. 生物技术通报, 2017, 33(4): 8-18. |
| [9] | 龙翔宇, 梁启福, 戚继艳, 方永军, 唐朝荣. 橡胶树半胱氨酸蛋白酶抑制剂HbCYS2的克隆与表达分析[J]. 生物技术通报, 2017, 33(3): 86-92. |
| [10] | 郭天璐, 张欢欢, 杜建中, 郝曜山, 王亦学, 孙毅. 大蒜半胱氨酸合成酶的mRNA表达及生物信息学分析[J]. 生物技术通报, 2016, 32(8): 96-102. |
| [11] | 邹同雷, 汪芳俊, 侯赛男, 孙雪, 徐年军. 两种水杨酸代谢相关酶在逆境龙须菜中的活性研究[J]. 生物技术通报, 2016, 32(5): 194-199. |
| [12] | 郑超, 李登高, 白薇. 植物富含半胱氨酸的类受体激酶的研究进展[J]. 生物技术通报, 2016, 32(11): 10-17. |
| [13] | 王德正, 吴辉, 李志敏, 叶勤. 重组大肠杆菌发酵生产谷胱甘肽的氨基酸添加策略优化[J]. 生物技术通报, 2015, 31(9): 197-203. |
| [14] | 邢朝晖, 苏跃龙, 张琦, 阮馨怡, 林燕, 王欣泽, 孔海南. 磁性纳米材料载体固定纤维素酶技术研究进展[J]. 生物技术通报, 2015, 31(8): 59-65. |
| [15] | 赵婧楠, 李志敏, 叶勤. Pseudomonas sp. F-12发酵优化及转化合成半胱氨酸的研究[J]. 生物技术通报, 2014, 0(10): 207-214. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||