








生物技术通报 ›› 2023, Vol. 39 ›› Issue (4): 124-135.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0996
马玉倩1( ), 孙东辉1, 岳浩峰1, 辛佳瑜2, 刘宁2(
), 孙东辉1, 岳浩峰1, 辛佳瑜2, 刘宁2( ), 曹志艳2,3(
), 曹志艳2,3( )
)
收稿日期:2022-08-16
出版日期:2023-04-26
发布日期:2023-05-16
通讯作者:
刘宁,女,博士,副教授,研究方向:资源利用与植物保护;E-mail: lning121@126.com;作者简介:马玉倩,女,硕士,研究方向:生物与医药;E-mail: 1633160470@qq.com
基金资助:
MA Yu-qian1( ), SUN Dong-hui1, YUE Hao-feng1, XIN Jia-yu2, LIU Ning2(
), SUN Dong-hui1, YUE Hao-feng1, XIN Jia-yu2, LIU Ning2( ), CAO Zhi-yan2,3(
), CAO Zhi-yan2,3( )
)
Received:2022-08-16
Published:2023-04-26
Online:2023-05-16
摘要:
糖苷水解酶61家族(GH61)属于一类同时具有氧化作用和水解作用的纤维素降解酶类,既具有微弱的纤维素内切酶活性,也可通过氧化作用破坏纤维素晶体结构促进纤维素酶对木质纤维素的降解,在生物质资源的利用方面具有潜在的应用价值。对大斑刚毛座腔菌Setosphaeria turcica的GH61家族基因进行鉴定及生物信息学分析,通过转录组数据分析及荧光定量PCR验证,筛选出受玉米秸秆木质纤维素底物诱导表达的GH61家族基因StGH61-11。将StGH61-11进行重组表达,使用2,6-二甲氧基苯酚氧化反应测定其酶活力并优化诱导条件,表征其酶学性质并检测其对纤维素酶水解木质纤维素的促进作用。结果表明,在S. turcica基因组中存在21个GH61家族基因,且S. turcica在玉米秸秆的诱导下滤纸酶活明显增加,对其进行转录组分析发现,在以玉米秸秆为碳源时GH61家族基因中有11个基因的表达量增加。将其中StGH61-11基因在大肠杆菌中诱导表达,最佳诱导条件为25℃、1 mmol/L IPTG诱导9 h,此时获得的蛋白比活力可达到(54.08±1.67)U/g。重组蛋白StGH61-11的最佳催化条件为温度50℃、pH 5,在最佳催化条件下可以显著提高纤维素酶水解玉米秸秆的活性,协同度最高可达2.5,糖化率最高可达46.5%。
马玉倩, 孙东辉, 岳浩峰, 辛佳瑜, 刘宁, 曹志艳. 具有辅助降解纤维素功能的大斑刚毛座腔菌糖苷水解酶GH61的鉴定、异源表达及功能分析[J]. 生物技术通报, 2023, 39(4): 124-135.
MA Yu-qian, SUN Dong-hui, YUE Hao-feng, XIN Jia-yu, LIU Ning, CAO Zhi-yan. Identification, Heterologous Expression and Functional Analysis of a GH61 Family Glycoside Hydrolase from Setosphaeria turcica with the Assisting Function in Degrading Cellulose[J]. Biotechnology Bulletin, 2023, 39(4): 124-135.
| 引物名称Primer name | 序列Sequence(5'-3') | 引物名称Primer name | 序列Sequence(5'-3') | |
|---|---|---|---|---|
| StGH61_1-F | GGCTCTGGAACTGGCAAGAT | StGH61_12-F | CTCCTGGTCCGTCACTGTTC | |
| StGH61_1-R | TAGTACTCGAGGTCCCTGGC | StGH61_12-R | GGCTGGAGAGATCGGTCATG | |
| StGH61_2-F | GTCAGTTTCCCGGGTGCTTA | StGH61_13-F | GGCAGAAGGACCAGATCGAG | |
| StGH61_2-R | GACCACGCTGTTAGTCCCAA | StGH61_13-R | CGCTGTAGAGACCGGGAATC | |
| StGH61_3-F | ACATCAACACGTGGGATCCC | StGH61_14-F | CGACGCCATCCTAGACACTC | |
| StGH61_3-R | TGCGCACGACAGGTAGAATT | StGH61_14-R | TTGAAGTCGATGCCTGGGTC | |
| StGH61_4-F | CGACTTCCGTTGCAACAAGG | StGH61_15-F | CACCAAAGTCGAGCCCTTCT | |
| StGH61_4-R | AACATCACCAGGAGCCTTGG | StGH61_15-R | TTGAGGGAGAGGGAGACGAG | |
| StGH61_5-F | TTGCGCAAATCACAACCTCG | StGH61_16-F | GTGCCAAGGGAGGTCTCTTC | |
