








生物技术通报 ›› 2023, Vol. 39 ›› Issue (10): 323-331.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0291
收稿日期:2023-03-30
出版日期:2023-10-26
发布日期:2023-11-28
通讯作者:
晁跃辉,男,博士,副教授,研究方向:草地植物生物技术;E-mail: chaoyuehui@bjfu.edu.cn作者简介:王欣怡,女,硕士研究生,研究方向:草地植物生物技术;E-mail: nqzxwxy@163.com;基金资助:
WANG Xin-yi1( ), WANG Xiao-qian2, WANG Hong-jun2, CHAO Yue-hui1(
), WANG Xiao-qian2, WANG Hong-jun2, CHAO Yue-hui1( )
)
Received:2023-03-30
Published:2023-10-26
Online:2023-11-28
摘要:
纳米抗体是一种新型的蛋白质工程抗体,其体积小、稳定性强、亲和力高等特性为科学研究提供了新的可能性。FLAG标签是一种广泛应用于生物学研究中的短肽标签,在生物学研究中具有重要的作用。为了制备FLAG标签的纳米抗体,利用酵母双杂交技术筛选出具有高亲和力的纳米抗体,并对制备的FLAG纳米抗体进行性能检验。通过DNA重组技术,构建包含FLAG的诱饵载体,利用酵母双杂交技术,在驼源纳米抗体酵母文库中,筛选针对FLAG标签纳米抗体。在酵母文库中筛选出5个单一的候选抗体DNA序列,为了排除载体本身表达蛋白序列对抗体筛选带来的干扰,通过“点对点”验证方法排除非特异性杂交的可能,通过该操作确认所筛选的5株纳米抗体均能与FLAG标签发生特异性亲和反应。为了制备纳米抗体,构建了5个纳米抗体原核表达载体,并利用大肠杆菌体系进行表达。通过SDS-PAGE和Western杂交(WB)分析,结果显示,成功获得2株可溶性表达的抗FLAG标签蛋白纳米抗体。对这2株纳米抗体与商品化的常规FLAG标签抗体进行效果比对,结果显示制备的2株纳米抗体与商品化抗体均能够识别FLAG多肽及含有FLAG标签的融合蛋白,且在特异性上没有明显区别,表明制备的FLAG纳米抗体具有较好的应用前景。基于酵母双杂交技术,成功筛选并制备了FLAG标签纳米抗体,这一成果不仅丰富了纳米抗体的类型,也为抗体开发及应用提供了新的途径。该研究为进一步研究FLAG标签在生物学研究中的应用,以及纳米抗体在生物工程中的应用提供有力支持。
王欣怡, 王晓倩, 王红军, 晁跃辉. FLAG标签纳米抗体的筛选、表达及验证[J]. 生物技术通报, 2023, 39(10): 323-331.
WANG Xin-yi, WANG Xiao-qian, WANG Hong-jun, CHAO Yue-hui. Screening, Expression, and Validation of Nanobodies with FLAG Tag[J]. Biotechnology Bulletin, 2023, 39(10): 323-331.
