








生物技术通报 ›› 2024, Vol. 40 ›› Issue (1): 1-11.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0510
• 综述与专论 • 下一篇
收稿日期:2023-05-30
出版日期:2024-01-26
发布日期:2024-02-06
通讯作者:
孙超,男,博士,研究员,研究方向:药用植物基因资源发掘与利用;E-mail: csun@implad.ac.cn作者简介:关智晶,女,硕士研究生,研究方向:药用植物基因资源发掘;E-mail: g18535776268@163.com
基金资助:Received:2023-05-30
Published:2024-01-26
Online:2024-02-06
摘要:
植物次生代谢存在多层次的区室化现象,区室化对于植物的生长发育和环境胁迫响应具有重要意义。本文综述了植物次生代谢在分子水平、亚细胞水平、细胞水平和组织器官水平的区室化研究进展。次生代谢的区室化需要中间产物在不同区室间进行转运,因此转运蛋白是植物次生代谢产物区室化合成体系的重要组成部分。次生代谢区室化及其相关转运蛋白研究,丰富了植物次生代谢的理论基础,并且为天然产物合成生物学提供了新的靶点和研究策略。
关智晶, 孙超. 植物次生代谢的区室化研究进展[J]. 生物技术通报, 2024, 40(1): 1-11.
GUAN Zhi-jing, SUN Chao. Research Progress in the Compartmentalization of Plant Specialized Metabolism[J]. Biotechnology Bulletin, 2024, 40(1): 1-11.
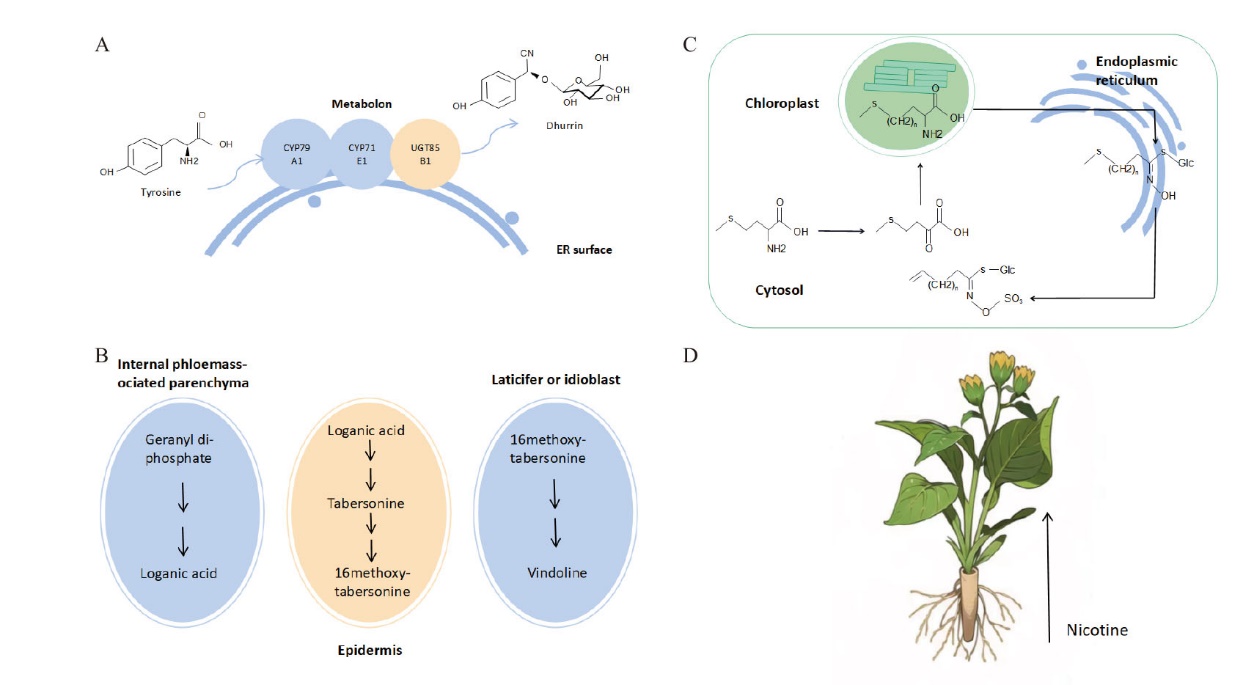
图1 植物不同水平上的次生代谢区室化 A:L-酪氨酸经过由3种酶(CYP79A1、CYP71E1和UGT85B1)组成的代谢区室,最终合成氰葡萄糖苷dhurrin;B:硫代葡萄糖苷的形成会涉及到3个不同的亚细胞区室,分别是胞质溶胶、叶绿体和内质网;C:文多灵的生物合成过程中会涉及到的4种细胞类型,分别是韧皮部内薄壁细胞、表皮细胞、乳汁细胞和异型细胞;D:尼古丁在根组织中合成后运输到地上部分并在叶中积累
Fig. 1 Plant specialized metabolic compartmentalization on different levels A: L-tyrosine undergoes a metabolon composed of three enzymes(CYP79A1, CYP71E1 and UGT85B1)to finally synthesize dhurrin. B: The biosynthesis of glucosinolates is involved three subcellular compartments, which are cytosol, chloroplast and endoplasmic reticulum. C: The biosynthesis of vindoline is involved four cell types, which are internal phloemassociated parenchyma, epidermal, laticifer and idioblast. D: Nicotine is synthesized in roots and transported to leaves for accumulation
| 转运蛋白家族 Transporter family | 次生代谢产物 Specialized metabolites | 转运蛋白 Transport protein | 转运蛋白序列号 GenBank | 基源植物 Original species | 细胞定位 Cell localization | 转运方向 Transport direction | 功能验证方式 Functional verification method | 参考文献 Reference |
|---|---|---|---|---|---|---|---|---|
| ABC | 小檗碱 | CjMDR1(ABC B) | AB043999.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ |
| CjABCB2(ABC B) | AB674325.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | ||
| CjABCB3(ABC B) | AB674326.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | ||
| 石蒜碱 | LaABCB11(ABC B) | YP_009745292.1 | 忽地笑Lycoris aurea | 细胞膜 | 向细胞外 | 酵母异源表达 | [ | |
| 花青素 | ZmMRP3(ABC C) | AAT37905.1 | 玉米Zea mays | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | VvABCC1(ABC C) | AGC23330.1 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC2(ABC C) | AEC09006.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC1(ABC C) | AEE31213.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC14(ABC C) | AEE80381.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 藏红花素 | CsABCC4a(ABC C) | QEY08349.1 | 番红花Crocus sativus | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| Sclareolide | NpPDR1(ABC G) | FE898762.1 | 皱叶烟草 Nicotiana plumbaginifolia | 细胞膜 | 向细胞外 | 诱导蛋白表达 检测底物浓度 | [ | |
| 西松烯 香紫苏醇 椒二醇 | NtPDR1(ABC G) | AB109388.1 | 烟草Nicotiana tabacum | 细胞膜 | — | 基因过表达 | [ | |
