








生物技术通报 ›› 2023, Vol. 39 ›› Issue (12): 320-328.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1469
魏婷柳1( ), 苗华彪1,2, 吴倩1,2, 黄遵锡1,2(
), 苗华彪1,2, 吴倩1,2, 黄遵锡1,2( )
)
收稿日期:2022-11-29
出版日期:2023-12-26
发布日期:2024-01-11
通讯作者:
黄遵锡,男,教授,博士生导师,研究方向:酶工程;E-mail: huangzunxi@163.com作者简介:魏婷柳,女,硕士研究生,研究方向:微生物;E-mail: wtl18468283070@163.com
基金资助:
WEI Ting-liu1( ), MIAO Hua-biao1,2, WU Qian1,2, HUANG Zun-xi1,2(
), MIAO Hua-biao1,2, WU Qian1,2, HUANG Zun-xi1,2( )
)
Received:2022-11-29
Published:2023-12-26
Online:2024-01-11
摘要:
为开发能高效降解游离棉酚的酶蛋白。从土壤中筛选得到一株Bacillus amyloliquefaciens XP-13,从其基因组扩增获得了基因BmLac,进而构建Ppic9k-BmLac重组质粒,将其导入毕赤酵母GS115实现了异源表达,对漆酶BmLac的酶学特性、棉酚降解效果进行研究。结果表明,以ABTS为底物时,该酶的最适反应条件为pH 4,55℃,最适条件下酶活是101.56 U/mL;在90℃下孵育1 h后,相对酶活仍保留70%以上;在pH 3-7孵育1 h,其相对酶活均保留96%以上。1 mmol/L的Cu2+能使该酶酶活提高1.93倍,但高浓度(10 mmol/L)的Fe2+、Fe3+和Al3+完全抑制该酶活性。在55℃下反应2 h后,该酶对棉酚的降解率高达94.57%(无介体)和98.73%(有ABTS)。综上,漆酶BmLac具有良好的热稳定性和广泛的pH适应性,并能有效降解棉酚,该酶为有效降解棉籽粕中的游离棉酚提供基础支撑。
魏婷柳, 苗华彪, 吴倩, 黄遵锡. 漆酶BmLac的异源表达、酶学特性及棉酚降解的研究[J]. 生物技术通报, 2023, 39(12): 320-328.
WEI Ting-liu, MIAO Hua-biao, WU Qian, HUANG Zun-xi. Heterologous Expression, Enzymatic Characterization of Laccase BmLac and Degradation of Gossypol by It[J]. Biotechnology Bulletin, 2023, 39(12): 320-328.

图1 XP-13 菌株鉴定 A:16S rDNA凝胶电泳图;B:菌株XP-13芽孢光学显微镜图;C:菌株XP-13基于16S rDNA构建系统发育树
Fig. 1 Identification of XP-13 strain A: Gel electrophoresis of 16S rDNA. B: Light micrograph of strain XP-13 bacteriop-hage. C: Phylogenetic tree construction of strain XP-13 based on 16S rDNA

图2 漆酶BmLac的分析 A:BmLac二级结构;B:BmLac以1GSK同源建模三级结构(紫色表示β-sheet,蓝色表示α-helix)
Fig. 2 Analysis of laccase BmLac A : The secondary structure of BmLac. B: The 3D structure of BmLac with 1GSK homology (purple indicates β-sheet. Blue indicates α-helix)

图3 BmLac和pPic9k凝胶电泳图 A:BmLac基因凝胶电泳图;B:pPic9k双酶切凝胶电泳图;1:目的条带;M:DNA maker
Fig. 3 BmLac and pPic9k gel electrophoresis A: Gel electrophoresis map of BmLac gene. B: Gel electrophoresis map of pPic9k double digestion. 1 : Target band; M: DNA maker
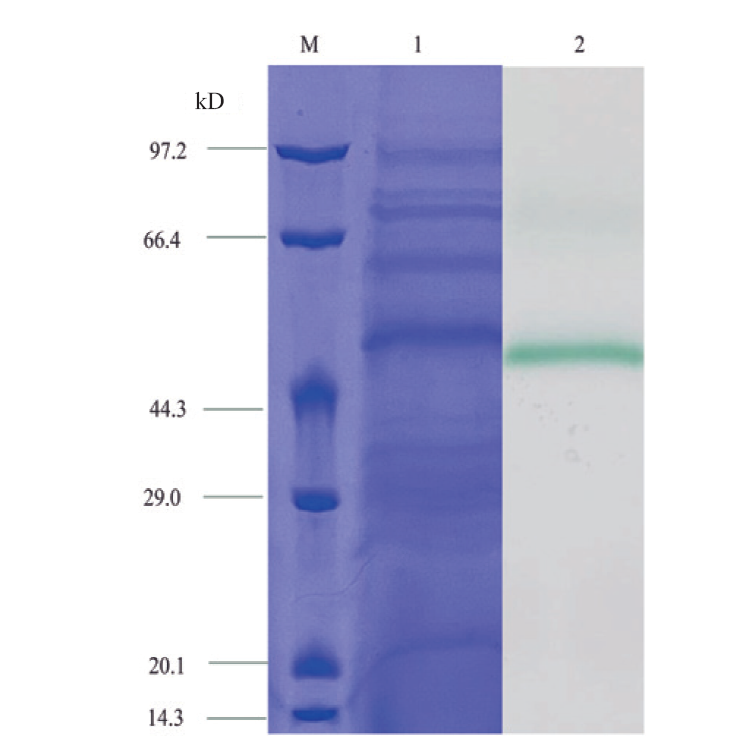
图4 BmLac的SDS-PAGE M:蛋白Maker;1:失活的BmLac粗酶液;2:0.5 mmol/L ABTS染色BmLac粗酶液1 h
Fig. 4 SDS-PAGE of BmLac M: Protein maker. 1: Inactivated BmLac crude enzyme solution. 2: 0.5 mmol/L ABTS staining BmLac crude enzyme solution for 1 h