| 引物名称 Primer name | 序列 Sequence(5'-3') |
|---|---|
| pGBKT7-FLAG-F | GGAGGCCGAATTCCCGGACTATAAAGACGACGACGATAAAGGGATCCGTCGACCT |
| pGBKT7-FLAG-R | AGGTCGACGGATCCCTTTATCGTCGTCGTCTTTATAGTCCGGGAATTCGGCCTCC |
| T7 promoter | AATACGACTCACTATAGGGC |
| 3'BD | TTTTCGTTTTAAAACCTAAGAGTC |
| 3'AD | AGATGGTGCACGATGCACAG |
| pCold-VHH-F | GATTACGCTCATATGGCCATGGAGGCCAGTCAGGTTCAGCTGCAGGAGTCTGGRGGAGG |
| pCold-VHH-R | GTTTTTCAGTATCTACGATTCATCTGCAGCTTACGCAGAAGAGACGGTGACCWGGGT |
| pCold-F | AAACCACTCCACTGCGTCGTCTG |
| pCold-R | CAGGGATCTTAGATTCTGTG |
表1 本试验所用引物序列
Table 1 Primer sequences used in the experiment
| 引物名称 Primer name | 序列 Sequence(5'-3') |
|---|---|
| pGBKT7-FLAG-F | GGAGGCCGAATTCCCGGACTATAAAGACGACGACGATAAAGGGATCCGTCGACCT |
| pGBKT7-FLAG-R | AGGTCGACGGATCCCTTTATCGTCGTCGTCTTTATAGTCCGGGAATTCGGCCTCC |
| T7 promoter | AATACGACTCACTATAGGGC |
| 3'BD | TTTTCGTTTTAAAACCTAAGAGTC |
| 3'AD | AGATGGTGCACGATGCACAG |
| pCold-VHH-F | GATTACGCTCATATGGCCATGGAGGCCAGTCAGGTTCAGCTGCAGGAGTCTGGRGGAGG |
| pCold-VHH-R | GTTTTTCAGTATCTACGATTCATCTGCAGCTTACGCAGAAGAGACGGTGACCWGGGT |
| pCold-F | AAACCACTCCACTGCGTCGTCTG |
| pCold-R | CAGGGATCTTAGATTCTGTG |
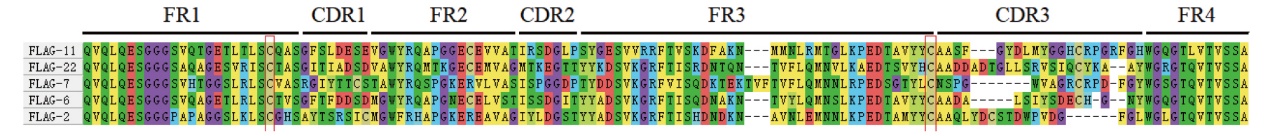
图2 FLAG标签纳米抗体序列及结构 FR1-4:4个骨架区;CDR1-3:3个抗原互补决定区
Fig. 2 Sequences and structures of FLAG-tag nanobody FR1-4: Four fragment regions. CDR1-3: Three complementarity determining regions
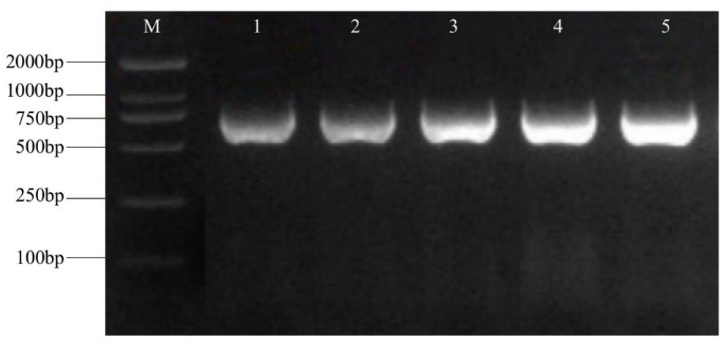
图4 纳米抗体原核表达载体PCR检测 M:MB2000 DNA marker;1-5:5种纳米抗体原核表达载体
Fig. 4 PCR detection of prokaryotic expression vector for nanobodies M: MB2000 DNA marker. 1-5: Prokaryotic expression vectors of 5 nanobodies
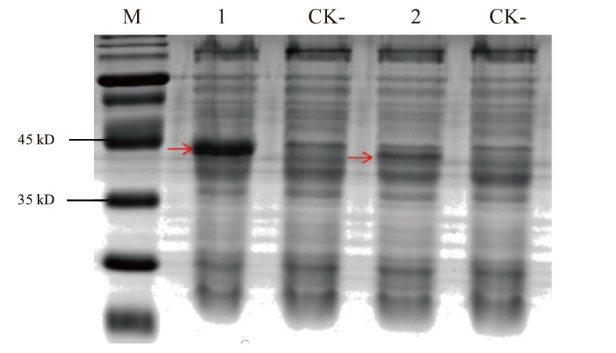
图5 原核表达 M:蛋白质marker;1: Nano-FLAG6;2:Nano-FLAG7;CK-:阴性对照;红色箭头:表达的纳米抗体
