








生物技术通报 ›› 2022, Vol. 38 ›› Issue (1): 228-235.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0448
李文姣1,2( ), 张忠峰3, 刘青4, 孙洁1,2, 杨利1,2, 王兴军1,2, 赵术珍1,2(
), 张忠峰3, 刘青4, 孙洁1,2, 杨利1,2, 王兴军1,2, 赵术珍1,2( )
)
收稿日期:2021-04-06
出版日期:2022-01-26
发布日期:2022-02-22
作者简介:李文姣,女,硕士研究生,研究方向:植物生理学;E-mail: 基金资助:
LI Wen-jiao1,2( ), ZHANG Zhong-feng3, LIU Qing4, SUN Jie1,2, YANG Li1,2, WANG Xing-jun1,2, ZHAO Shu-zhen1,2(
), ZHANG Zhong-feng3, LIU Qing4, SUN Jie1,2, YANG Li1,2, WANG Xing-jun1,2, ZHAO Shu-zhen1,2( )
)
Received:2021-04-06
Published:2022-01-26
Online:2022-02-22
摘要:
油菜素甾体(brassinosteroids,BRs)是植物界普遍存在的一类多羟基化的植物甾体激素,不仅调节植物的生长发育过程,还参与植物对生物和非生物胁迫的响应。概述了BRs的生物合成途径以及信号转导途径,重点阐述了BRs参与非生物胁迫应答的分子机制,展望了BRs未来的研究方向,为深入理解BRs介导的非生物胁迫调控网络、提高作物抵抗非生物胁迫的能力提供理论依据。
李文姣, 张忠峰, 刘青, 孙洁, 杨利, 王兴军, 赵术珍. BRs在植物响应非生物胁迫中的作用[J]. 生物技术通报, 2022, 38(1): 228-235.
LI Wen-jiao, ZHANG Zhong-feng, LIU Qing, SUN Jie, YANG Li, WANG Xing-jun, ZHAO Shu-zhen. Role of BRs in Plant Response to Abiotic Stress[J]. Biotechnology Bulletin, 2022, 38(1): 228-235.
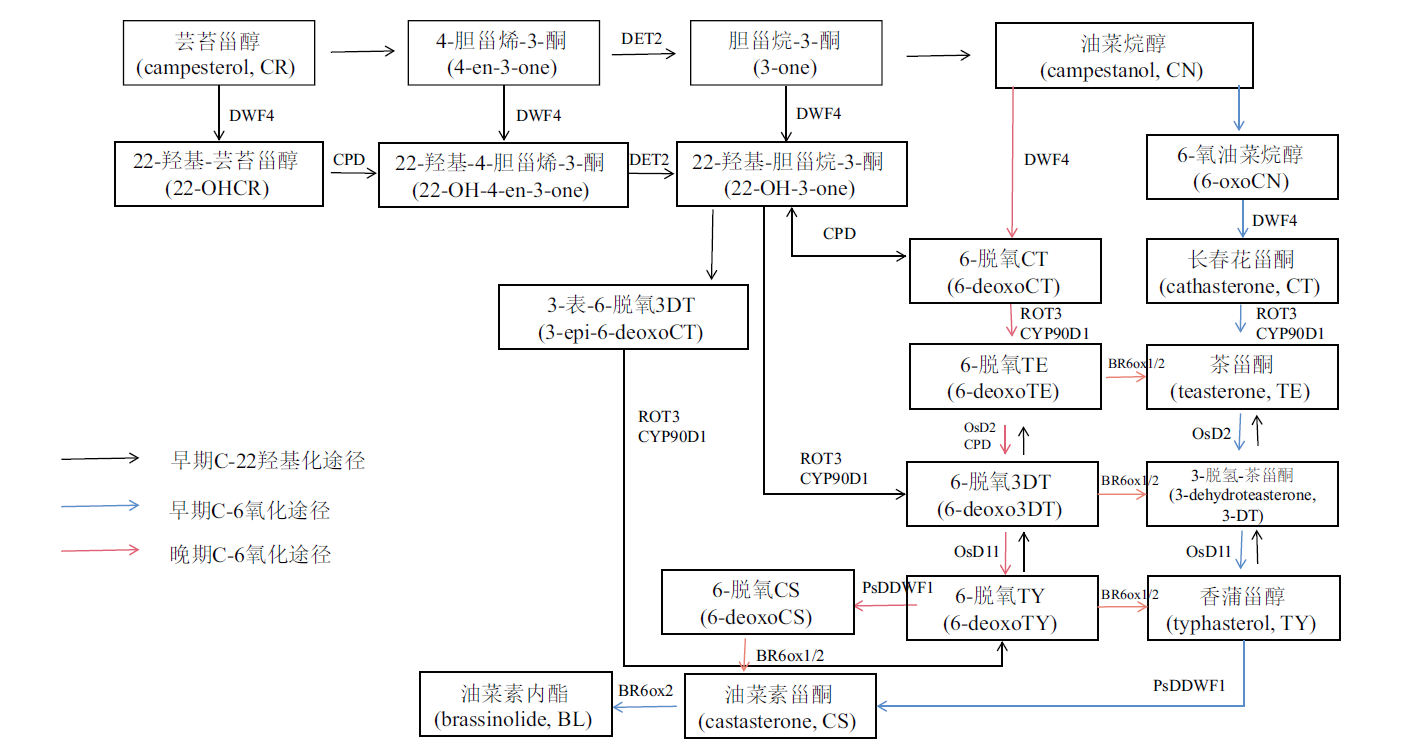
图1 BRs的生物合成途径模式图 BRs特有的前体芸苔甾醇(CR)可经过多条途径转化形成具有生物活性的CS和BL[6]。这些途径主要包括C-6位的早期C-6氧化途径、晚期C-6氧化途径以及C-22位的早期C-22羟基化途径,其中,DWF4是C-22位羟化酶,CPD是C-3位氧化酶,DET2是一种还原酶,ROT3与CYP90D1是C-23位羟化酶,PsDDWF1是C-2位羟化酶,BR6ox1和BR6ox2是拟南芥中的C-6位氧化酶,OsD2和OsD11分别是C-3位脱氢酶和C-3位羟化酶