| 椒二醇 | NbABCG1(ABC G) | LC015759.1 | 本氏烟草Nicotiana benthamiana | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| NbABCG2(ABC G) | LC015761 | 本氏烟草Nicotiana benthamiana | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | ||
| β-石竹烯 | AaPDR3(ABC G) | KR153482.1 | 黄花蒿Artemisia annua | 细胞膜 | 向细胞内 | 基因过表达、酵母异源表达 | [ | |
| 单萜 | PbABCG1(ABC G) | JQ088099.1 | 荧光蝴蝶兰Phalaenopsis bellina | 细胞膜 | 向细胞外 | RNA干扰基因沉默、 病毒诱导的基因沉默 | [ | |
| 挥发性有机 化合物 | PhABCG1(ABC G) | AFC36404.1 | 碧冬茄Petunia×hybrida | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | |
| 长春质碱 | CrTPT2(ABC G) | KC511771.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| 植保素Camalexin | AtABCG34(ABC G) | AEC09246.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 香豆素 | AtABCG37(ABC G) | AEE79095.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 香豆素 | NtABCG3(ABC G) | CAJ19055.1 | 烟草Nicotiana tabacum | 细胞膜 | — | 病毒诱导的基因沉默、 基因过表达 | [ | |
| 苜蓿素Medicarpin | MtABCG10(ABC G) | XP_003597819 | 蒺藜苜蓿Medicago truncatula | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 木质素 | PgrABCG14(ABC G) | Pgr018151.1 | 石榴Punica granatum | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 角质层 | AtABCG32(ABC G) | AEC07906.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因敲除 | [ | |
| 角质层 | SIABCG42(ABC G) | XP_006353655.1 | 番茄Solanum lycopersicum | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | |
| SlABCG36(ABC G) | XP_006338166.1 | 番茄Solanum lycopersicum | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | ||
| 蜡质 | ZxABCG11(ABC G) | YP_009990684.1 | 霸王Zygophyllum xanthoxylum | — | — | 基因过表达 | [ | |
| 脱落酸 | AtABCG40(ABC G) | AEE29332.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达、酵母异源表达 | [ | |
| MATE | 尼古丁 | NtMATE1 | AB286963.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 基因过表达、酵母异源表达 | [ |
| NtMATE2 | AB286962.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 基因过表达、酵母异源表达 | [ | ||
| NtJAT1 | BAG68655.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| NtJAT2 | BAG68656.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 小檗碱 | CjMATE1 | BAX73926.1 | 日本黄连Coptis japonica | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 黄酮醇 | AtFFT | OAP00740.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| 原花青素 | AtTT12 | OAP05921.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 原花青素 | MtMATE1 | ACX37118.1 | 蒺藜苜蓿 Medicago truncatula | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 类黄酮 | MtMATE2 | ADV04045.1 | 蒺藜苜蓿 Medicago truncatula | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 原花青素 | MdMATE1 | GU064954 | 栽培苹果Malus x domestica Borkh. | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| MdMATE2 | GU064956 | 栽培苹果Malus x domestica Borkh. | 液泡膜 | 向液泡内 | 突变体验证 | [ | ||
| 原花青素 | GhTT12 | AGW32085.1 | 陆地棉Gossypium hirsutum | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| 原花青素 | FaTT12-1 | AUA60209.1 | 草莓Fragaria×ananassa | 液泡膜 | 向液泡内 | 病毒诱导的基因沉默 | [ | |