Fig. 5 Prokaryotic expression M: Protein marker. 1: Nano-FLAG6. 2: Nano-FLAG7. CK-: Negative controls. Red arrows: Expressed nanobodies
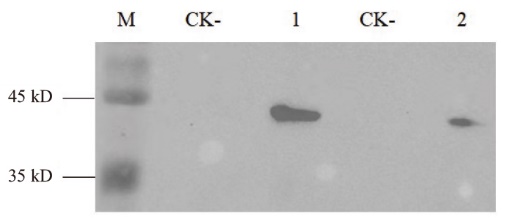
图6 Western blot检测 M:蛋白质marker;1:Nano-FLAG6;2:Nano-FLAG7;CK-:阴性对照
Fig. 6 Western blot detection M: Protein marker. 1: Nano-FLAG6. 2: Nano-FLAG7. CK-: Negative controls
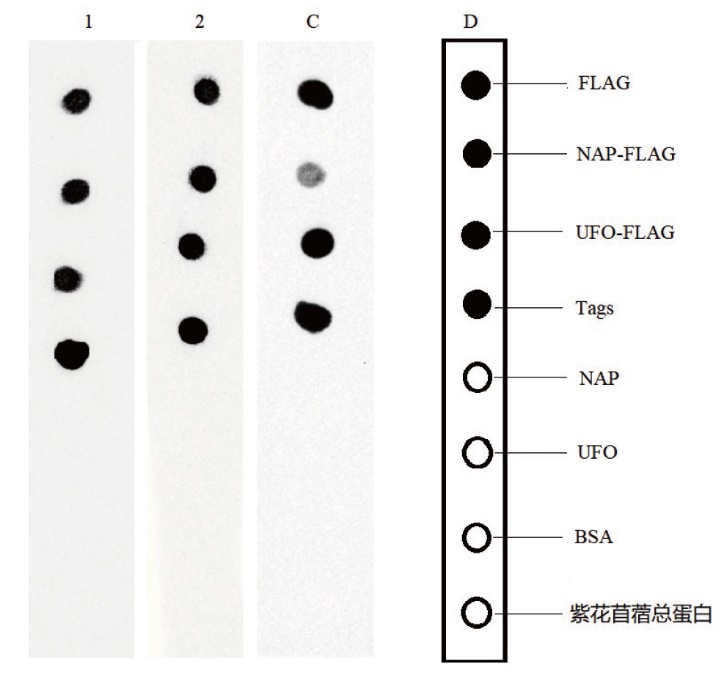
图7 纳米抗体Western blot验证 1:Nano-FLAG6;2:Nano-FLAG7;C:商品化FLAG单抗;D:示意图
Fig. 7 Western blot validation of nanobodies 1: Nano-FLAG6. 2: Nano-FLAG7. C: Commercialized FLAG monoclonal antibody. D: Schematic diagram
| [1] |
Hamers-Casterman C, Atarhouch T, Muyldermans S, et al. Naturally occurring antibodies devoid of light chains[J]. Nature, 1993, 363(6428): 446-448.
doi: 10.1038/363446a0 |
| [2] |
Muyldermans S. Nanobodies: natural single-domain antibodies[J]. Annu Rev Biochem, 2013, 82: 775-797.
doi: 10.1146/annurev-biochem-063011-092449 pmid: 23495938 |
| [3] |
Greenberg AS, Avila D, Hughes M, et al. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks[J]. Nature, 1995, 374(6518): 168-173.
doi: 10.1038/374168a0 |
| [4] | 姜忍忍, 许超, 周小理, 等. 纳米抗体的应用及其研究新进展[J]. 生命的化学, 2013, 33(3): 307-315. |
| Jiang RR, Xu C, Zhou XL, et al. Application and the research progress of nanobodies[J]. Chem Life, 2013, 33(3): 307-315. | |
| [5] |
De Meyer T, Muyldermans S, Depicker A. Nanobody-based products as research and diagnostic tools[J]. Trends Biotechnol, 2014, 32(5): 263-270.
doi: 10.1016/j.tibtech.2014.03.001 pmid: 24698358 |
| [6] |
Wesolowski J, Alzogaray V, Reyelt J, et al. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity[J]. Med Microbiol Immunol, 2009, 198(3): 157-174.