图6 BmLac最适温度及温度稳定性 A:最适温度;B:温度稳定性
Fig. 6 Optimal temperature and temperature stability of the BmLac A : Optimal temperature. B : Temperature stability
| 试剂 Reagent | 相对酶活1 Relative activity 1/% | 相对酶活2 Relative activity 2/% |
|---|---|---|
| None | 100±1.43 | 100±4.20 |
| CuSO4 | 193.09±5.05 | 98.71±2.44 |
| MnSO4 | 102.05±1.99 | 96.56±3.25 |
| Pb(CH3CO2)2 | 99.69±2.78 | 69.35±6.03 |
| MgSO4 | 98.36±2.15 | 97.15±4.36 |
| ZnSO4 | 98.26±1.13 | 86.13±4.69 |
| NiSO4 | 94.32±1.63 | 61.77±0.43 |
| KCl | 93.15±3.11 | 58.60±5.83 |
| NaCl | 90.74±0.49 | 59.52±1.77 |
| CaCl2 | 90.33±2.07 | 45.32±4.09 |
| FeCl3 | 90.43±1.83 | 0.00 |
| LiCl | 88.34±1.78 | 65.11±3.04 |
| CoCl2 | 84.30±3.28 | 19.84±1.26 |
| AlCl3 | 71.92±2.70 | 0.00 |
| Fe SO4 | 2.92±0.80 | 0.00 |
| Guanidine hydrochloride | 95.40±3.40 | 69.68±2.12 |
| Urea | 92.53±2.93 | 103.49±3.87 |
| EDTA | 86.75±1.29 | 42.37±1.25 |
表1 金属离子和化学试剂对BmLac的影响
Table 1 Effects of metal ions and chemical reagents on BmLac
| 试剂 Reagent | 相对酶活1 Relative activity 1/% | 相对酶活2 Relative activity 2/% |
|---|---|---|
| None | 100±1.43 | 100±4.20 |
| CuSO4 | 193.09±5.05 | 98.71±2.44 |
| MnSO4 | 102.05±1.99 | 96.56±3.25 |
| Pb(CH3CO2)2 | 99.69±2.78 | 69.35±6.03 |
| MgSO4 | 98.36±2.15 | 97.15±4.36 |
| ZnSO4 | 98.26±1.13 | 86.13±4.69 |
| NiSO4 | 94.32±1.63 | 61.77±0.43 |
| KCl | 93.15±3.11 | 58.60±5.83 |
| NaCl | 90.74±0.49 | 59.52±1.77 |
| CaCl2 | 90.33±2.07 | 45.32±4.09 |
| FeCl3 | 90.43±1.83 | 0.00 |
| LiCl | 88.34±1.78 | 65.11±3.04 |
| CoCl2 | 84.30±3.28 | 19.84±1.26 |
| AlCl3 | 71.92±2.70 | 0.00 |
| Fe SO4 | 2.92±0.80 | 0.00 |
| Guanidine hydrochloride | 95.40±3.40 | 69.68±2.12 |
| Urea | 92.53±2.93 | 103.49±3.87 |
| EDTA | 86.75±1.29 | 42.37±1.25 |

图8 BmLac降解棉酚的HPLC分析 A:不加介体ABTS;B:加介体1.25 mmol/L ABTS;C:棉酚降解率
Fig. 8 HPLC analysis of BmLac degrading gossypol A:Without mediator ABTS. B:With 1.25 mmol/L ABTS. C:Gossypol degradation rate
| [24] |
Givaudan A, Effosse A, Faure D, et al. Polyphenol oxidase in Azos-pirillum lipoferum isolated from rice rhizosphere: evidence for laccase activity in non-motile strains of Azospirillum lipoferum[J]. FEMS Microbiol Lett, 1993, 108(2): 205-210.