| StGH61_5-R | TCGTGGCATGTCATGTTGGA | StGH61_16-R | GGTCGGCAGTGAAAGAGACA | |
| StGH61_6-F | CACCCAGACTGTCACCATCC | StGH61_17-F | GGTTCAAGGTGCAGGAGGAA | |
| StGH61_6-R | CACCCAGACTGTCACCATCC | StGH61_17-R | CTACCCGTCACCTTGAGCTG | |
| StGH61_7-F | GGTCCAAGTTCTCGCAGGAA | StGH61_18-F | AGCTCGACTGCCATGATCTG | |
| StGH61_7-R | CATTCACATTCGAGCCGCTG | StGH61_18-R | GGGGAGCTTGACGTTGATGA | |
| StGH61_8-F | CCACAAGAATGCTAGCCCCA | StGH61_19-F | CAAGGTCTCCAACGCAGCTA | |
| StGH61_8-R | GCACCCTCAATCTTGGTCCA | StGH61_19-R | AAAATTGAGCACCGCCAACC | |
| StGH61_9-F | TGGTTCAAGGTTTCCAGCGA | StGH61_20-F | CATCCCTGCTTGTATTGCGC | |
| StGH61_9-R | AAATGTAGAATTGCGCGCCG | StGH61_20-R | GGAACGAGACGGTTGATGGT | |
| StGH61_10-F | GGGCAGTGATGTGAAGAGCT | StGH61_21-F | AAGATCGACGAGCAAGGCAT | |
| StGH61_10-R | GGAGCACGCGAGGTAGAATT | StGH61_21-R | GACGCATACCCAGGAATCGT | |
| StGH61_11-F | AGCCTCTCTTCCTCGGACAT | β-tubulin-F | GTGCGCAAGGAGGCTGAGGG | |
| StGH61_11-R | TTTTGGCGTCGCTGACTTTG | β-tubulin-R | CATGAAGAAATGGAGACGGGGGAA |
表1 实时荧光定量PCR引物序列
Table 1 Primer sequences used in RT-qPCR
| 引物名称Primer name | 序列Sequence(5'-3') | 引物名称Primer name | 序列Sequence(5'-3') | |
|---|---|---|---|---|
| StGH61_1-F | GGCTCTGGAACTGGCAAGAT | StGH61_12-F | CTCCTGGTCCGTCACTGTTC | |
| StGH61_1-R | TAGTACTCGAGGTCCCTGGC | StGH61_12-R | GGCTGGAGAGATCGGTCATG | |
| StGH61_2-F | GTCAGTTTCCCGGGTGCTTA | StGH61_13-F | GGCAGAAGGACCAGATCGAG | |
| StGH61_2-R | GACCACGCTGTTAGTCCCAA | StGH61_13-R | CGCTGTAGAGACCGGGAATC | |
| StGH61_3-F | ACATCAACACGTGGGATCCC | StGH61_14-F | CGACGCCATCCTAGACACTC | |
| StGH61_3-R | TGCGCACGACAGGTAGAATT | StGH61_14-R | TTGAAGTCGATGCCTGGGTC | |
| StGH61_4-F | CGACTTCCGTTGCAACAAGG | StGH61_15-F | CACCAAAGTCGAGCCCTTCT | |
| StGH61_4-R | AACATCACCAGGAGCCTTGG | StGH61_15-R | TTGAGGGAGAGGGAGACGAG | |
| StGH61_5-F | TTGCGCAAATCACAACCTCG | StGH61_16-F | GTGCCAAGGGAGGTCTCTTC | |
| StGH61_5-R | TCGTGGCATGTCATGTTGGA | StGH61_16-R | GGTCGGCAGTGAAAGAGACA | |
| StGH61_6-F | CACCCAGACTGTCACCATCC | StGH61_17-F | GGTTCAAGGTGCAGGAGGAA | |
| StGH61_6-R | CACCCAGACTGTCACCATCC | StGH61_17-R | CTACCCGTCACCTTGAGCTG | |
| StGH61_7-F | GGTCCAAGTTCTCGCAGGAA | StGH61_18-F | AGCTCGACTGCCATGATCTG | |
| StGH61_7-R | CATTCACATTCGAGCCGCTG | StGH61_18-R | GGGGAGCTTGACGTTGATGA | |
| StGH61_8-F | CCACAAGAATGCTAGCCCCA | StGH61_19-F | CAAGGTCTCCAACGCAGCTA | |