doi: 10.1007/s00430-009-0116-7 URL |
| [7] |
Oliveira S, Heukers R, Sornkom J, et al. Targeting tumors with nanobodies for cancer imaging and therapy[J]. J Control Release, 2013, 172(3): 607-617.
doi: 10.1016/j.jconrel.2013.08.298 URL |
| [8] |
Chakravarty R, Goel S, Cai WB. Nanobody: the magic bullet for molecular imaging?[J]. Theranostics, 2014, 4(4): 386-398.
doi: 10.7150/thno.8006 pmid: 24578722 |
| [9] |
Schumacher D, Helma J, Schneider AFL, et al. Nanobodies: chemical functionalization strategies and intracellular applications[J]. Angew Chem Int Ed Engl, 2018, 57(9): 2314-2333.
doi: 10.1002/anie.v57.9 URL |
| [10] |
Alirahimi E, Kazemi-Lomedasht F, Shahbazzadeh D, et al. Nanobodies as novel therapeutic agents in envenomation[J]. Biochim Biophys Acta Gen Subj, 2018, 1862(12): 2955-2965.
doi: 10.1016/j.bbagen.2018.08.019 URL |
| [11] |
Revets H, De Baetselier P, Muyldermans S. Nanobodies as novel agents for cancer therapy[J]. Expert Opin Biol Ther, 2005, 5(1): 111-124.
pmid: 15709914 |
| [12] | 曹飞婷, 邹阳, 方利. 抗小鼠IgG纳米抗体的制备及应用[J]. 中国生物制品学杂志, 2022, 35(8): 974-980. |
| Cao FT, Zou Y, Fang L. Preparation and application of anti-mouse IgG nanobody[J]. Chin J Biol, 2022, 35(8): 974-980. | |
| [13] |
Orlov I, Hemmer C, Ackerer L, et al. Structural basis of nanobody recognition of grapevine fanleaf virus and of virus resistance loss[J]. PNAS, 2020, 117(20): 10848-10855.
doi: 10.1073/pnas.1913681117 pmid: 32371486 |
| [14] |
Ahmadi S, Knerr JM, Argemi L, et al. Scorpion venom: detriments and benefits[J]. Biomedicines, 2020, 8(5): 118.
doi: 10.3390/biomedicines8050118 URL |
| [15] |
Uchański T, Masiulis S, Fischer B, et al. Megabodies expand the nanobody toolkit for protein structure determination by single-particle cryo-EM[J]. Nat Methods, 2021, 18(1): 60-68.
doi: 10.1038/s41592-020-01001-6 pmid: 33408403 |
| [16] | 詹万雷, 崔东, 郑文岭, 等. FLAG融合短肽在重组蛋白质纯化中的应用[J]. 生命的化学, 2004, 24(2): 155-156. |
| Zhan WL, Cui D, Zheng WL, et al. Application of FLAG fusion peptide in purification of recombinant protein[J]. Chem Life, 2004, 24(2): 155-156. | |
| [17] | 李永进, 陈媛媛, 毕利军. 融合标签技术及其应用[J]. 生物工程学报, 2006, 22(4): 523-527. |
| Li YJ, Chen YY, Bi LJ. Fusion tags technology and their applications[J]. Chin J Biotechnol, 2006, 22(4): 523-527. | |
| [18] | 李忠信. FLAG标签单克隆抗体的制备、鉴定与应用研究[D]. 郑州: 河南工业大学, 2010. |
| Li ZX. Preparation and characteristic of monoclonal antibodies to tag of FLAG and its application[D]. Zhengzhou: Henan University of Technology, 2010. | |
| [19] | 刘相叶, 邓洪宽, 吴秀萍, 等. 噬菌体展示技术及其应用[J]. 动物医学进展, 2008, 29(1): 60-63. |
| Liu XY, Deng HK, Wu XP, et al. Phage display technology and its application[J]. Prog Vet Med, 2008, 29(1): 60-63. | |
| [20] | 李丽芳, 张映. 噬菌体展示文库的筛选技术[J]. 生物技术通报, 2005(4): 36-38. |
| Li LF, Zhang Y. The selection techniques of phage display libraries[J]. Biotechnol Inf, 2005(4): 36-38. | |
| [21] |
王婷, 葛怀娜, 郭宏. 酵母双杂交技术应用进展[J]. 生物技术进展, 2015, 5(5): 392-396.
