








生物技术通报 ›› 2022, Vol. 38 ›› Issue (4): 86-96.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0185
• 作物品质遗传与改良专题(专题主编: 刘巧泉 教授) • 上一篇 下一篇
王荣花( ), 王树彬, 张志刚, 赵智中, 李巧云, 王立华, 刘栓桃
), 王树彬, 张志刚, 赵智中, 李巧云, 王立华, 刘栓桃
收稿日期:2022-02-15
出版日期:2022-04-26
发布日期:2022-05-06
作者简介:王荣花,女,博士,助理研究员,研究方向:大白菜分子遗传育种;E-mail: wangrh1101@163.com
基金资助:
WANG Rong-hua( ), WANG Shu-bin, ZHANG Zhi-gang, ZHAO Zhi-zhong, LI Qiao-yun, WANG Li-hua, LIU Shuan-tao
), WANG Shu-bin, ZHANG Zhi-gang, ZHAO Zhi-zhong, LI Qiao-yun, WANG Li-hua, LIU Shuan-tao
Received:2022-02-15
Published:2022-04-26
Online:2022-05-06
摘要:
β-酮脂酰辅酶A合成酶(β-ketoacyl-CoA synthase,KCS)是超长链脂肪酸合成中的限速酶,通过对大白菜KCS基因家族鉴定分析,为揭示KCS在控制大白菜蜡质性状中的具体功能研究奠定了基础。利用生物信息学的分析方法鉴定大白菜KCS基因家族成员,分析其亲缘关系、蛋白质理化性质、染色体定位、共线性关系、基因结构、蛋白保守域结构和基因表达模式。从大白菜全基因组共鉴定到32个BrKCS基因成员分成4个亚组,不均匀地分布在10条染色体上。BrKCS氨基酸长度和分子量的范围分别为181-755 aa和20.54-84.83 kD。共线性分析表明BrKCS5、BrKCS8和BrKCS13等7个家族成员与拟南芥共线性的BrKCS基因发生两倍化,1个家族成员(BrKCS9)发生了三倍化。基因结构分析显示,不同成员编码的氨基酸序列外显子数目及位置存在差异。转录组分析表明,BrKCS5、BrKCS6b与BrKCS10b基因在大白菜不同器官/组织尤其在花器官中可能参与蜡粉的合成。qRT-PCR分析结果验证了转录组数据的可靠性。综上所述,从大白菜全基因组共鉴定到32个BrKCS,其中3个基因在蜡粉近等基因系中表达量高且差异显著,为解析BrKCS基因在控制大白菜蜡质性状中的分子机制奠定了基础。
王荣花, 王树彬, 张志刚, 赵智中, 李巧云, 王立华, 刘栓桃. 大白菜KCS基因家族鉴定及在蜡粉近等基因系中表达分析[J]. 生物技术通报, 2022, 38(4): 86-96.
WANG Rong-hua, WANG Shu-bin, ZHANG Zhi-gang, ZHAO Zhi-zhong, LI Qiao-yun, WANG Li-hua, LIU Shuan-tao. Genome-wide Characterization of KCS Gene Family in Brassica rapa and Their Expression Profiling in Waxy Near-isogenic Lines[J]. Biotechnology Bulletin, 2022, 38(4): 86-96.
| 基因名称Gene | 正向引物Forward primer sequence(5'-3') | 反向引物Reverse primer sequence(5'-3') |
|---|---|---|
| BrKCS1a | CCGCTACAAGATGAGAGAAGACA | GGTTAGAGAGGAGAATCGCTGC |
| BrKCS2a | CGTCCTCGCAAAGTATTCCTC | GGAAAGTAAGTCTTCTGTCCTAAACCG |
| BrKCS5 | GCTATTCACTACATCCCTCCAACTC | CGAGAGAGAAGGCGTTGGT |
| BrKCS6a | CCTCAACGACAAGCCTAAGC | GCCTCACTTGTAGCCTCGTTC |
| BrKCS6b | GTGTCCCCTTCGCAACTTTC | GGTTGGTGTCGGAGGAATG |
| BrKCS7a | GGGAGCAAGACAAGCCTAATG | TGAGGACCTAACTCCACCACG |
| BrKCS7b | ACCCTGATGATGCTCGCCT | GTAAGTGCCCTTGTGCTCATCA |
| BrKCS9a | GGTGTAGTGCTGGTGTTATCTCC | GGATCAGCATCGCCTTCTTA |
| BrKCS9b | TGAGGGTTTGCACTGCGT | GTCGGGTTGAACAAGCTGC |