| StGH61_8-R | GCACCCTCAATCTTGGTCCA | StGH61_19-R | AAAATTGAGCACCGCCAACC | |
| StGH61_9-F | TGGTTCAAGGTTTCCAGCGA | StGH61_20-F | CATCCCTGCTTGTATTGCGC | |
| StGH61_9-R | AAATGTAGAATTGCGCGCCG | StGH61_20-R | GGAACGAGACGGTTGATGGT | |
| StGH61_10-F | GGGCAGTGATGTGAAGAGCT | StGH61_21-F | AAGATCGACGAGCAAGGCAT | |
| StGH61_10-R | GGAGCACGCGAGGTAGAATT | StGH61_21-R | GACGCATACCCAGGAATCGT | |
| StGH61_11-F | AGCCTCTCTTCCTCGGACAT | β-tubulin-F | GTGCGCAAGGAGGCTGAGGG | |
| StGH61_11-R | TTTTGGCGTCGCTGACTTTG | β-tubulin-R | CATGAAGAAATGGAGACGGGGGAA |
| 基因 Gene | 检索号 Accession number | 氨基酸数目 Number of amino acids | 分子量 Molecular weight/kD | 等电点 pI | 信号肽长度 Signal peptide length/aa | 蛋白定位 Protein location |
|---|---|---|---|---|---|---|
| StGH61-1 | XP_008027730 | 250 | 26.69 | 8.26 | 16 | 胞外Extracellular |
| StGH61-2 | XP_008031219 | 334 | 36.13 | 5.77 | 18 | 胞外Extracellular |
| StGH61-3 | XP_008024527 | 221 | 23.46 | 8.33 | 17 | 胞外Extracellular |
| StGH61-4 | XP_008025833 | 418 | 43.38 | 7.63 | 17 | 胞外Extracellular |
| StGH61-5 | XP_008021808 | 266 | 28.81 | 8.24 | 21 | 胞外Extracellular |
| StGH61-6 | XP_008023212 | 291 | 32.06 | 6.16 | 22 | 膜结合溶酶体Lysosomes |
| StGH61-7 | XP_008024957 | 248 | 26.32 | 7.69 | 20 | 胞外Extracellular |
| StGH61-8 | XP_008025253 | 329 | 34.56 | 5.98 | 22 | 分泌到胞外 Extracellular(Secreted) |
| StGH61-9 | XP_008030985 | 149 | 15.76 | 7.83 | — | 分泌到胞外 Extracellular(Secreted) |
| StGH61-10 | XP_008022828 | 236 | 25.55 | 9.00 | 20 | 膜结合线粒体Mitochondria |
| StGH61-11 | XP_008023697 | 229 | 23.52 | 8.49 | 16 | 胞外Extracellular |
| StGH61-12 | XP_008023696 | 258 | 27.49 | 5.47 | 22 | 胞外Extracellular |
| StGH61-13 | XP_008028494 | 325 | 34.02 | 6.31 | 18 | 胞外Extracellular |
| StGH61-14 | XP_008028319 | 292 | 30.73 | 6.28 | 21 | 胞外Extracellular |
| StGH61-15 | XP_008025489 | 472 | 49.64 | 7.44 | 19 | 胞外Extracellular |
| StGH61-16 | XP_008027303 | 222 | 23.26 | 7.69 | 20 | 胞外Extracellular |
| StGH61-17 | XP_008027295 | 246 | 25.92 | 8.44 | 18 | 胞外Extracellular |
| StGH61-18 | XP_008024355 | 344 | 34.75 | 6.49 | 17 | 胞外Extracellular |
| StGH61-19 | XP_008022590 | 243 | 25.06 | 8.90 | 17 | 胞外Extracellular |
| StGH61-20 | XP_008026441 | 233 | 24.21 | 7.67 | 18 | 胞外Extracellular |
表2 大斑刚毛座腔菌糖苷水解酶GH61家族蛋白性质预测
Table 2 Prediction of the protein properties of the glycoside hydrolase GH61 family from S. turcica