| 花青素 | VvAM1 | FJ264202 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| VvAM3 | FJ264203 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 异黄酮 | GmMATE1 | KRG94946.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| GmMATE2 | KAG4396127.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 异黄酮 | GmMATE4 | KRH64938.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| CCoumaroylagmatine | AtDTX18 | AEE76776.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 突变体验证 | [ | |
| 黄酮醇 | NtMATE21 | XP_016475205.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞外 | 突变体验证 | [ | |
| NtMATE22 | XP_016477351.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞外 | 突变体验证 | [ | ||
| 染料木素 | LaMATE2 | KY464927 | 白羽扇豆Lupinus albus | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| 苦味素 | CsMATE1 | AXN55888.1 | 黄瓜Cucumis sativus | 液泡膜 | 向液泡内 | 基因过表达 | [ | |
| 水杨酸 | EDS5 | ABZ03276.1 | 拟南芥Arabidopsis thaliana | 叶绿体被膜 | 向叶绿体外 | 酵母异源表达 | [ | |
| PUP | 尼古丁 | NUP1 | ADP30799.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞内 | 酵母异源表达 | [ |
| 苄基异喹啉生物碱 | BUP1 | QBG64391.1 | 罂粟Papaver somniferum | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | |
| 咖啡因 | CsPUP1 | TEA003596 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | |
| CsPUP3.1 | TEA029223 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | ||
| CsPUP10.1 | TEA023430 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | ||
| NPF | 硫代葡萄糖苷 | AtNPF2.9(AtGTR3) | Q9M9V7.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ |
| 硫代葡萄糖苷 | AtNPF2.10(AtGTR1) | Q944G5.3 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 硫代葡萄糖苷 | AtNPF2.11(AtGTR2) | BAH19623.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 黄酮醇苷 | AtFST1(NPF2.8) | Q3E8X3.2 | 拟南芥Arabidopsis thaliana | 细胞膜 | — | 大肠杆菌异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF2.9 | AQM73449.1 | 长春花Catharanthus roseus | 液泡膜 | 向液泡外 | 病毒诱导的基因沉默 | [ | |
| 长春碱和长春新碱 | CrNPF2.4 | ALE20039.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF 2.5 | ALE20040.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF 2.6 | ALE20041.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 番茄碱 | SlNPF1.5 | OP765903.1 | 番茄Solanum lycopersicum | 液泡膜 | 向液泡外 | 爪蟾卵母细胞异源表达 | [ |
表1 运输植物次生代谢产物的转运蛋白
Table 1 Transport proteins that transport plant specialized metabolites
| 转运蛋白家族 Transporter family | 次生代谢产物 Specialized metabolites | 转运蛋白 Transport protein | 转运蛋白序列号 GenBank | 基源植物 Original species | 细胞定位 Cell localization | 转运方向 Transport direction | 功能验证方式 Functional verification method | 参考文献 Reference |
|---|---|---|---|---|---|---|---|---|
| ABC | 小檗碱 | CjMDR1(ABC B) | AB043999.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ |
| CjABCB2(ABC B) | AB674325.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | ||
| CjABCB3(ABC B) | AB674326.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | ||
| 石蒜碱 | LaABCB11(ABC B) | YP_009745292.1 | 忽地笑Lycoris aurea | 细胞膜 | 向细胞外 | 酵母异源表达 | [ | |