Fig. 1 Biosynthetic pathway pattern of BRs[6,7] During BR biosynthesis,a unique precursor molecule known as brassinosterol(CR),which can be converted into the bioactive form of CS and BL via several pathways[6]. These pathways consist of the early C-6 oxidation pathway,the late C-6 oxidation pathway at C-6,as well as the early C-22 hydroxylation pathway at C-22. Amidst these pathways,DWF4 and CPD were identified as C-22 hydroxylase and C-3 oxidase respectively in Arabidopsis thaliana followed by ROT3 and CYP90D1 as C-23 hydroxylases,PsDDWF1 as C-2 hydroxylase,BR6ox1 and BR6ox2 as C-6 oxidases,and DET2 as the reductase. In addition,OsD2 and OsD11 were identified as the C-3 dehydrogenase and C-3 hydroxylase respectively

图2 BRs对非生物胁迫的应答 BRI1和BAK1分别是BRs的受体和共受体,BIN2是BRs信号通路中的负调节因子,BZR1和BES1为BRs信号转导通路的关键转录因子,通过与下游目的基因互作调控其表达;在植物遭受非生物胁迫时,可与许多其它转录因子协同拮抗作用,共同参与非生物胁迫应答
Fig. 2 Response of BRs to abiotic stress BR1 and BAK1 were identified as BR receptors and co-receptors,respectively. BIN2 was reported as the negative regulator during the signaling pathway BRs. Moreover,BZR1 and BES1 were identified as important transcription factors,which controlled their expression by interacting with downstream target genes in the BRs signaling pathway. Plants may be synergistically antagonized with other transcription factors to be involved in the abiotic stresses when they are exposed to various abiotic stresses
| [1] |
Mitchell JW, Mandava N, Worley JF, et al. Brassins——a new family of plant hormones from rape pollen[J]. Nature, 1970, 225(5237):1065-1066.