doi: 10.1111/fml.1993.108.issue-2 URL |
| [25] |
Yang S, Long Y, et al. Gene cloning, identification, and characterization of the multicopper oxidase CumA from Pseudomonas sp. 593[J]. Biotechnol Appl Biochem, 2017, 64(3): 347-355.
doi: 10.1002/bab.2017.64.issue-3 URL |
| [26] |
Zeng J, Lin XG, Zhang J, et al. Oxidation of polycyclic aromatic hydrocarbons by the bacterial laccase CueO from E. coli[J]. Appl Microbiol Biotechnol, 2011, 89(6): 1841-1849.
doi: 10.1007/s00253-010-3009-1 pmid: 21120471 |
| [27] |
Qiao WC, Chu JP, Ding SJ, et al. Characterization of a thermo-alkali-stable laccase from Bacillus subtilis cjp3 and its application in dyes decolorization[J]. J Environ Sci Health A Tox Hazard Subst Environ Eng, 2017, 52(8): 710-717.
doi: 10.1080/10934529.2017.1301747 URL |
| [28] |
Brander S, Mikkelsen JD, Kepp KP. Characterization of an alkali- and halide-resistant laccase expressed in E. coli: CotA from Bacil-lus clausii[J]. PLoS One, 2014, 9(6): e99402.
doi: 10.1371/journal.pone.0099402 URL |
| [29] |
Wang HB, Huang L, Li YZ, et al. Characterization and application of a novel laccase derived from Bacillus amyloliquefaciens[J]. Int J Biol Macromol, 2020, 150: 982-990.
doi: 10.1016/j.ijbiomac.2019.11.117 URL |
| [30] |
Lu L, Wang TN, Xu TF, et al. Cloning and expression of thermo-alkali-stable laccase of Bacillus licheniformis in Pichia pastoris and its characterization[J]. Bioresour Technol, 2013, 134: 81-86.
doi: 10.1016/j.biortech.2013.02.015 URL |
| [31] |
Reiss R, Ihssen J, Thöny-Meyer L. Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum[J]. BMC Biotechnol, 2011, 11: 9.
doi: 10.1186/1472-6750-11-9 |
| [32] |
Wan J, Sun XW, Liu C, et al. Decolorization of textile dye RB19 using volcanic rock matrix immobilized Bacillus thuringiensis cells with surface displayed laccase[J]. World J Microbiol Biotechnol, 2017, 33(6): 123.
doi: 10.1007/s11274-017-2290-x URL |
| [33] |
Wang L, Chen M, Luo XC, et al. Intramolecular annulation of gossypol by laccase to produce safe cottonseed protein[J]. Front Chem, 2020, 8: 583176.
doi: 10.3389/fchem.2020.583176 URL |
| [34] | 张乐珊. 重组漆酶OhLac的分子改造及其降解性能研究[D]. 杨凌: 西北农林科技大学, 2022. |
| Zhang LS. Molecular modification and degradation of recombinant laccase OhLac[D]. Yangling: Northwest A & F University, 2022. | |
| [35] |
周梦宇, 古丽斯坦·阿不来提, 姚军, 等. 高效液相色谱法同时测定棉籽油中游离棉酚及其降解产物四甲氧基棉酚含量[J]. 食品科学, 2019, 40(16): 261-266.