| 花青素 | ZmMRP3(ABC C) | AAT37905.1 | 玉米Zea mays | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | VvABCC1(ABC C) | AGC23330.1 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC2(ABC C) | AEC09006.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC1(ABC C) | AEE31213.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC14(ABC C) | AEE80381.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 藏红花素 | CsABCC4a(ABC C) | QEY08349.1 | 番红花Crocus sativus | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| Sclareolide | NpPDR1(ABC G) | FE898762.1 | 皱叶烟草 Nicotiana plumbaginifolia | 细胞膜 | 向细胞外 | 诱导蛋白表达 检测底物浓度 | [ | |
| 西松烯 香紫苏醇 椒二醇 | NtPDR1(ABC G) | AB109388.1 | 烟草Nicotiana tabacum | 细胞膜 | — | 基因过表达 | [ | |
| 椒二醇 | NbABCG1(ABC G) | LC015759.1 | 本氏烟草Nicotiana benthamiana | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| NbABCG2(ABC G) | LC015761 | 本氏烟草Nicotiana benthamiana | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | ||
| β-石竹烯 | AaPDR3(ABC G) | KR153482.1 | 黄花蒿Artemisia annua | 细胞膜 | 向细胞内 | 基因过表达、酵母异源表达 | [ | |
| 单萜 | PbABCG1(ABC G) | JQ088099.1 | 荧光蝴蝶兰Phalaenopsis bellina | 细胞膜 | 向细胞外 | RNA干扰基因沉默、 病毒诱导的基因沉默 | [ | |
| 挥发性有机 化合物 | PhABCG1(ABC G) | AFC36404.1 | 碧冬茄Petunia×hybrida | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | |
| 长春质碱 | CrTPT2(ABC G) | KC511771.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| 植保素Camalexin | AtABCG34(ABC G) | AEC09246.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 香豆素 | AtABCG37(ABC G) | AEE79095.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 香豆素 | NtABCG3(ABC G) | CAJ19055.1 | 烟草Nicotiana tabacum | 细胞膜 | — | 病毒诱导的基因沉默、 基因过表达 | [ | |
| 苜蓿素Medicarpin | MtABCG10(ABC G) | XP_003597819 | 蒺藜苜蓿Medicago truncatula | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 木质素 | PgrABCG14(ABC G) | Pgr018151.1 | 石榴Punica granatum | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 角质层 | AtABCG32(ABC G) | AEC07906.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因敲除 | [ | |
| 角质层 | SIABCG42(ABC G) | XP_006353655.1 | 番茄Solanum lycopersicum | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | |
| SlABCG36(ABC G) | XP_006338166.1 | 番茄Solanum lycopersicum | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | ||
| 蜡质 | ZxABCG11(ABC G) | YP_009990684.1 | 霸王Zygophyllum xanthoxylum | — | — | 基因过表达 | [ | |
| 脱落酸 | AtABCG40(ABC G) | AEE29332.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达、酵母异源表达 | [ | |
| MATE | 尼古丁 | NtMATE1 | AB286963.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 基因过表达、酵母异源表达 | [ |
| NtMATE2 | AB286962.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 基因过表达、酵母异源表达 | [ | ||
| NtJAT1 | BAG68655.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| NtJAT2 | BAG68656.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 小檗碱 | CjMATE1 | BAX73926.1 | 日本黄连Coptis japonica | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 黄酮醇 | AtFFT | OAP00740.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| 原花青素 | AtTT12 | OAP05921.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 原花青素 | MtMATE1 | ACX37118.1 | 蒺藜苜蓿 Medicago truncatula | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 类黄酮 | MtMATE2 | ADV04045.1 | 蒺藜苜蓿 Medicago truncatula | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 原花青素 | MdMATE1 | GU064954 | 栽培苹果Malus x domestica Borkh. | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| MdMATE2 | GU064956 | 栽培苹果Malus x domestica Borkh. | 液泡膜 | 向液泡内 | 突变体验证 | [ | ||