doi: 10.1038/2251065a0 URL |
| [2] | Mitchell JW, Gregory LE. Enhancement of overall plant growth, a new response to brassins[J]. Nat New Biol, 1972, 239(95):253-254. |
| [3] |
Grove MD, Spencer GF, Rohwedder WK, et al. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen[J]. Nature, 1979, 281(5728):216-217.
doi: 10.1038/281216a0 URL |
| [4] | Clouse SD. Brassinosteroids. Plant counterparts to animal steroid hormones?[J]. Vitam Horm, 2002, 65:195-223. |
| [5] |
Asami T, Yoshida S. Brassinosteroid biosynjournal inhibitors[J]. Trends Plant Sci, 1999, 4(9):348-353.
pmid: 10462767 |
| [6] | 任鸿雁, 王莉, 马青秀, 等. 油菜素内酯生物合成途径的研究进展[J]. 植物学报, 2015, 50(6):768-778. |
| Ren HY, Wang L, Ma QX, et al. Progress in biosynthetic pathways of brassinosteroids[J]. Chin Bull Bot, 2015, 50(6):768-778. | |
| [7] |
Zhao B, Li J. Regulation of brassinosteroid biosynjournal and inactivation[J]. J Integr Plant Biol, 2012, 54(10):746-759.
doi: 10.1111/jipb.2012.54.issue-10 URL |
| [8] |
Li JM, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction[J]. Cell, 1997, 90(5):929-938.
pmid: 9298904 |
| [9] |
Peng P, Yan Z, Zhu Y, et al. Regulation of the Arabidopsis GSK3-like kinase BRASSINOSTEROID-INSENSITIVE 2 through proteasome-mediated protein degradation[J]. Mol Plant, 2008, 1(2):338-346.
doi: 10.1093/mp/ssn001 pmid: 18726001 |
| [10] |
Wang ZY, Nakano T, Gendron J, et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynjournal[J]. Dev Cell, 2002, 2(4):505-513.
doi: 10.1016/S1534-5807(02)00153-3 URL |
| [11] | 肖瑞雪, 郭丽丽, 贾琦石, 等. 油菜素内酯调控植物生长发育及产量品质研究进展[J]. 江苏农业科学, 2019, 47(10):16-21. |
| Xiao RX, Guo LL, Jia QS, et al. Research progress on regulation of plant growth, yield and quality by brassinolide[J]. Jiangsu Agric Sci, 2019, 47(10):16-21. | |
| [12] |
Nolan TM, Vukašinović N, Liu D, et al. Brassinosteroids:multidimensional regulators of plant growth, development, and stress responses[J]. Plant Cell, 2020, 32(2):295-318.
doi: 10.1105/tpc.19.00335 URL |
| [13] | 齐琪, 马书荣, 徐维东. 盐胁迫对植物生长的影响及耐盐生理机制研究进展[J]. 分子植物育种, 2020, 18(8):2741-2746. |
| Qi Q, Ma SR, Xu WD. Advances in the effects of salt stress on plant growth and physiological mechanisms of salt tolerance[J]. Mol Plant Breed, 2020, 18(8):2741-2746. | |
| [14] |
Kim TW, Michniewicz M, Bergmann DC, et al. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway[J]. Nature, 2012, 482(7385):419-422.
doi: 10.1038/nature10794 URL |
| [15] |
Shang Y, Dai C, Lee MM, et al. BRI1-associated receptor kinase 1 regulates guard cell ABA signaling mediated by open stomata 1 in Arabidopsis[J]. Mol Plant, 2016, 9(3):447-460.
doi: S1674-2052(15)00467-0 pmid: 26724418 |
| [16] | Tao JJ, Chen HW, Ma B, et al. The role of ethylene in plants under salinity stress[J]. Front Plant Sci, 2015, 6:1059. |
| [17] |
Sun Y, Fan XY, Cao DM, et al. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis[J]. Dev Cell, 2010, 19(5):765-777.