doi: 10.7506/spkx1002-6630-20180926-282 |
| Zhou MY, Abulaiti G, Yao J, et al. Simultaneous determination of free gossypol and its degradation product tetramethoxy gossypol in commercially available cottonseed oil by high performance liquid chromatography[J]. Food Sci, 2019, 40(16): 261-266. | |
| [36] | Gadelha ICN, Fonseca NBS, Oloris SCS, et al. Gossypol toxicity from cottonseed products[J]. Sci World J, 2014, 231635. |
| [37] |
王雨辰, 丁尊丹, 关菲菲, 等. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-1422 |
| Wang YC, Ding ZD, Guan FF, et al. Identification and characterization of heat-resistant laccase Ba4 gene[J]. Biotechnol Bull, 2022, 38(8): 252-260. | |
| [38] |
Sharma P, Goel R, Capalash N. Bacterial laccases[J]. World J Microbiol Biotechnol, 2007, 23(6): 823-832.
doi: 10.1007/s11274-006-9305-3 URL |
| [39] |
El-Bendary MA, Ezzat SM, et al. Optimization of spore laccase production by Bacillus amyloliquefaciens isolated from wastewater and its potential in green biodecolorization of synthetic textile dyes[J]. Prep Biochem Biotechnol, 2021, 51(1): 16-27.
doi: 10.1080/10826068.2020.1786698 URL |
| [40] | You LF, Liu ZM, Lin JF, et al. Molecular cloning of a laccase gene from Ganoderma lucidum and heterologous expression in Pichia pastoris[J]. J Basic Microbiol, 2014, 54(Suppl 1): S134-S141. |
| [41] | Radveikienė I, Vidžiūnaitė R, Meškienė R, et al. Characterization of a yellow laccase from Botrytis cinerea 241[J]. J Fungi(Basel), 2021, 7(2): 143. |
| [42] |
Li T, Chu XX, Yuan ZT, et al. Biochemical and structural properties of a high-temperature-active laccase from Bacillus pumilus and its application in the decolorization of food dyes[J]. Foods, 2022, 11(10): 1387.
doi: 10.3390/foods11101387 URL |
| [43] |
Zhang WR, Wang WW, Wang JH, et al. Isolation and characterization of a novel laccase for lignin degradation, LacZ1[J]. Appl Environ Microbiol, 2021, 87(23): e0135521.
doi: 10.1128/AEM.01355-21 URL |
| [44] |
Lin JH, Liu YJ, Chen S, et al. Reversible immobilization of laccase onto metal-ion-chelated magnetic microspheres for bisphenol A removal[J]. Int J Biol Macromol, 2016, 84: 189-199.
doi: 10.1016/j.ijbiomac.2015.12.013 pmid: 26691384 |
| [45] | Irshad M. Production and optimization of ligninolytic enzymes by white rot fungus Schizophyllum commune IBL-06 in solid state medium banana stalks[J]. Afr J Biotechnol, 2011, 10(79): 18234-18242. |
| [1] |
Rehemujiang H, Yimamu A, Wang YL. Effect of dietary cotton stalk on nitrogen and free gossypol metabolism in sheep[J]. Asian-Australas J Anim Sci, 2019, 32(2): 233-240.
doi: 10.5713/ajas.18.0057 URL |
| [2] |
Zhang YH, Zhang ZY, et al. Isolation and characterization of a novel gossypol-degrading bacteria Bacillus subtilis strain Rumen Bacillus Subtilis[J]. Asian-Australas J Anim Sci, 2018, 31(1): 63-70.
doi: 10.5713/ajas.17.0018 URL |
| [3] |
Wang WK, Wang YL, Li WJ, et al. In situ rumen degradation characteristics and bacterial colonization of whole cottonseed, cottonseed hull and cottonseed meal with different gossypol content[J]. AMB Expr, 2021, 11(1): 91.
doi: 10.1186/s13568-021-01244-2 |
| [4] |
宣秋希, 乔琳, 侯晓林, 等. 固态生料发酵棉籽粕菌种筛选及发酵工艺的研究[J]. 动物营养学报, 2022, 34(5): 3376-3391.