| BrKCS9c | GCGAGGAGTCGTCTTTGGA | CTGGAGAGGTGGAACGCTATG |
| BrKCS10a | GTTTCTTTAGGATGGGGTGCTC | GTCGGCAGCCTTATGAGTC |
| BrKCS10b | CGACTCCGTCACTATCTGCCA | GGCTATGATTCCCGCTGAG |
| BrKCS13 | GCCAAAGGTGCTCAACTCTACA | GAGGTCAGCATGCAACGTG |
| BrKCS20a | CTATCCTCTACAACCACCTCCG | AAGACACGGCGAGGACGA |
| G6PD | GGGTATGCCAGGACTAAGCTC | GAATCATAAGGGCCACTCACAT |
表1 用于qRT-PCR分析的引物序列
Table 1 Primer sequences for qRT-PCR analysis
| 基因名称Gene | 正向引物Forward primer sequence(5'-3') | 反向引物Reverse primer sequence(5'-3') |
|---|---|---|
| BrKCS1a | CCGCTACAAGATGAGAGAAGACA | GGTTAGAGAGGAGAATCGCTGC |
| BrKCS2a | CGTCCTCGCAAAGTATTCCTC | GGAAAGTAAGTCTTCTGTCCTAAACCG |
| BrKCS5 | GCTATTCACTACATCCCTCCAACTC | CGAGAGAGAAGGCGTTGGT |
| BrKCS6a | CCTCAACGACAAGCCTAAGC | GCCTCACTTGTAGCCTCGTTC |
| BrKCS6b | GTGTCCCCTTCGCAACTTTC | GGTTGGTGTCGGAGGAATG |
| BrKCS7a | GGGAGCAAGACAAGCCTAATG | TGAGGACCTAACTCCACCACG |
| BrKCS7b | ACCCTGATGATGCTCGCCT | GTAAGTGCCCTTGTGCTCATCA |
| BrKCS9a | GGTGTAGTGCTGGTGTTATCTCC | GGATCAGCATCGCCTTCTTA |
| BrKCS9b | TGAGGGTTTGCACTGCGT | GTCGGGTTGAACAAGCTGC |
| BrKCS9c | GCGAGGAGTCGTCTTTGGA | CTGGAGAGGTGGAACGCTATG |
| BrKCS10a | GTTTCTTTAGGATGGGGTGCTC | GTCGGCAGCCTTATGAGTC |
| BrKCS10b | CGACTCCGTCACTATCTGCCA | GGCTATGATTCCCGCTGAG |
| BrKCS13 | GCCAAAGGTGCTCAACTCTACA | GAGGTCAGCATGCAACGTG |
| BrKCS20a | CTATCCTCTACAACCACCTCCG | AAGACACGGCGAGGACGA |
| G6PD | GGGTATGCCAGGACTAAGCTC | GAATCATAAGGGCCACTCACAT |
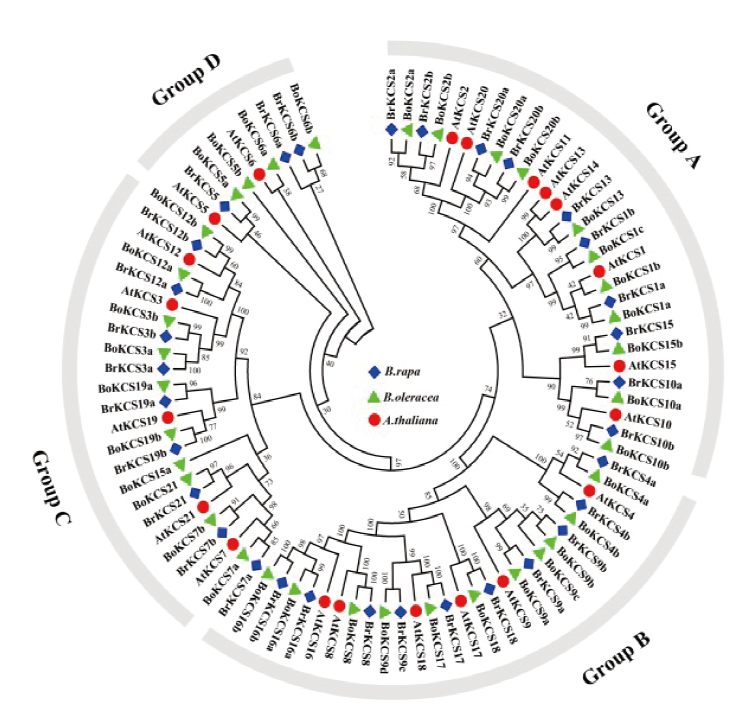
图1 大白菜(Br)、甘蓝(Bo)、拟南芥(At)KCS蛋白系统进化树分析
Fig. 1 Phylogenetic tree analysis of KCS proteins in Bras-sica rapa(Br),Brassica oleracea(Bo)and Ara-bidopsis thaliana(At)
| 拟南芥A. thaliana | 大白菜B. rapa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 基因名称 Gene Name | 基因编号 Gene ID | 基因名称 Gene name | 基因编号 Gene ID | 染色体 Chromosome | 亚基因组 Subgenome | 氨基酸大小 No. of amino acids/ aa | 分子量 Molecular weight/kD | 理论等电点 pI | ||
| AtKCS1 | AT1G01120 | BrKCS1a | BraA10g000750.3C | A10 | LF | 523 | 59.36 | 8.57 | ||
| BrKCS1b | BraA09g065990.3C | A09 | MF2 | 491 | 55.11 | 9.02 | ||||
| AtKCS2 | AT1G04220 | BrKCS2a | BraA10g002770.3C | A10 | LF | 529 | 59.58 | 9.27 | ||
| BrKCS2b | BraA08g034990.3C | A08 | MF1 | 181 | 20.54 | 9.34 | ||||