doi: 10.1016/j.devcel.2010.10.010 pmid: 21074725 |
| [18] |
Zeng HT, Tang Q, Hua XJ. Arabidopsis brassinosteroid mutants det2-1 and bin2-1 display altered salt tolerance[J]. J Plant Growth Regul, 2010, 29(1):44-52.
doi: 10.1007/s00344-009-9111-x URL |
| [19] |
Cui F, Liu LJ, Zhao QZ, et al. Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance[J]. Plant Cell, 2012, 24(1):233-244.
doi: 10.1105/tpc.111.093062 URL |
| [20] | Li J, Zhou H, Zhang Y, et al. The GSK3-like kinase BIN2 is a molecular switch between the salt stress response and growth recovery in Arabidopsis thaliana[J]. Dev Cell, 2020, 55(3):367-380. e6. |
| [21] |
Jia C, Zhao S, Bao T, et al. Tomato BZR/BES transcription factor SlBZR1 positively regulates BR signaling and salt stress tolerance in tomato and Arabidopsis[J]. Plant Sci, 2021, 302:110719.
doi: 10.1016/j.plantsci.2020.110719 URL |
| [22] |
Srivastava M, Srivastava AK, Orosa-Puente B, et al. SUMO conjugation to BZR1 enables brassinosteroid signaling to integrate environmental cues to shape plant growth[J]. Curr Biol, 2021, 31(3):668-669.
doi: 10.1016/j.cub.2021.01.060 URL |
| [23] |
Zia R, Nawaz MS, Siddique MJ, et al. Plant survival under drought stress:Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation[J]. Microbiol Res, 2021, 242:126626.
doi: 10.1016/j.micres.2020.126626 URL |
| [24] |
Wang H, Tang J, Liu J, et al. Abscisic acid signaling inhibits brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2[J]. Mol Plant, 2018, 11(2):315-325.
doi: 10.1016/j.molp.2017.12.013 URL |
| [25] |
Cui XY, Gao Y, Guo J, et al. BES/BZR transcription factor TaBZR2 positively regulates drought responses by activation of TaGST1[J]. Plant Physiol, 2019, 180(1):605-620.
doi: 10.1104/pp.19.00100 URL |
| [26] | Chen J, Nolan TM, Ye H, et al. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses[J]. Plant Cell, 2017, 29(6):1425-1439. |
| [27] |
Xie Z, Nolan T, Jiang H, et al. The AP2/ERF transcription factor TINY modulates brassinosteroid-regulated plant growth and drought responses in Arabidopsis[J]. Plant Cell, 2019, 31(8):1788-1806.
doi: 10.1105/tpc.18.00918 URL |
| [28] |
Ye H, Liu S, Tang B, et al. RD26 mediates crosstalk between drought and brassinosteroid signalling pathways[J]. Nat Commun, 2017, 8:14573.
doi: 10.1038/ncomms14573 URL |
| [29] |
Jiang H, Tang B, Xie Z, et al. GSK3-like kinase BIN2 phosphorylates RD26 to potentiate drought signaling in Arabidopsis[J]. Plant J, 2019, 100(5):923-937.
doi: 10.1111/tpj.14484 |
| [30] |
Quint M, Delker C, Franklin KA, et al. Molecular and genetic control of plant thermomorphogenesis[J]. Nat Plants, 2016, 2:15190.
doi: 10.1038/nplants.2015.190 URL |
| [31] |
Ramirez VE, Poppenberger B. Modes of brassinosteroid activity in cold stress tolerance[J]. Front Plant Sci, 2020, 11:583666.
doi: 10.3389/fpls.2020.583666 URL |
| [32] |
Gilmour SJ, Zarka DG, Stockinger EJ, et al. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression[J]. Plant J, 1998, 16(4):433-442.
pmid: 9881163 |
| [33] |
Novillo F, Alonso JM, Ecker JR, et al. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis[J]. PNAS, 2004, 101(11):3985-3990.