| 基因 Gene | 检索号 Accession number | 氨基酸数目 Number of amino acids | 分子量 Molecular weight/kD | 等电点 pI | 信号肽长度 Signal peptide length/aa | 蛋白定位 Protein location |
|---|---|---|---|---|---|---|
| StGH61-1 | XP_008027730 | 250 | 26.69 | 8.26 | 16 | 胞外Extracellular |
| StGH61-2 | XP_008031219 | 334 | 36.13 | 5.77 | 18 | 胞外Extracellular |
| StGH61-3 | XP_008024527 | 221 | 23.46 | 8.33 | 17 | 胞外Extracellular |
| StGH61-4 | XP_008025833 | 418 | 43.38 | 7.63 | 17 | 胞外Extracellular |
| StGH61-5 | XP_008021808 | 266 | 28.81 | 8.24 | 21 | 胞外Extracellular |
| StGH61-6 | XP_008023212 | 291 | 32.06 | 6.16 | 22 | 膜结合溶酶体Lysosomes |
| StGH61-7 | XP_008024957 | 248 | 26.32 | 7.69 | 20 | 胞外Extracellular |
| StGH61-8 | XP_008025253 | 329 | 34.56 | 5.98 | 22 | 分泌到胞外 Extracellular(Secreted) |
| StGH61-9 | XP_008030985 | 149 | 15.76 | 7.83 | — | 分泌到胞外 Extracellular(Secreted) |
| StGH61-10 | XP_008022828 | 236 | 25.55 | 9.00 | 20 | 膜结合线粒体Mitochondria |
| StGH61-11 | XP_008023697 | 229 | 23.52 | 8.49 | 16 | 胞外Extracellular |
| StGH61-12 | XP_008023696 | 258 | 27.49 | 5.47 | 22 | 胞外Extracellular |
| StGH61-13 | XP_008028494 | 325 | 34.02 | 6.31 | 18 | 胞外Extracellular |
| StGH61-14 | XP_008028319 | 292 | 30.73 | 6.28 | 21 | 胞外Extracellular |
| StGH61-15 | XP_008025489 | 472 | 49.64 | 7.44 | 19 | 胞外Extracellular |
| StGH61-16 | XP_008027303 | 222 | 23.26 | 7.69 | 20 | 胞外Extracellular |
| StGH61-17 | XP_008027295 | 246 | 25.92 | 8.44 | 18 | 胞外Extracellular |
| StGH61-18 | XP_008024355 | 344 | 34.75 | 6.49 | 17 | 胞外Extracellular |
| StGH61-19 | XP_008022590 | 243 | 25.06 | 8.90 | 17 | 胞外Extracellular |
| StGH61-20 | XP_008026441 | 233 | 24.21 | 7.67 | 18 | 胞外Extracellular |
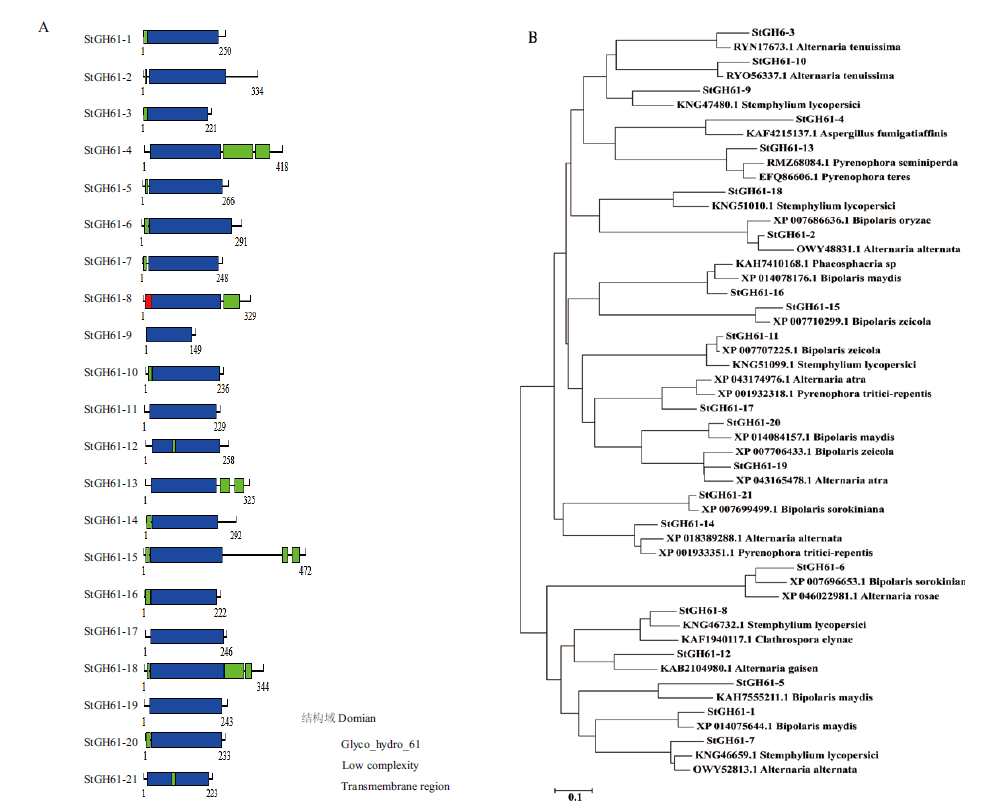
图1 大斑刚毛座腔菌糖苷水解酶GH61家族蛋白结构域及进化分析 A:大斑刚毛座腔菌糖苷水解酶GH61家族蛋白结构域;B:大斑刚毛座腔菌糖苷水解酶GH61家族蛋白进化关系
Fig. 1 Protein domains and evolutionary analysis of the GH61 family of glycoside hydrolases from S. turcica A: Domain of GH61 family of glycoside hydrolases from S. turcica. B: Evolutionary relationship of glycoside hydrolases GH61 family proteins from S. turcica
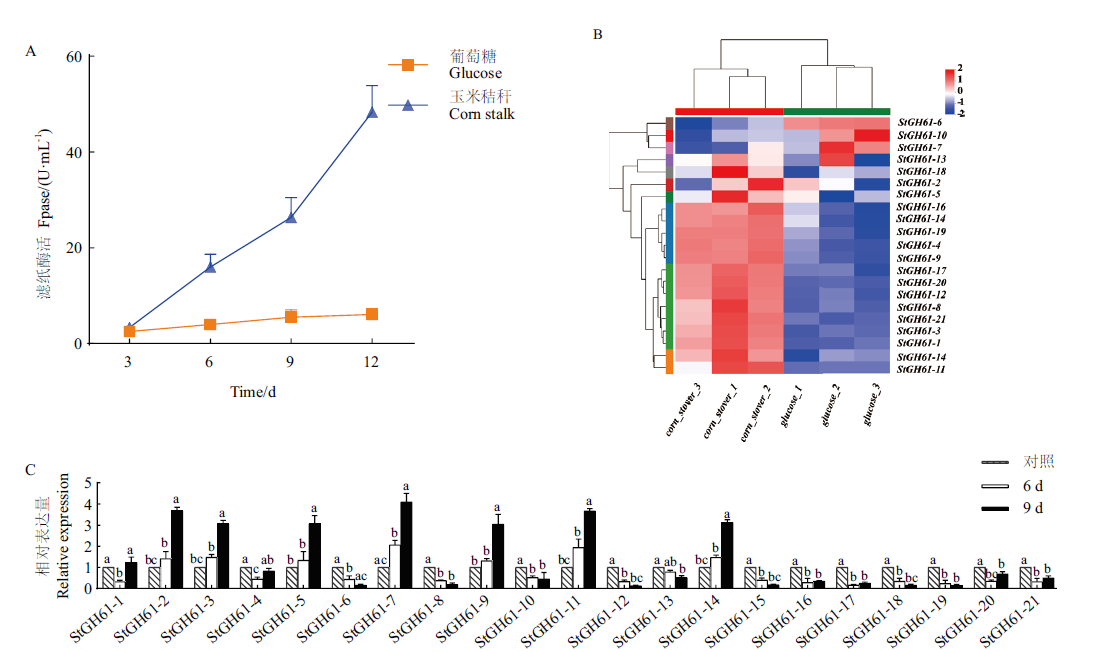
图2 大斑刚毛座腔菌纤维素酶的滤纸酶活及GH61家族基因表达分析 A:大斑刚毛座腔菌纤维素酶的滤纸酶活;B:大斑刚毛座腔菌中GH61家族糖苷水解酶基因的表达图谱;C:RT-qPCR验证大斑刚毛座腔菌中GH61家族糖苷水解酶基因相对表达量。不同小写字母表示同一基因在不同处理下存在显著差异(P<0.05)