| 原花青素 | GhTT12 | AGW32085.1 | 陆地棉Gossypium hirsutum | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| 原花青素 | FaTT12-1 | AUA60209.1 | 草莓Fragaria×ananassa | 液泡膜 | 向液泡内 | 病毒诱导的基因沉默 | [ | |
| 花青素 | VvAM1 | FJ264202 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| VvAM3 | FJ264203 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 异黄酮 | GmMATE1 | KRG94946.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| GmMATE2 | KAG4396127.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 异黄酮 | GmMATE4 | KRH64938.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| CCoumaroylagmatine | AtDTX18 | AEE76776.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 突变体验证 | [ | |
| 黄酮醇 | NtMATE21 | XP_016475205.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞外 | 突变体验证 | [ | |
| NtMATE22 | XP_016477351.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞外 | 突变体验证 | [ | ||
| 染料木素 | LaMATE2 | KY464927 | 白羽扇豆Lupinus albus | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| 苦味素 | CsMATE1 | AXN55888.1 | 黄瓜Cucumis sativus | 液泡膜 | 向液泡内 | 基因过表达 | [ | |
| 水杨酸 | EDS5 | ABZ03276.1 | 拟南芥Arabidopsis thaliana | 叶绿体被膜 | 向叶绿体外 | 酵母异源表达 | [ | |
| PUP | 尼古丁 | NUP1 | ADP30799.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞内 | 酵母异源表达 | [ |
| 苄基异喹啉生物碱 | BUP1 | QBG64391.1 | 罂粟Papaver somniferum | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | |
| 咖啡因 | CsPUP1 | TEA003596 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | |
| CsPUP3.1 | TEA029223 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | ||
| CsPUP10.1 | TEA023430 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | ||
| NPF | 硫代葡萄糖苷 | AtNPF2.9(AtGTR3) | Q9M9V7.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ |
| 硫代葡萄糖苷 | AtNPF2.10(AtGTR1) | Q944G5.3 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 硫代葡萄糖苷 | AtNPF2.11(AtGTR2) | BAH19623.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 黄酮醇苷 | AtFST1(NPF2.8) | Q3E8X3.2 | 拟南芥Arabidopsis thaliana | 细胞膜 | — | 大肠杆菌异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF2.9 | AQM73449.1 | 长春花Catharanthus roseus | 液泡膜 | 向液泡外 | 病毒诱导的基因沉默 | [ | |
| 长春碱和长春新碱 | CrNPF2.4 | ALE20039.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF 2.5 | ALE20040.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF 2.6 | ALE20041.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 番茄碱 | SlNPF1.5 | OP765903.1 | 番茄Solanum lycopersicum | 液泡膜 | 向液泡外 | 爪蟾卵母细胞异源表达 | [ |
| [1] |
Gani U, Vishwakarma RA, Misra P. Membrane transporters: the key drivers of transport of secondary metabolites in plants[J]. Plant Cell Rep, 2021, 40(1): 1-18.