doi: 10.1073/pnas.0303029101 URL |
| [34] | Ye K, Li H, Ding Y, et al. BRASSINOSTEROID-INSENSITIVE2 negatively regulates the stability of transcription factor ICE1 in response to cold stress in Arabidopsis[J]. Plant Cell, 2019, 31(11):2682-2696. |
| [35] |
Li H, Ye K, Shi Y, et al. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis[J]. Mol Plant, 2017, 10(4):545-559.
doi: 10.1016/j.molp.2017.01.004 URL |
| [36] |
Chi C, Li XM, Fang PP, et al. Brassinosteroids act as a positive regulator of NBR1-dependent selective autophagy in response to chilling stress in tomato[J]. J Exp Bot, 2020, 71(3):1092-1106.
doi: 10.1093/jxb/erz466 URL |
| [37] |
Hasanuzzaman M, Nahar K, Alam MM, et al. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants[J]. Int J Mol Sci, 2013, 14(5):9643-9684.
doi: 10.3390/ijms14059643 pmid: 23644891 |
| [38] |
Lamaoui M, Jemo M, Datla R, et al. Heat and drought stresses in crops and approaches for their mitigation[J]. Front Chem, 2018, 6:26.
doi: 10.3389/fchem.2018.00026 pmid: 29520357 |
| [39] |
Fariduddin Q, Yusuf M, Ahmad I, et al. Brassinosteroids and their role in response of plants to abiotic stresses[J]. Biol Plant, 2014, 58(1):9-17.
doi: 10.1007/s10535-013-0374-5 URL |
| [40] |
Zhang XW, Zhou LY, Qin YK, et al. A temperature-sensitive misfolded bri1-301 receptor requires its kinase activity to promote growth[J]. Plant Physiol, 2018, 178(4):1704-1719.
doi: 10.1104/pp.18.00452 URL |
| [41] |
Setsungnern A, Muñoz P, Pérez-Llorca M, et al. A defect in BRI1-EMS-SUPPRESSOR 1(bes1)-mediated brassinosteroid signaling increases photoinhibition and photo-oxidative stress during heat stress in Arabidopsis[J]. Plant Sci, 2020, 296:110470.
doi: S0168-9452(20)30072-8 pmid: 32540000 |
| [42] | Martínez C, Espinosa-Ruíz A, Lucas M, et al. PIF 4-induced BR synjournal is critical to diurnal and thermomorphogenic growth[J]. EMBO J, 2018, 37(23):e99552. |
| [43] | Ibañez C, Delker C, Martinez C, et al. Brassinosteroids dominate hormonal regulation of plant thermomorphogenesis via BZR1[J]. Curr Biol, 2018, 28(2):303-310. e3. |
| [44] |
Rajewska I, Talarek M, Bajguz A. Brassinosteroids and response of plants to heavy metals action[J]. Front Plant Sci, 2016, 7:629.
doi: 10.3389/fpls.2016.00629 pmid: 27242833 |
| [45] |
Betti C, Della Rovere F, Piacentini D, et al. Jasmonates, ethylene and brassinosteroids control adventitious and lateral rooting as stress avoidance responses to heavy metals and metalloids[J]. Biomolecules, 2021, 11(1):77.
doi: 10.3390/biom11010077 URL |
| [46] |
Hayat S, Alyemeni MN, Hasan SA. Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress[J]. Saudi J Biol Sci, 2012, 19(3):325-335.
doi: 10.1016/j.sjbs.2012.03.005 URL |
| [47] |
Allagulova CR, Maslennikova DR, Avalbaev AM, et al. Influence of 24-epibrassinolide on growth of wheat plants and the content of dehydrins under cadmium stress[J]. Russ J Plant Physiol, 2015, 62(4):465-471.
doi: 10.1134/S1021443715040020 URL |
| [48] |
Yusuf M, Fariduddin Q, Hayat S, et al. Protective response of 28-homobrassinolide in cultivars of Triticum aestivum with different levels of nickel[J]. Arch Environ Contam Toxicol, 2011, 60(1):68-76.