doi: 10.3969/j.issn.2095-2341.2015.05.12 |
|
Wang T, Ge HN, Guo H. Progress on application of yeast two-hybrid technique[J]. Curr Biotechnol, 2015, 5(5): 392-396.
doi: 10.3969/j.issn.2095-2341.2015.05.12 |
|
| [22] |
Fields S, Song O. A novel genetic system to detect protein-protein interactions[J]. Nature, 1989, 340(6230): 245-246.
doi: 10.1038/340245a0 |
| [23] |
Fu XJ, Gao XL, He SF, et al. Design and selection of a camelid single-chain antibody yeast two-hybrid library produced de novo for the cap protein of porcine circovirus type 2(PCV2)[J]. PLoS One, 2013, 8(3): e56222.
doi: 10.1371/journal.pone.0056222 URL |
| [24] |
Gao XL, Hu XY, Tong LN, et al. Construction of a camelid VHH yeast two-hybrid library and the selection of VHH against haemagglutinin-neuraminidase protein of the Newcastle disease virus[J]. BMC Vet Res, 2016, 12: 39.
doi: 10.1186/s12917-016-0664-1 pmid: 26920806 |
| [25] |
Banerjee S, Singh A, Rawat J, et al. Dataset of next-generation sequence reads of nanobody clones in phage display library derived from Indian desert camel(Camelus dromedarius L.)[J]. Data Brief, 2020, 34: 106663.
doi: 10.1016/j.dib.2020.106663 URL |
| [26] |
Terpe K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems[J]. Appl Microbiol Biotechnol, 2003, 60(5): 523-533.
pmid: 12536251 |
| [27] |
Waugh DS. Making the most of affinity tags[J]. Trends Biotechnol, 2005, 23(6): 316-320.
pmid: 15922084 |
| [28] | 姜茵, 奚月, 李妍. 幽门杆菌Catalase/GST融合蛋白的表达、标签切除及鉴定[J]. 生物技术通报, 2012(2): 102-106. |
| Jiang Y, Xi Y, Li Y. Expression, tag cleavage and identification of catalase/GST fusion protein of Helicobacter pylori[J]. Biotechnol Bull, 2012(2): 102-106. | |
| [29] | 李志要, 孙阳阳, 鲍恩东, 等. 利用猪源SUMO3标签蛋白可溶性表达猪腺病毒3型的Hexon基因高变区蛋白[J]. 畜牧与兽医, 2020, 52(3): 107-111. |
| Li ZY, Sun YY, Bao ED, et al. Soluble expression of Hexon protein of porcine adenovirus serotype 3 using the pigSUMO3 fusion tag[J]. Anim Husb Vet Med, 2020, 52(3): 107-111. | |
| [30] | 汪小杰, 毛若雨, 张勇, 等. SUMO在蛋白表达中的应用[J]. 生物技术通报, 2013(10): 28-33. |
| Wang XJ, Mao RY, Zhang Y, et al. Application of SUMO protein in fusion expression system[J]. Biotechnol Bull, 2013(10): 28-33. | |
| [31] | 陈爱春, 彭伟, 汪生鹏. 亲和标签在重组蛋白表达与纯化中的应用[J]. 中国生物工程杂志, 2012, 32(12): 93-103. |
| Chen AC, Peng W, Wang SP. Progress in the application of affinity tags for the expression and purification of recombinant proteins[J]. China Biotechnol, 2012, 32(12): 93-103. | |
| [32] | 汪婷婷, 戴佳咪, 郑永祥, 等. 抗菌肽-裂解酶双机制抗菌蛋白质的设计与可溶性表达[J]. 华西药学杂志, 2022, 37(1): 6-10. |
| Wang TT, Dai JM, Zheng YX, et al. Design and soluble expression of antibacterial peptide-endolysin antibacterial protein with dual-mechanism[J]. West China J Pharm Sci, 2022, 37(1): 6-10. | |
| [33] | 黄昕畑, 张白曦, 陈海琴, 等. 重组融合蛋白MBP-PAI的表达、纯化及酶活测定[J]. 食品与生物技术学报, 2020, 39(2): 10-15. |
| Huang XT, Zhang BX, Chen HQ, et al. Expression, purification and enzyme activity determination of recombination fusion protein MBP-PAI[J]. J Food Sci Biotechnol, 2020, 39(2): 10-15. | |
| [34] |
Burnette WN. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate—polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A[J]. Anal Biochem, 1981, 112(2): 195-203.