doi: 10.3969/j.issn.1006-267x.2022.05.062 |
| Xuan QX, Qiao L, Hou XL, et al. Screening of strains for solid-state fermentation of raw cottonseed meal and study on fermentation technology[J]. Chin J Animal Nutr, 2022, 34(5): 3376-3391. | |
| [5] |
Tian X, Ruan JX, Huang JQ, et al. Gossypol: phytoalexin of cotton[J]. Sci China Life Sci, 2016, 59(2): 122-129.
doi: 10.1007/s11427-016-5003-z pmid: 26803304 |
| [6] |
Santana AT, Guelfi M, Medeiros HCD, et al. Mechanisms involved in reproductive damage caused by gossypol in rats and protective effects of vitamin E[J]. Biol Res, 2015, 48(1): 43.
doi: 10.1186/s40659-015-0026-7 URL |
| [7] |
Mena H, Santos JE, Huber JT, et al. The effects of feeding varying amounts of gossypol from whole cottonseed and cottonseed meal in lactating dairy cows[J]. J Dairy Sci, 2001, 84(10): 2231-2239.
pmid: 11699455 |
| [8] |
Tang JW, Sun H, et al. Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers[J]. Asian-Australas J Anim Sci, 2012, 25(3): 393-400.
doi: 10.5713/ajas.2011.11381 URL |
| [9] |
Liu YX, Wang LL, Zhao L, et al. Structure, properties of gossypol and its derivatives—from physiological activities to drug discovery and drug design[J]. Nat Prod Rep, 2022, 39(6): 1282-1304.
doi: 10.1039/D1NP00080B URL |
| [10] |
Wang X, Howell CP, Chen F, et al. Gossypol—a polyphenolic compound from cotton plant[J]. Adv Food Nutr Res, 2009, 58: 215-263.
doi: 10.1016/S1043-4526(09)58006-0 pmid: 19878861 |
| [11] | 胡雷雨, 徐方旭, 高岩, 等. 棉籽饼粕综合利用及发展趋势研究[J]. 园艺与种苗, 2017, 37(11): 74-76. |
| Hu LY, Xu FX, Gao Y, et al. Study on the comprehensive utilization and development trend of cottonseed cake[J]. Hortic Seed, 2017, 37(11): 74-76. | |
| [12] |
Zhang WJ, Xu ZR, Sun JY, et al. Effect of selected fungi on the reduction of gossypol levels and nutritional value during solid substrate fermentation of cottonseed meal[J]. J Zhejiang Univ - Sci B, 2006, 7(9): 690-695.
doi: 10.1631/jzus.2006.B0690 URL |
| [13] |
Nikolaivits E, Siaperas R, Agrafiotis A, et al. Functional and transcriptomic investigation of laccase activity in the presence of PCB29 identifies two novel enzymes and the multicopper oxidase repertoire of a marine-derived fungus[J]. Sci Total Environ, 2021, 775: 145818.
doi: 10.1016/j.scitotenv.2021.145818 URL |
| [14] |
Janusz G, Pawlik A, Świderska-Burek U, et al. Laccase properties, physiological functions, and evolution[J]. Int J Mol Sci, 2020, 21(3): 966.
doi: 10.3390/ijms21030966 URL |
| [15] |
Malhotra M, Suman SK. Laccase-mediated delignification and detoxification of lignocellulosic biomass: removing obstacles in energy generation[J]. Environ Sci Pollut Res, 2021, 28(42): 58929-58944.
doi: 10.1007/s11356-021-13283-0 |
| [16] |
Mao GT, Wang K, Wang FY, et al. An engineered thermostable laccase with great ability to decolorize and detoxify malachite green[J]. Int J Mol Sci, 2021, 22(21): 11755.
doi: 10.3390/ijms222111755 URL |
| [17] |
Gupta V, Balda S, Gupta N, et al. Functional substitution of domain 3(T1 copper center)of a novel laccase with Cu ions[J]. Int J Biol Macromol, 2019, 123: 1052-1061.
doi: 10.1016/j.ijbiomac.2018.11.174 URL |
| [18] |
Karittapattawan P, Benchawattananon R. Evaluation of laccase production by monokaryotic strains of edible mushrooms[J]. Pak J Biol Sci, 2021, 24(4): 454-460.