| AtKCS3 | AT1G07720 | BrKCS3a | BraA06g005040.3C | A06 | LF | 427 | 48.19 | 9.31 | ||
| BrKCS3b | BraA08g033410.3C | A08 | MF1 | 391 | 43.97 | 8.92 | ||||
| AtKCS4 | AT1G19440 | BrKCS4a | BraA06g015010.3C | A06 | LF | 506 | 56.71 | 9.17 | ||
| BrKCS4b | BraA09g056620.3C | A09 | MF2 | 511 | 57.13 | 9.01 | ||||
| AtKCS5 | AT1G25450 | BrKCS5 | BraA09g037930.3C | A09 | LF | 497 | 56.26 | 9.02 | ||
| AtKCS6 | AT1G68530 | BrKCS6a | BraA02g018480.3C | A02 | MF1 | 231 | 25.68 | 9.19 | ||
| BrKCS6b | BraA07g030740.3C | A07 | MF2 | 497 | 56.36 | 9.03 | ||||
| AtKCS7 | AT1G71160 | BrKCS7a | BraA02g020280.3C | A02 | MF1 | 449 | 50.04 | 8.01 | ||
| BrKCS7b | BraA07g029700.3C | A07 | MF2 | 755 | 84.83 | 8.41 | ||||
| AtKCS8 | AT2G15090 | BrKCS8 | BraA07g005530.3C | A07 | LF | 461 | 51.80 | 9.00 | ||
| AtKCS9 | AT2G16280 | BrKCS9a | BraA07g004540.3C | A07 | LF | 507 | 57.08 | 9.18 | ||
| BrKCS9b | BraA09g010440.3C | A09 | MF1 | 456 | 51.26 | 8.87 | ||||
| BrKCS9c | BraA03g043030.3C | A03 | MF2 | 487 | 54.81 | 9.27 | ||||
| AtKCS10 | AT2G26250 | BrKCS10a | BraA09g052820.3C | A09 | LF | 547 | 61.48 | 9.10 | ||
| BrKCS10b | BraA04g019210.3C | A04 | MF1 | 549 | 61.81 | 9.14 | ||||
| AtKCS11 | AT2G26640 | NA | NA | NA | NA | NA | NA | NA | ||
| AtKCS12 | AT2G28630 | BrKCS12a | BraA07g018970.3C | A07 | LF | 437 | 49.39 | 9.27 | ||
| BrKCS12b | BraA04g020490.3C | A04 | MF1 | 476 | 54.00 | 9.15 | ||||
| AtKCS13 | AT2G46720 | BrKCS13 | BraA05g001130.3C | A05 | LF | 587 | 65.18 | 9.39 | ||
| AtKCS14 | AT3G10280 | NA | NA | NA | NA | NA | NA | NA | ||
| AtKCS15 | AT3G52160 | BrKCS15 | BraA03g045900.3C | A03 | MF2 | 457 | 51.92 | 9.40 | ||
| AtKCS16 | AT4G34250 | BrKCS16a | BraA01g004000.3C | A01 | LF | 459 | 51.60 | 9.25 | ||
| BrKCS16b | BraA03g058730.3C | A03 | MF1 | 482 | 53.97 | 9.19 | ||||
| AtKCS17 | AT4G34510 | BrKCS17 | BraA08g015890.3C | A08 | MF2 | 506 | 56.37 | 9.26 | ||
| AtKCS18 | AT4G34520 | BrKCS18 | BraA08g015900.3C | A08 | MF2 | 474 | 53.81 | 9.31 | ||
| AtKCS19 | AT5G04530 | BrKCS19a | BraA10g031780.3C | A10 | LF | 463 | 52.25 | 8.81 | ||
| BrKCS19b | BraA02g001400.3C | A02 | MF2 | 429 | 48.3 | 8.79 | ||||