Fig. 2 Filter paper enzymatic activity and GH61 family gene expression analysis of cellulase from S. turcica A: Filter paper enzymatic activity of cellulase from S. turcica. B: Expression map of GH61 family glycoside hydrolase genes in S. turcica. C: RT-qPCR verifies the relative expression of GH61 family glycoside hydrolase genes in S. turcica. Different normal letters indicate significant differences among different treatments at 0.05 level

图3 StGH61-11重组蛋白异源表达及优化诱导条件 A:目的片段StGH61-11的PCR扩增产物琼脂糖凝胶电泳结果, 1-3:StGH61-11的cDNA片段, M:DNA marker;B:PCR验证重组转化子的琼脂糖凝胶电泳结果, 1-3:Pet32a-StGH61-11 重组质粒, M:DNA marker;C:SDS-PAGE 检测pET32a-StGH61-11(BL21)重组菌表达蛋白, 0:Pet32a-StGH61-11-BL21(DE3)重组菌未诱导对照, 1 :Pet32a-StGH61-11-BL21(DE3)重组菌经IPTG诱导后, M :预染蛋白分子量标准;D:诱导时间对重组蛋白StGH61-11酶活的影响;E:IPTG浓度对重组蛋白StGH61-11酶活的影响
Fig. 3 Heterologous expression of StGH61-11 recombinant protein and optimized induction conditions A: Agarose gel electrophoresis results of the target gene StGH61-11 PCR products, 1-3: GH61-11 cDNA fragment. M:DNA marker. B: Agarose gel electrophoresis results of PCR for recombinant transformants, 1-3: Pet32a-StGH61-11 recombinant plasmid, M:DNA marker. C: SDS-PAGE detection of whole bacterial lysate expressed by recombinant pET32a-StGH61-11(BL21), 0 : Pet32a-StGH61-11-BL21(DE3)not induce control, 1 : Pet32a-StGH61-11-BL21(DE3)the recombinant bacteria were induced by IPTG, M : Standard for molecular weight of pre-dyed protein. D: Effect of induction time on the enzymatic activity of recombinant protein StGH61-11. E: Effect of IPTG concentration on the enzymatic activity of recombinant protein StGH61-11
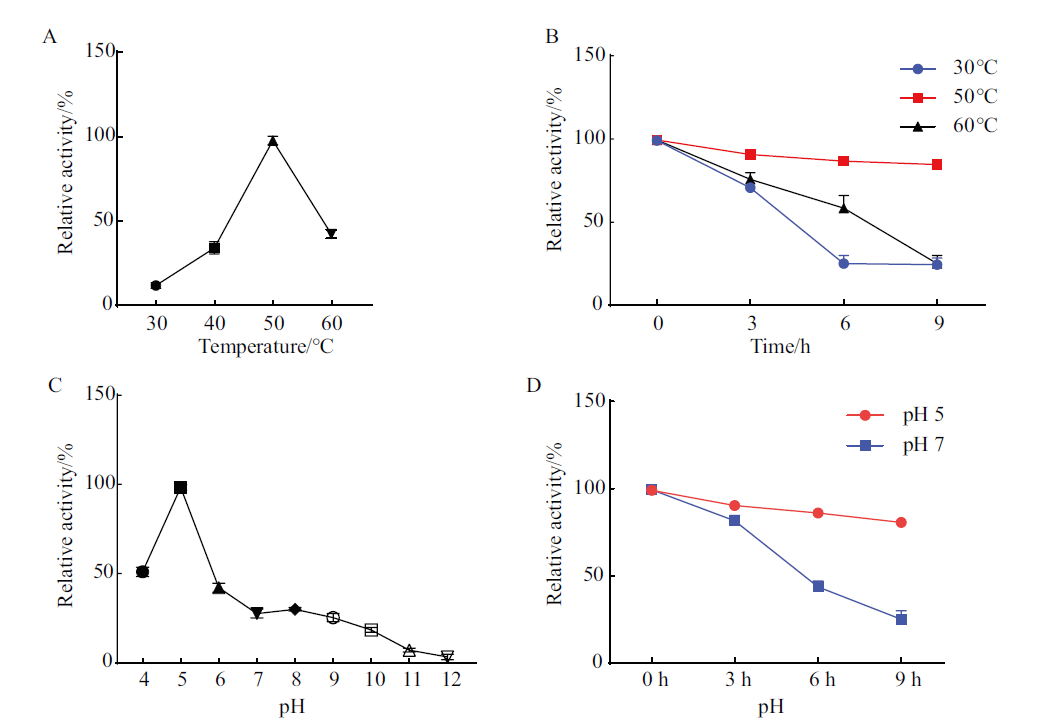
图4 StGH61-11重组蛋白的最适反应温度和稳定性及pH和稳定性 A:温度对StGH61-11纤维素酶活力的影响;B:温度对StGH61-11纤维素酶活力的稳定性影响;C:pH对StGH61-11纤维素酶活力的影响;D:pH对StGH61-11纤维素酶活力稳定性的影响
Fig. 4 Optimal reaction temperature and stability and pH and stability of StGH61-11 recombinant protein A: Effects of temperature on the cellulase activity of StGH61-11. B: Effect of temperature on the stability of StGH61-11 cellulase activity. C: Effects of pH on StGH61-11 cellulase activity. D: Effect of pH on the viability stability of StGH61-11 cellulase

图5 StGH61-11 与纤维素酶水解玉米秸秆的协同反应 A:StGH61-11对纤维素酶活性的促进作用;B:StGH61-11与纤维素酶共同作用的协同度
Fig. 5 Synergistic reaction of StGH61-11 with cellulase hydrolysis of corn stover A: Promoting effect of StGH61-11 on cellulase activity. B: Synergism degree of StGH61-11 and cellulase