doi: 10.1007/s00299-020-02599-9 |
| [2] |
O'Connor SE. Engineering of secondary metabolism[J]. Annu Rev Genet, 2015, 49: 71-94.
doi: 10.1146/annurev-genet-120213-092053 pmid: 26393965 |
| [3] |
Shi YS, Wang D, Li RS, et al. Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides[J]. Metab Eng, 2021, 67: 104-111.
doi: 10.1016/j.ymben.2021.06.002 pmid: 34153454 |
| [4] |
Zhang YJ, Fernie AR. Metabolons, enzyme-enzyme assemblies that mediate substrate channeling, and their roles in plant metabolism[J]. Plant Commun, 2020, 2(1): 100081.
doi: 10.1016/j.xplc.2020.100081 URL |
| [5] |
Zhang YH. Substrate channeling and enzyme complexes for biotechnological applications[J]. Biotechnol Adv, 2011, 29(6): 715-725.
doi: 10.1016/j.biotechadv.2011.05.020 pmid: 21672618 |
| [6] |
Laursen T, Borch J, Knudsen C, et al. Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum[J]. Science, 2016, 354(6314): 890-893.
pmid: 27856908 |
| [7] |
Achnine L, Blancaflor EB, Rasmussen S, et al. Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis[J]. Plant Cell, 2004, 16(11): 3098-3109.
doi: 10.1105/tpc.104.024406 pmid: 15472080 |
| [8] |
Verma P, Mathur AK, Srivastava A, et al. Emerging trends in research on spatial and temporal organization of terpenoid indole alkaloid pathway in Catharanthus roseus: a literature update[J]. Protoplasma, 2012, 249(2): 255-268.
doi: 10.1007/s00709-011-0291-4 URL |
| [9] |
Li J, Kristiansen KA, Hansen BG, et al. Cellular and subcellular localization of flavin-monooxygenases involved in glucosinolate biosynthesis[J]. J Exp Bot, 2011, 62(3): 1337-1346.
doi: 10.1093/jxb/erq369 pmid: 21078824 |
| [10] |
Heinig U, Gutensohn M, Dudareva N, et al. The challenges of cellular compartmentalization in plant metabolic engineering[J]. Curr Opin Biotechnol, 2013, 24(2): 239-246.
doi: 10.1016/j.copbio.2012.11.006 URL |
| [11] |
Brillouet JM, Verdeil JL, Odoux E, et al. Phenol homeostasis is ensured in vanilla fruit by storage under solid form in a new chloroplast-derived organelle, the phenyloplast[J]. J Exp Bot, 2014, 65(9): 2427-2435.
doi: 10.1093/jxb/eru126 URL |
| [12] |
Brillouet JM, Romieu C, Schoefs B, et al. The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta[J]. Ann Bot, 2013, 112(6): 1003-1014.