| AtKCS20 | AT5G43760 | BrKCS20a | BraA06g043600.3C | A06 | LF | 526 | 58.99 | 9.21 | ||
| BrKCS20b | BraA02g030820.3C | A02 | MF1 | 526 | 58.99 | 9.10 | ||||
| AtKCS21 | AT5G49070 | BrKCS21 | BraA02g039980.3C | A02 | MF1 | 457 | 51.55 | 9.17 | ||
表2 大白菜和拟南芥KCS基因对应关系及大白菜KCS基因基本信息
Table 2 Corresponding relationship between B. rapa and A. thaliana KCS genes and basic information of KCS genes in B. rapa
| 拟南芥A. thaliana | 大白菜B. rapa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 基因名称 Gene Name | 基因编号 Gene ID | 基因名称 Gene name | 基因编号 Gene ID | 染色体 Chromosome | 亚基因组 Subgenome | 氨基酸大小 No. of amino acids/ aa | 分子量 Molecular weight/kD | 理论等电点 pI | ||
| AtKCS1 | AT1G01120 | BrKCS1a | BraA10g000750.3C | A10 | LF | 523 | 59.36 | 8.57 | ||
| BrKCS1b | BraA09g065990.3C | A09 | MF2 | 491 | 55.11 | 9.02 | ||||
| AtKCS2 | AT1G04220 | BrKCS2a | BraA10g002770.3C | A10 | LF | 529 | 59.58 | 9.27 | ||
| BrKCS2b | BraA08g034990.3C | A08 | MF1 | 181 | 20.54 | 9.34 | ||||
| AtKCS3 | AT1G07720 | BrKCS3a | BraA06g005040.3C | A06 | LF | 427 | 48.19 | 9.31 | ||
| BrKCS3b | BraA08g033410.3C | A08 | MF1 | 391 | 43.97 | 8.92 | ||||
| AtKCS4 | AT1G19440 | BrKCS4a | BraA06g015010.3C | A06 | LF | 506 | 56.71 | 9.17 | ||
| BrKCS4b | BraA09g056620.3C | A09 | MF2 | 511 | 57.13 | 9.01 | ||||
| AtKCS5 | AT1G25450 | BrKCS5 | BraA09g037930.3C | A09 | LF | 497 | 56.26 | 9.02 | ||
| AtKCS6 | AT1G68530 | BrKCS6a | BraA02g018480.3C | A02 | MF1 | 231 | 25.68 | 9.19 | ||
| BrKCS6b | BraA07g030740.3C | A07 | MF2 | 497 | 56.36 | 9.03 | ||||
| AtKCS7 | AT1G71160 | BrKCS7a | BraA02g020280.3C | A02 | MF1 | 449 | 50.04 | 8.01 | ||
| BrKCS7b | BraA07g029700.3C | A07 | MF2 | 755 | 84.83 | 8.41 | ||||
| AtKCS8 | AT2G15090 | BrKCS8 | BraA07g005530.3C | A07 | LF | 461 | 51.80 | 9.00 | ||
| AtKCS9 | AT2G16280 | BrKCS9a | BraA07g004540.3C | A07 | LF | 507 | 57.08 | 9.18 | ||
| BrKCS9b | BraA09g010440.3C | A09 | MF1 | 456 | 51.26 | 8.87 | ||||
| BrKCS9c | BraA03g043030.3C | A03 | MF2 | 487 | 54.81 | 9.27 | ||||
| AtKCS10 | AT2G26250 | BrKCS10a | BraA09g052820.3C | A09 | LF | 547 | 61.48 | 9.10 | ||
| BrKCS10b | BraA04g019210.3C | A04 | MF1 | 549 | 61.81 | 9.14 | ||||
| AtKCS11 | AT2G26640 | NA | NA | NA | NA | NA | NA | NA | ||