doi: 10.1016/0003-2697(81)90281-5 pmid: 6266278 |
| [1] | 林红妍, 郭晓蕊, 刘迪, 李慧, 陆海. 转录组分析转录因子AtbHLH68调控细胞壁发育的分子机制[J]. 生物技术通报, 2023, 39(9): 105-116. |
| [2] | 王子颖, 龙晨洁, 范兆宇, 张蕾. 利用酵母双杂交系统筛选水稻中与OsCRK5互作蛋白[J]. 生物技术通报, 2023, 39(9): 117-125. |
| [3] | 温晓蕾, 李建嫄, 李娜, 张娜, 杨文香. 小麦叶锈菌与小麦互作的酵母双杂交cDNA文库构建与应用[J]. 生物技术通报, 2023, 39(9): 136-146. |
| [4] | 娄慧, 朱金成, 杨洋, 张薇. 抗、感品种棉花根系分泌物对尖孢镰刀菌生长及基因表达的影响[J]. 生物技术通报, 2023, 39(9): 156-167. |
| [5] | 吴巧茵, 施友志, 李林林, 彭政, 谭再钰, 刘利平, 张娟, 潘勇. 类胡萝卜素降解菌株的原位筛选及其在雪茄提质增香中的应用[J]. 生物技术通报, 2023, 39(9): 192-201. |
| [6] | 杨志晓, 侯骞, 刘国权, 卢志刚, 曹毅, 芶剑渝, 王轶, 林英超. 不同抗性烟草品系Rubisco及其活化酶对赤星病胁迫的响应[J]. 生物技术通报, 2023, 39(9): 202-212. |
| [7] | 江海溶, 崔若琪, 王玥, 白淼, 张明露, 任连海. NH3和H2S降解功能菌的分离鉴定及降解特性研究[J]. 生物技术通报, 2023, 39(9): 246-254. |
| [8] | 薛宁, 王瑾, 李世新, 刘叶, 程海娇, 张玥, 毛雨丰, 王猛. 多基因同步调控结合高通量筛选构建高产L-苯丙氨酸的谷氨酸棒杆菌工程菌株[J]. 生物技术通报, 2023, 39(9): 268-280. |
| [9] | 陈中元, 王玉红, 代为俊, 张艳敏, 叶倩, 刘旭平, 谭文松, 赵亮. 柠檬酸铁铵对悬浮HEK293细胞转染的影响机制探究[J]. 生物技术通报, 2023, 39(9): 311-318. |
| [10] | 王佳蕊, 孙培媛, 柯瑾, 冉彬, 李洪有. 苦荞糖基转移酶基因FtUGT143的克隆及表达分析[J]. 生物技术通报, 2023, 39(8): 204-212. |
| [11] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [12] | 付钰, 贾瑞瑞, 何荷, 王良桂, 杨秀莲. 两种砧木楸树嫁接苗生长差异及转录组比较分析[J]. 生物技术通报, 2023, 39(8): 251-261. |
| [13] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [14] | 孙明慧, 吴琼, 刘丹丹, 焦小雨, 王文杰. 茶树CsTMFs的克隆与表达分析[J]. 生物技术通报, 2023, 39(7): 151-159. |
| [15] | 马俊秀, 吴皓琼, 姜威, 闫更轩, 胡基华, 张淑梅. 蔬菜软腐病菌广谱拮抗细菌菌株筛选鉴定及防效研究[J]. 生物技术通报, 2023, 39(7): 228-240. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||