doi: 10.3923/pjbs.2021.454.460 pmid: 34486304 |
| [19] |
Le TT, Murugesan K, Lee CS, et al. Degradation of synthetic pollutants in real wastewater using laccase encapsulated in core-shell magnetic copper alginate beads[J]. Bioresour Technol, 2016, 216: 203-210.
doi: 10.1016/j.biortech.2016.05.077 URL |
| [20] |
Martins LO, Durão P, Brissos V, et al. Laccases of prokaryotic origin: enzymes at the interface of protein science and protein technology[J]. Cell Mol Life Sci, 2015, 72(5): 911-922.
doi: 10.1007/s00018-014-1822-x pmid: 25572294 |
| [21] |
Cheng CM, Patel AK, Singhania RR, et al. Heterologous expression of bacterial CotA-laccase, characterization and its application for biodegradation of malachite green[J]. Bioresour Technol, 2021, 340: 125708.
doi: 10.1016/j.biortech.2021.125708 URL |
| [22] |
Chauhan PS, Goradia B, Saxena A. Bacterial laccase: recent update on production, properties and industrial applications[J]. 3 Biotech, 2017, 7(5): 323.
doi: 10.1007/s13205-017-0955-7 pmid: 28955620 |
| [23] |
Guan ZB, Luo Q, Wang HR, et al. Bacterial laccases: promising biological green tools for industrial applications[J]. Cell Mol Life Sci, 2018, 75(19): 3569-3592.
doi: 10.1007/s00018-018-2883-z |
| [1] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [2] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [3] | 蒋晶晶, 周昭旭, 杜蕙, 吕昭龙, 王春明, 郭建国, 张新瑞, 李继平. 甘肃部分地区苹果褐腐病病原分离鉴定及拮抗细菌筛选[J]. 生物技术通报, 2023, 39(10): 209-218. |
| [4] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| [5] | 贾晨波, 苏一黄, 马秀梅, 王春利, 徐春燕. 端梗霉Z45产漆酶培养基的优化及其对染料的脱色[J]. 生物技术通报, 2022, 38(6): 252-260. |
| [6] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [7] | 常晴, 束月蓉, 王文韬, 蒋昊, 延泉德, 钱政, 高雪纯, 吴金鸿, 张勇. 来自Yeosuana marina sp. JLT21内切型海藻酸裂解酶的异源表达及酶学表征[J]. 生物技术通报, 2022, 38(2): 123-131. |
| [8] | 邱益彬, 马艳琴, 沙媛媛, 朱逸凡, 苏二正, 雷鹏, 李莎, 徐虹. 解淀粉芽孢杆菌分子遗传操作及其应用研究进展[J]. 生物技术通报, 2022, 38(2): 205-217. |
| [9] | 王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268. |
| [10] | 岑潇龙, 雷曦, 马诗云, 陈倩茹, 黄遵锡, 周峻沛, 张蕊. 金黄色葡萄球菌透明质酸裂解酶HylS的异源表达与特性研究[J]. 生物技术通报, 2022, 38(1): 157-167. |
| [11] | 蔡国磊, 陆小凯, 娄水珠, 杨海英, 杜刚. 芽孢杆菌LM基于全基因组的分类鉴定及抑菌原理的研究[J]. 生物技术通报, 2021, 37(8): 176-185. |
| [12] | 田嘉慧, 封佳丽, 卢俊桦, 毛林静, 胡著然, 王莹, 楚杰. 一色齿毛菌漆酶LacT-1的分离纯化与性质研究[J]. 生物技术通报, 2021, 37(8): 186-194. |
| [13] | 陈明雨, 倪烜, 司友斌, 孙凯. 固定化真菌漆酶在环境有机污染修复中的应用研究进展[J]. 生物技术通报, 2021, 37(6): 244-258. |
| [14] | 张瑶心, 王亮节, 郑文, 徐汉琴, 郑恋, 钟静. 产几丁质酶的无色杆菌ZWW8的发酵产酶及酶学性质研究[J]. 生物技术通报, 2021, 37(4): 96-106. |
| [15] | 熊雪, 李鹏, 张贵合, 向准, 陶文广, 周光燕, 和耀威. 不同栽培基质诱导对香菇液体发酵产漆酶活性的影响[J]. 生物技术通报, 2021, 37(12): 50-59. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||