| AtKCS12 | AT2G28630 | BrKCS12a | BraA07g018970.3C | A07 | LF | 437 | 49.39 | 9.27 | ||
| BrKCS12b | BraA04g020490.3C | A04 | MF1 | 476 | 54.00 | 9.15 | ||||
| AtKCS13 | AT2G46720 | BrKCS13 | BraA05g001130.3C | A05 | LF | 587 | 65.18 | 9.39 | ||
| AtKCS14 | AT3G10280 | NA | NA | NA | NA | NA | NA | NA | ||
| AtKCS15 | AT3G52160 | BrKCS15 | BraA03g045900.3C | A03 | MF2 | 457 | 51.92 | 9.40 | ||
| AtKCS16 | AT4G34250 | BrKCS16a | BraA01g004000.3C | A01 | LF | 459 | 51.60 | 9.25 | ||
| BrKCS16b | BraA03g058730.3C | A03 | MF1 | 482 | 53.97 | 9.19 | ||||
| AtKCS17 | AT4G34510 | BrKCS17 | BraA08g015890.3C | A08 | MF2 | 506 | 56.37 | 9.26 | ||
| AtKCS18 | AT4G34520 | BrKCS18 | BraA08g015900.3C | A08 | MF2 | 474 | 53.81 | 9.31 | ||
| AtKCS19 | AT5G04530 | BrKCS19a | BraA10g031780.3C | A10 | LF | 463 | 52.25 | 8.81 | ||
| BrKCS19b | BraA02g001400.3C | A02 | MF2 | 429 | 48.3 | 8.79 | ||||
| AtKCS20 | AT5G43760 | BrKCS20a | BraA06g043600.3C | A06 | LF | 526 | 58.99 | 9.21 | ||
| BrKCS20b | BraA02g030820.3C | A02 | MF1 | 526 | 58.99 | 9.10 | ||||
| AtKCS21 | AT5G49070 | BrKCS21 | BraA02g039980.3C | A02 | MF1 | 457 | 51.55 | 9.17 | ||
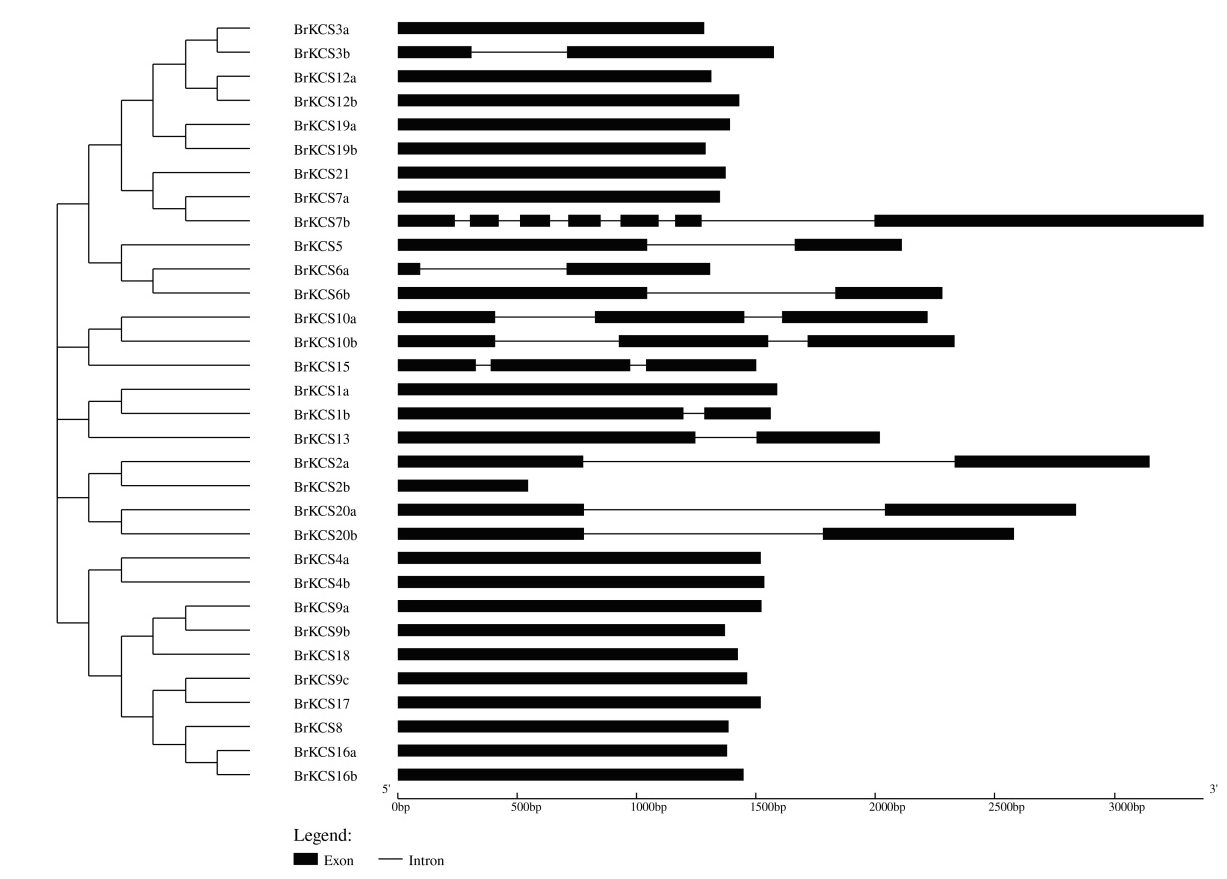
图4 大白菜KCS基因家族外显子-内含子结构分析 黑色矩形为外显子,黑色线条为内含子
Fig. 4 Analysis of exon-intron structure of KCS gene family in B. rapa Black rectangles are exons,and black lines are introns
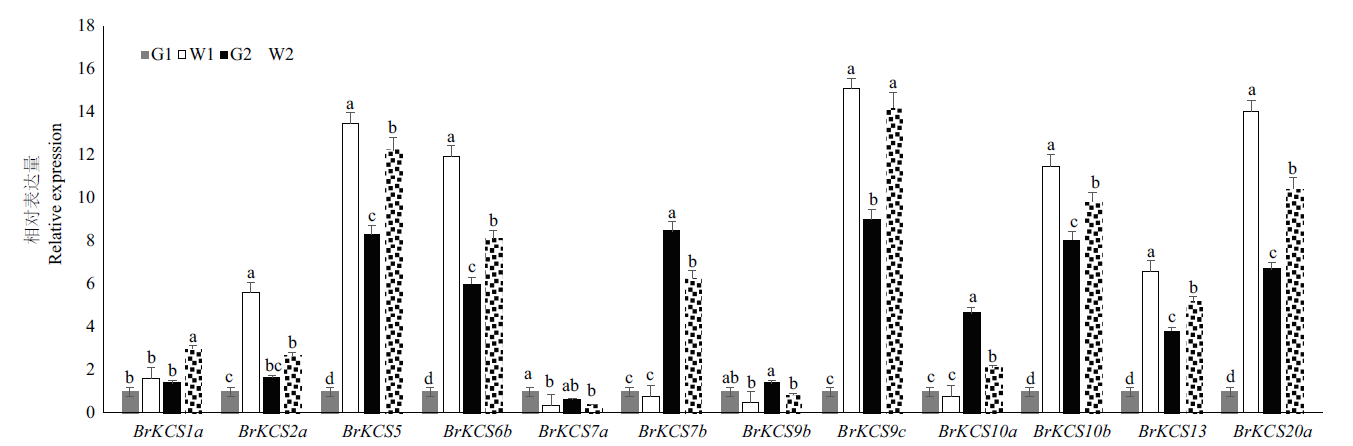
图8 大白菜KCS基因在大白菜有/无蜡粉高代自交系及近等基因系表皮中的相对表达量
Fig. 8 Relative expression of BrKCS gene in waxy/non-waxy high-generation inbred lines and near-isogenic lines of B. rapa
| [1] |
Kunst L, Samuels AL. Biosynjournal and secretion of plant cuticular wax[J]. Prog Lipid Res, 2003, 42(1):51-80.
pmid: 12467640 |
| [2] |
Haslam TM, Kunst L. Extending the story of very-long-chain fatty acid elongation[J]. Plant Sci, 2013, 210:93-107.
doi: 10.1016/j.plantsci.2013.05.008 pmid: 23849117 |
| [3] |
Denic V, Weissman JS. A molecular caliper mechanism for determining very long-chain fatty acid length[J]. Cell, 2007, 130(4):663-677.
doi: 10.1016/j.cell.2007.06.031 URL |
| [4] |
James DW Jr, Lim E, Keller J, et al. Directed tagging of the Arabidopsis FATTY ACID ELONGATION1(FAE1)gene with the maize transposon activator[J]. Plant Cell, 1995, 7(3):309-319.