| [1] |
Huang Z, Ni GR, Zhao XY, et al. Characterization of a GH8 β-1, 4-glucanase from Bacillus subtilis B111 and its saccharification potential for agricultural straws[J]. J Microbiol Biotechnol, 2021, 31(10): 1446-1454.
doi: 10.4014/jmb.2105.05026 URL |
| [2] | 许从峰, 艾士奇, 申贵男, 等. 木质纤维素的微生物降解[J]. 生物工程学报, 2019, 35(11): 2081-2091. |
| Xu CF, Ai SQ, Shen GN, et al. Microbial degradation of lignocellulose[J]. Chin J Biotechnol, 2019, 35(11): 2081-2091. | |
| [3] |
Østby H, Hansen LD, Horn SJ, et al. Enzymatic processing of lignocellulosic biomass: principles, recent advances and perspectives[J]. J Ind Microbiol Biotechnol, 2020, 47(9/10): 623-657.
doi: 10.1007/s10295-020-02301-8 URL |
| [4] |
Zhang WR, Wang WW, Wang JH, et al. Isolation and characterization of a novel laccase for lignin degradation, LacZ1[J]. Appl Environ Microbiol, 2021, 87(23): e0135521.
doi: 10.1128/AEM.01355-21 URL |
| [5] |
Kucharska K, Rybarczyk P, Hołowacz I, et al. Pretreatment of lignocellulosic materials as substrates for fermentation processes[J]. Molecules, 2018, 23(11): 2937.
doi: 10.3390/molecules23112937 URL |
| [6] |
Wang YS, Shao Y, Zou XY, et al. Synergistic action between extracellular products from white-rot fungus and cellulase significantly improves enzymatic hydrolysis[J]. Bioengineered, 2018, 9(1): 178-185.
doi: 10.1080/21655979.2017.1308991 pmid: 28384075 |
| [7] | 饶佳, 鲍大鹏, 李燕, 等. 草菇GH61家族基因的生物信息学分析及金属离子对其表达水平的影响[J]. 菌物学报, 2016, 35(5): 586-596. |
| Rao J, Bao DP, Li Y, et al. Bioinformatic and gene expression analyses of the GH61 family genes of Volvariella volvacea[J]. Mycosystema, 2016, 35(5): 586-596. | |
| [8] |
Sun PC, Valenzuela SV, Chunkrua P, et al. Oxidized product profiles of AA9 lytic polysaccharide monooxygenases depend on the type of cellulose[J]. ACS Sustain Chem Eng, 2021, 9(42): 14124-14133.
doi: 10.1021/acssuschemeng.1c04100 pmid: 34722005 |
| [9] |
Waghmare PR, Waghmare PP, Gao LW, et al. Efficient constitutive expression of cellulolytic enzymes in Penicillium oxalicum for improved efficiency of lignocellulose degradation[J]. J Microbiol Biotechnol, 2021, 31(5): 740-746.
doi: 10.4014/jmb.2101.01003 URL |
| [10] |
Vaaje-Kolstad G, Westereng B, Horn SJ, et al. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides[J]. Science, 2010, 330(6001): 219-222.