doi: 10.1007/s00244-010-9535-0 URL |
| [49] |
Wu C, Li F, Xu H, et al. The potential role of brassinosteroids (BRs)in alleviating antimony(Sb)stress in Arabidopsis thaliana[J]. Plant Physiol Biochem, 2019, 141:51-59.
doi: 10.1016/j.plaphy.2019.05.011 URL |
| [1] | 赵雪婷, 高利燕, 王俊刚, 沈庆庆, 张树珍, 李富生. 甘蔗AP2/ERF转录因子基因ShERF3的克隆、表达及其编码蛋白的定位[J]. 生物技术通报, 2023, 39(6): 208-216. |
| [2] | 李苑虹, 郭昱昊, 曹燕, 祝振洲, 王飞飞. 外源植物激素调控微藻生长及目标产物积累研究进展[J]. 生物技术通报, 2023, 39(6): 61-72. |
| [3] | 冯珊珊, 王璐, 周益, 王幼平, 方玉洁. WOX家族基因调控植物生长发育和非生物胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(5): 1-13. |
| [4] | 翟莹, 李铭杨, 张军, 赵旭, 于海伟, 李珊珊, 赵艳, 张梅娟, 孙天国. 异源表达大豆转录因子GmNF-YA19提高转基因烟草抗旱性[J]. 生物技术通报, 2023, 39(5): 224-232. |
| [5] | 姚姿婷, 曹雪颖, 肖雪, 李瑞芳, 韦小妹, 邹承武, 朱桂宁. 火龙果溃疡病菌实时荧光定量PCR内参基因的筛选[J]. 生物技术通报, 2023, 39(5): 92-102. |
| [6] | 杨春洪, 董璐, 陈林, 宋丽. 大豆VAS1基因家族的鉴定及参与侧根发育的研究[J]. 生物技术通报, 2023, 39(3): 133-142. |
| [7] | 崔俊美, 魏家萍, 董小云, 王莹, 郑国强, 刘自刚. PIP/PIPL:一类调控植物逆境响应和发育的植物内源性多肽[J]. 生物技术通报, 2023, 39(3): 35-42. |
| [8] | 苗淑楠, 高宇, 李昕儒, 蔡桂萍, 张飞, 薛金爱, 季春丽, 李润植. 大豆GmPDAT1参与油脂合成和非生物胁迫应答的功能分析[J]. 生物技术通报, 2023, 39(2): 96-106. |
| [9] | 许睿, 祝英方. 中介体复合物在植物非生物胁迫应答中的功能[J]. 生物技术通报, 2023, 39(11): 54-60. |
| [10] | 陈广霞, 李秀杰, 蒋锡龙, 单雷, 张志昌, 李勃. 植物小分子信号肽参与非生物逆境胁迫应答的研究进展[J]. 生物技术通报, 2023, 39(11): 61-73. |
| [11] | 孙雨桐, 刘德帅, 齐迅, 冯美, 黄栩筝, 姚文孔. 茉莉酸调控植物生长发育和胁迫的研究进展[J]. 生物技术通报, 2023, 39(11): 99-109. |
| [12] | 杨旭妍, 赵爽, 马天意, 白玉, 王玉书. 三个甘蓝WRKY基因的克隆及其对非生物胁迫的表达[J]. 生物技术通报, 2023, 39(11): 261-269. |
| [13] | 安昌, 陆琳, 沈梦千, 陈盛圳, 叶康卓, 秦源, 郑平. 植物bHLH基因家族研究进展及在药用植物中的应用前景[J]. 生物技术通报, 2023, 39(10): 1-16. |
| [14] | 位欣欣, 兰海燕. 植物MYB转录因子调控次生代谢及逆境响应的研究进展[J]. 生物技术通报, 2022, 38(8): 12-23. |
| [15] | 王慧, 马艺文, 乔正浩, 常彦彩, 术琨, 丁海萍, 聂永心, 潘光堂. AOX基因家族的结构和功能特征分析[J]. 生物技术通报, 2022, 38(7): 160-170. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||