pmid: 7734965 |
| [5] |
Joubès J, Raffaele S, Bourdenx B, et al. The VLCFA elongase gene family in Arabidopsis thaliana:phylogenetic analysis, 3D modelling and expression profiling[J]. Plant Mol Biol, 2008, 67(5):547-566.
doi: 10.1007/s11103-008-9339-z URL |
| [6] |
Todd J, Post-Beittenmiller D, Jaworski JG. KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynjournal in Arabidopsis thaliana[J]. Plant J, 1999, 17(2):119-130.
pmid: 10074711 |
| [7] |
Franke R, Höfer R, Briesen I, et al. The DAISY gene from Arabidopsis encodes a fatty acid elongase condensing enzyme involved in the biosynjournal of aliphatic suberin in roots and the chalaza-micropyle region of seeds[J]. Plant J, 2009, 57(1):80-95.
doi: 10.1111/j.1365-313X.2008.03674.x URL |
| [8] | Costaglioli P, Joubès J, Garcia C, et al. Profiling candidate genes involved in wax biosynjournal in Arabidopsis thaliana by microarray analysis[J]. Biochim Biophys Acta BBA Mol Cell Biol Lipids, 2005, 1734(3):247-258. |
| [9] |
Hooker TS, Millar AA, Kunst L. Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis[J]. Plant Physiol, 2002, 129(4):1568-1580.
pmid: 12177469 |
| [10] |
Kim J, Jung JH, Lee SB, et al. Arabidopsis 3-ketoacyl-coenzyme a synthase9 is involved in the synjournal of tetracosanoic acids as precursors of cuticular waxes, suberins, sphingolipids, and phospholipids[J]. Plant Physiol, 2013, 162(2):567-580.
doi: 10.1104/pp.112.210450 URL |
| [11] |
Pruitt RE, Vielle-Calzada JP, Ploense SE, et al. FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme[J]. Proc Natl Acad Sci USA, 2000, 97(3):1311-1316.
doi: 10.1073/pnas.97.3.1311 URL |
| [12] |
Gray JE, Holroyd GH, van der Lee FM, et al. The HIC signalling pathway links CO2 perception to stomatal development[J]. Nature, 2000, 408(6813):713-716.
doi: 10.1038/35047071 URL |
| [13] |
Lee SB, Jung SJ, Go YS, et al. Two Arabidopsis 3-ketoacyl CoA synthase genes, KCS20 and KCS2/DAISY, are functionally redundant in cuticular wax and root suberin biosynjournal, but differentially controlled by osmotic stress[J]. Plant J, 2009, 60(3):462-475.