doi: 10.1126/science.1192231 pmid: 20929773 |
| [11] |
Morgenstern I, Powlowski J, Tsang A. Fungal cellulose degradation by oxidative enzymes: from dysfunctional GH61 family to powerful lytic polysaccharide monooxygenase family[J]. Brief Funct Genomics, 2014, 13(6): 471-481.
doi: 10.1093/bfgp/elu032 pmid: 25217478 |
| [12] |
Pierce BC, Agger JW, Zhang ZH, et al. A comparative study on the activity of fungal lytic polysaccharide monooxygenases for the depolymerization of cellulose in soybean spent flakes[J]. Carbohydr Res, 2017, 449: 85-94.
doi: 10.1016/j.carres.2017.07.004 URL |
| [13] |
Zhang RQ. Functional characterization of cellulose-degrading AA9 lytic polysaccharide monooxygenases and their potential exploitation[J]. Appl Microbiol Biotechnol, 2020, 104(8): 3229-3243.
doi: 10.1007/s00253-020-10467-5 pmid: 32076777 |
| [14] |
Mazurkewich S, Seveso A, Hüttner S, et al. Structure of a C1/C4-oxidizing AA9 lytic polysaccharide monooxygenase from the thermophilic fungus Malbranchea cinnamomea[J]. Acta Crystallogr D Struct Biol, 2021, 77(Pt 8): 1019-1026.
doi: 10.1107/S2059798321006628 URL |
| [15] |
Meng YN, Zeng FL, Hu JJ, et al. Novel factors contributing to fungal pathogenicity at early stages of Setosphaeria turcica infection[J]. Mol Plant Pathol, 2022, 23(1): 32-44.
doi: 10.1111/mpp.13140 URL |
| [16] | 王晶晶. StPP2A-c基因调控玉米大斑病菌致病性的机制研究[D]. 保定: 河北农业大学, 2013. |
| Wang JJ. The mechanism of StPP2A-c gene regulating the pathogenicity in Setosphaeria turcica[D]. Baoding: Hebei Agricultural University, 2013. | |
| [17] |
常晴, 束月蓉, 王文韬, 等. 来自Yeosuana marina sp. JLT21内切型海藻酸裂解酶的异源表达及酶学表征[J]. 生物技术通报, 2022, 38(2): 123-131.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0539 |
| Chang Q, Shu YR, Wang WT, et al. Heterologous expression and characterization of endo-type alginate lyase from Yeosuana marina sp. JLT21[J]. Biotechnol Bull, 2022, 38(2): 123-131. | |
| [18] |
薛鲜丽, 王静然, 毕杭杭, 等. 过表达Spt7对黑曲霉生长及抗逆性影响[J]. 生物技术通报, 2022, 38(5): 112-122.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0946 |
| Xue XL, Wang JR, Bi HH, et al. Effect of Spt7 overexpression of on the growth and stress resistance of Aspergillus niger[J]. Biotechnol Bull, 2022, 38(5): 112-122. | |
| [19] |
Breslmayr E, Hanžek M, Hanrahan A, et al. A fast and sensitive activity assay for lytic polysaccharide monooxygenase[J]. Biotechnol Biofuels, 2018, 11: 79.
doi: 10.1186/s13068-018-1063-6 pmid: 29588664 |
| [20] |
de Gouvêa PF, Bernardi AV, Gerolamo LE, et al. Transcriptome and secretome analysis of Aspergillus fumigatus in the presence of sugarcane bagasse[J]. BMC Genomics, 2018, 19(1): 232.
doi: 10.1186/s12864-018-4627-8 |
| [21] | 白雪, 张梦娣, 张东远, 等. 丝状真菌溶解性多糖单加氧酶的研究进展[J]. 基因组学与应用生物学, 2018, 37(12): 5339-5348. |
| Bai X, Zhang MD, Zhang DY, et al. Research progress on lytic polysaccharide monooxygenases(LPMOs)in filamentous fungi[J]. Genom Appl Biol, 2018, 37(12): 5339-5348. | |
| [22] |
Midorikawa GEO, Correa CL, Noronha EF, et al. Analysis of the transcriptome in Aspergillus tamarii during enzymatic degradation of sugarcane bagasse[J]. Front Bioeng Biotechnol, 2018, 6: 123.
doi: 10.3389/fbioe.2018.00123 URL |
| [23] |
de Gouvêa PF, Gerolamo LE, Bernardi AV, et al. Lytic polysaccharide monooxygenase from Aspergillus fumigatus can improve enzymatic cocktail activity during sugarcane bagasse hydrolysis[J]. Protein Pept Lett, 2019, 26(5): 377-385.
doi: 10.2174/0929866526666190228163629 URL |
| [24] | 郭宵, 安亚静, 柴成程, 等. 大肠杆菌分泌表达裂解性多糖单加氧酶发酵条件的优化[J]. 食品与发酵工业, 2020, 46(5): 31-37. |
| Guo X, An YJ, Chai CC, et al. Fermentation condition optimization of recombinant lytic polysaccharide monooxygenase extracellularly expressed in Escherichia coli[J]. Food Ferment Ind, 2020, 46(5): 31-37. | |
| [25] |
Bernardi AV, Gerolamo LE, de Gouvêa PF, et al. LPMO af AA9_B and cellobiohydrolase af Cel6A from A. fumigatus boost enzymatic saccharification activity of cellulase cocktail[J]. Int J Mol Sci, 2020, 22(1): 276.