doi: 10.1111/j.1365-313X.2009.03973.x URL |
| [14] |
Lee SB, Suh MC. Recent advances in cuticular wax biosynjournal and its regulation in Arabidopsis[J]. Mol Plant, 2013, 6(2):246-249.
doi: 10.1093/mp/sss159 URL |
| [15] |
Millar AA, Kunst L. The natural genetic variation of the fatty-acyl composition of seed oils in different ecotypes of Arabidopsis thaliana[J]. Phytochemistry, 1999, 52(6):1029-1033.
pmid: 10643668 |
| [16] |
Fiebig A, Mayfield JA, Miley NL, et al. Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems[J]. Plant Cell, 2000, 12(10):2001-2008.
pmid: 11041893 |
| [17] |
Qin YM, Hu CY, Pang Y, et al. Saturated very-long-chain fatty acids promote cotton fiber and Arabidopsis cell elongation by activating ethylene biosynjournal[J]. Plant Cell, 2007, 19(11):3692-3704.
doi: 10.1105/tpc.107.054437 URL |
| [18] |
Chai MF, Queralta Castillo I, Sonntag A, et al. A seed coat-specific β-ketoacyl-CoA synthase, KCS12, is critical for preserving seed physical dormancy[J]. Plant Physiol, 2021, 186(3):1606-1615.
doi: 10.1093/plphys/kiab152 URL |
| [19] | Yang HB, Mei WJ, Wan HL, et al. Comprehensive analysis of KCS gene family in Citrinae reveals the involvement of CsKCS2 and CsKCS11 in fruit cuticular wax synjournal at ripening[J]. Plant Sci, 2021, 310:110972. |
| [20] | 武玉花. 甘蓝型油菜KCS基因家族表达及功能分析[D]. 北京:中国农业科学院, 2012. |
| Wu YH. Expression profile and functional characterization of KCS gene family in Brassica napus[D]. Beijing:Chinese Academy of Agricultural Sciences, 2012. | |
| [21] | 易婷, 张志硕, 汤冰倩, 等. 辣椒β-酮脂酰辅酶A合酶基因家族的鉴定与表达分析[J]. 园艺学报, 2020, 47(2):370-380. |
| Yi T, Zhang ZS, Tang BQ, et al. Identification and expression analysis of the KCS gene family in pepper[J]. Acta Hortic Sin, 2020, 47(2):370-380. | |
| [22] | 张高阳, 单士莲, 邓接楼, 等. 黄麻β-酮脂酰-CoA合酶(KCS)基因分离与功能鉴定[J]. 分子植物育种, 2018, 16(20):6718-6722. |
| Zhang GY, Shan SL, Deng JL, et al. The segregation and functional identification of jute β-ketoyl-CoA synthase gene(KCS)[J]. Mol Plant Breed, 2018, 16(20):6718-6722. | |
| [23] | 王荣花, 王树彬, 刘栓桃, 等. 大白菜花茎蜡粉近等基因系转录组分析[J]. 园艺学报, 2022, 49(1):62-72. |
| Wang RH, Wang SB, Liu ST, et al. Transcriptome analysis of waxy near-isogenic lines in Chinese cabbage floral axis[J]. Acta Hortic Sin, 2022, 49(1):62-72. | |
| [24] |
Liu ST, Wang RH, Zhang ZG, et al. High-resolution mapping of quantitative trait loci controlling main floral stalk length in Chinese cabbage(Brassica rapa L. ssp. pekinensis)[J]. BMC Genomics, 2019, 20(1):437.
doi: 10.1186/s12864-019-5810-2 URL |
| [25] |
Ayaz A, Saqib S, Huang HD, et al. Genome-wide comparative analysis of long-chain acyl-CoA synthetases(LACSs)gene family:a focus on identification, evolution and expression profiling related to lipid synjournal[J]. Plant Physiol Biochem, 2021, 161:1-11.
doi: 10.1016/j.plaphy.2021.01.042 URL |
| [26] |
Zhang L, Cai X, Wu J, et al. Improved Brassica rapa reference genome by single-molecule sequencing and chromosome conformation capture technologies[J]. Hortic Res, 2018, 5:50.
doi: 10.1038/s41438-018-0071-9 URL |
| [27] |
Liu SY, Liu YM, Yang XH, et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes[J]. Nat Commun, 2014, 5:3930.
doi: 10.1038/ncomms4930 URL |
| [28] |
Kumar S, Stecher G, Tamura K. MEGA7:molecular evolutionary genetics analysis version 7. 0 for bigger datasets[J]. Mol Biol Evol, 2016, 33(7):1870-1874.
doi: 10.1093/molbev/msw054 URL |
| [29] |
Artimo P, Jonnalagedda M, Arnold K, et al. ExPASy:SIB bioinformatics resource portal[J]. Nucleic Acids Res, 2012, 40(Web Server issue):W597-W603.