doi: 10.3390/ijms22010276 URL |
| [26] | 冯玉和, 孙小宝, 陈书昕, 等. 米曲霉裂解性多糖单加氧酶的异源表达与性质分析[J]. 微生物学报, 2020, 60(1): 183-199. |
| Feng YH, Sun XB, Chen SX, et al. Heterologous expression and characterization of Aspergillus oryzae lytic polysaccharide monooxygenases[J]. Acta Microbiol Sin, 2020, 60(1): 183-199. | |
| [27] | 夏东慧, 刘宁, 郭秀娜, 等. 嗜热毛壳菌多糖单加氧酶的氧化特性及协同作用[J]. 菌物学报, 2022, 41(7): 1068-1079. |
| Xia DH, Liu N, Guo XN, et al. The oxidation properties and synergism of polysaccharide monooxygenase from Chaetomium thermophilum[J]. Mycosystema, 2022, 41(7): 1068-1079. | |
| [28] |
Singh RK, Blossom BM, Russo DA, et al. Thermal unfolding and refolding of a lytic polysaccharide monooxygenase from Thermoascus aurantiacus[J]. RSC Adv, 2019, 9(51): 29734-29742.
doi: 10.1039/C9RA05920B URL |
| [29] |
Tuveng TR, Jensen MS, Fredriksen L, et al. A thermostable bacterial lytic polysaccharide monooxygenase with high operational stability in a wide temperature range[J]. Biotechnol Biofuels, 2020, 13(1): 194.
doi: 10.1186/s13068-020-01834-5 pmid: 33292445 |
| [30] | 马清. 黑曲霉多糖单加氧酶的克隆表达与协同性研究[D]. 天津: 天津科技大学, 2018. |
| Ma Q. Cloning of lytic polysaccharide monooxygenases genes from asperillus Niger and research on its synergism activity[D]. Tianjin: Tianjin University of Science & Technology, 2018. |
| [1] | 饶紫环, 谢志雄. 一株Olivibacter jilunii 纤维素降解菌株的分离鉴定与降解能力分析[J]. 生物技术通报, 2023, 39(8): 283-290. |
| [2] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [3] | 张晶, 张浩睿, 曹云, 黄红英, 曲萍, 张志萍. 嗜热纤维素降解菌研究进展[J]. 生物技术通报, 2023, 39(6): 73-87. |
| [4] | 陈楠楠, 王春来, 蒋振忠, 焦鹏, 关淑艳, 马义勇. 玉米ZmDHN15基因在烟草中的遗传转化及抗冷性分析[J]. 生物技术通报, 2023, 39(4): 259-267. |
| [5] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [6] | 杨俊钊, 张新蕊, 孙清扬, 郑菲. Loop B3对GH7内切纤维素酶功能的影响机制[J]. 生物技术通报, 2023, 39(10): 281-291. |
| [7] | 张开平, 刘燕丽, 涂绵亮, 李继伟, 吴文标. 烟曲霉A-16产纤维素酶工艺优化及酶学特性[J]. 生物技术通报, 2022, 38(9): 215-225. |
| [8] | 王新光, 田磊, 王恩泽, 钟成, 田春杰. 玉米秸秆高效降解微生物复合菌系的构建及降解效果评价[J]. 生物技术通报, 2022, 38(4): 217-229. |
| [9] | 牛馨, 张莹, 王茂军, 刘文龙, 路福平, 李玉. 解淀粉芽胞杆菌不同整合位点对外源碱性蛋白酶表达的影响[J]. 生物技术通报, 2022, 38(4): 253-260. |
| [10] | 王玥, 高庆华, 董聪, 罗同阳, 王庆庆. 密码子优化的吡喃糖氧化酶基因在毕赤酵母中的表达[J]. 生物技术通报, 2022, 38(4): 269-277. |
| [11] | 张功友, 王一涵, 郭敏, 张婷婷, 王兵, 刘红美. 重楼中一株产纤维素酶内生真菌的分离及鉴定[J]. 生物技术通报, 2022, 38(2): 95-104. |
| [12] | 王博雅, 姜勇, 黄艳, 曹颖, 胡尚连. 慈竹纤维素合酶BeCesA4的克隆及功能分析[J]. 生物技术通报, 2022, 38(11): 185-193. |
| [13] | 王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268. |
| [14] | 张彤彤, 郑登俞, 吴忠义, 张中保, 于荣. 玉米NF-Y转录因子基因ZmNF-YB13响应干旱和盐胁迫的功能分析[J]. 生物技术通报, 2022, 38(10): 115-123. |
| [15] | 唐昊, 孙灿, 李沅秋, 罗朝兵. 纤维素降解菌Raoultella ornithinolytica LL1的筛选及基因组测序[J]. 生物技术通报, 2021, 37(6): 85-96. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||