doi: 10.1093/nar/gks400 URL |
| [30] |
Chen CJ, Chen H, Zhang Y, et al. TBtools:an integrative toolkit developed for interactive analyses of big biological data[J]. Mol Plant, 2020, 13(8):1194-1202.
doi: 10.1016/j.molp.2020.06.009 URL |
| [31] |
Hu B, Jin JP, Guo AY, et al. GSDS 2. 0:an upgraded gene feature visualization server[J]. Bioinformatics, 2015, 31(8):1296-1297.
doi: 10.1093/bioinformatics/btu817 URL |
| [32] |
Yang SJ, Liu HL, Wei XC, et al. BrWAX2 plays an essential role in cuticular wax biosynjournal in Chinese cabbage(Brassica rapa L. ssp. pekinensis)[J]. Theor Appl Genet, 2022, 135(2):693-707.
doi: 10.1007/s00122-021-03993-x URL |
| [1] | 王佳蕊, 孙培媛, 柯瑾, 冉彬, 李洪有. 苦荞糖基转移酶基因FtUGT143的克隆及表达分析[J]. 生物技术通报, 2023, 39(8): 204-212. |
| [2] | 孙明慧, 吴琼, 刘丹丹, 焦小雨, 王文杰. 茶树CsTMFs的克隆与表达分析[J]. 生物技术通报, 2023, 39(7): 151-159. |
| [3] | 赵雪婷, 高利燕, 王俊刚, 沈庆庆, 张树珍, 李富生. 甘蔗AP2/ERF转录因子基因ShERF3的克隆、表达及其编码蛋白的定位[J]. 生物技术通报, 2023, 39(6): 208-216. |
| [4] | 王春语, 李政君, 王平, 张丽霞. 高粱表皮蜡质缺失突变体sb1抗旱生理生化分析[J]. 生物技术通报, 2023, 39(5): 160-167. |
| [5] | 姜晴春, 杜洁, 王嘉诚, 余知和, 王允, 柳忠玉. 虎杖转录因子PcMYB2的表达特性和功能分析[J]. 生物技术通报, 2023, 39(5): 217-223. |
| [6] | 姚姿婷, 曹雪颖, 肖雪, 李瑞芳, 韦小妹, 邹承武, 朱桂宁. 火龙果溃疡病菌实时荧光定量PCR内参基因的筛选[J]. 生物技术通报, 2023, 39(5): 92-102. |
| [7] | 王艺清, 王涛, 韦朝领, 戴浩民, 曹士先, 孙威江, 曾雯. 茶树SMAS基因家族的鉴定及互作分析[J]. 生物技术通报, 2023, 39(4): 246-258. |
| [8] | 刘思佳, 王浩楠, 付宇辰, 闫文欣, 胡增辉, 冷平生. ‘西伯利亚’百合LiCMK基因克隆及功能分析[J]. 生物技术通报, 2023, 39(3): 196-205. |
| [9] | 王涛, 漆思雨, 韦朝领, 王艺清, 戴浩民, 周喆, 曹士先, 曾雯, 孙威江. CsPPR和CsCPN60-like在茶树白化叶片中的表达分析及互作蛋白验证[J]. 生物技术通报, 2023, 39(3): 218-231. |
| [10] | 庞强强, 孙晓东, 周曼, 蔡兴来, 张文, 王亚强. 菜心BrHsfA3基因克隆及其对高温胁迫的响应[J]. 生物技术通报, 2023, 39(2): 107-115. |
| [11] | 苗淑楠, 高宇, 李昕儒, 蔡桂萍, 张飞, 薛金爱, 季春丽, 李润植. 大豆GmPDAT1参与油脂合成和非生物胁迫应答的功能分析[J]. 生物技术通报, 2023, 39(2): 96-106. |
| [12] | 葛雯冬, 王腾辉, 马天意, 范震宇, 王玉书. 结球甘蓝PRX基因家族全基因组鉴定与逆境条件下的表达分析[J]. 生物技术通报, 2023, 39(11): 252-260. |
| [13] | 杨旭妍, 赵爽, 马天意, 白玉, 王玉书. 三个甘蓝WRKY基因的克隆及其对非生物胁迫的表达[J]. 生物技术通报, 2023, 39(11): 261-269. |
| [14] | 陈楚怡, 杨小梅, 陈胜艳, 陈斌, 岳莉然. ABA和干旱胁迫下菊花脑ZF-HD基因家族的表达分析[J]. 生物技术通报, 2023, 39(11): 270-282. |
| [15] | 尤垂淮, 谢津津, 张婷, 崔天真, 孙欣路, 臧守建, 武奕凝, 孙梦瑶, 阙友雄, 苏亚春. 钩吻脂氧合酶基因 GeLOX1 的鉴定及低温胁迫表达分析[J]. 生物技术通报, 2023, 39(11): 318-327. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||