doi: 10.1093/aob/mct168 URL |
| [13] |
Jacobowitz JR, Weng JK. Exploring uncharted territories of plant specialized metabolism in the postgenomic era[J]. Annu Rev Plant Biol, 2020, 71: 631-658.
doi: 10.1146/annurev-arplant-081519-035634 pmid: 32176525 |
| [14] |
Sun SJ, Shen XF, Li Y, et al. Single-cell RNA sequencing provides a high-resolution roadmap for understanding the multicellular compartmentation of specialized metabolism[J]. Nat Plants, 2023, 9(1): 179-190.
doi: 10.1038/s41477-022-01291-y |
| [15] |
Ozber N, Facchini PJ. Phloem-specific localization of benzylisoquinoline alkaloid metabolism in opium poppy[J]. J Plant Physiol, 2022, 271: 153641.
doi: 10.1016/j.jplph.2022.153641 URL |
| [16] |
de Brito Francisco R, Martinoia E. The vacuolar transportome of plant specialized metabolites[J]. Plant Cell Physiol, 2018, 59(7): 1326-1336.
doi: 10.1093/pcp/pcy039 pmid: 29452376 |
| [17] |
Nogia P, Pati PK. Plant secondary metabolite transporters: diversity, functionality, and their modulation[J]. Front Plant Sci, 2021, 12: 758202.
doi: 10.3389/fpls.2021.758202 URL |
| [18] |
Dechorgnat J, Nguyen CT, Armengaud P, et al. From the soil to the seeds: the long journey of nitrate in plants[J]. J Exp Bot, 2011, 62(4): 1349-1359.
doi: 10.1093/jxb/erq409 pmid: 21193579 |
| [19] |
Léran S, Varala K, Boyer JC, et al. A unified nomenclature of nitrate transporter 1/peptide transporter family members in plants[J]. Trends Plant Sci, 2014, 19(1): 5-9.
doi: 10.1016/j.tplants.2013.08.008 pmid: 24055139 |
| [20] |
Shitan N, Bazin I, Dan K, et al. Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica[J]. Proc Natl Acad Sci USA, 2003, 100(2): 751-756.
doi: 10.1073/pnas.0134257100 URL |
| [21] |
Shitan N, Dalmas F, Dan K, et al. Characterization of Coptis japonica CjABCB2, an ATP-binding cassette protein involved in alkaloid transport[J]. Phytochemistry, 2013, 91: 109-116.
doi: 10.1016/j.phytochem.2012.02.012 URL |
| [22] |
Wang R, Liu YT, Xu S, et al. An ATP-binding cassette transporter, LaABCB11, contributes to alkaloid transport in Lycoris aurea[J]. Int J Mol Sci, 2021, 22(21): 11458.
doi: 10.3390/ijms222111458 URL |
| [23] |
Goodman CD, Casati P, Walbot V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays[J]. Plant Cell, 2004, 16(7): 1812-1826.
doi: 10.1105/tpc.022574 URL |
| [24] |
Francisco RM, Regalado A, Ageorges A, et al. ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3-O-Glucosides[J]. Plant Cell, 2013, 25(5): 1840-1854.
doi: 10.1105/tpc.112.102152 URL |
| [25] |
Behrens CE, Smith KE, Iancu CV, et al. Transport of anthocyanins and other flavonoids by the Arabidopsis ATP-binding cassette transporter AtABCC2[J]. Sci Rep, 2019, 9(1): 437.
doi: 10.1038/s41598-018-37504-8 pmid: 30679715 |
| [26] |
Dean JV, Willis M, Shaban L. Transport of acylated anthocyanins by the Arabidopsis ATP-binding cassette transporters AtABCC1, AtABCC2, and AtABCC14[J]. Physiol Plant, 2022, 174(5): e13780.
doi: 10.1111/ppl.v174.5 URL |
| [27] | Demurtas OC, de Brito Francisco R, Diretto G, et al. ABCC transporters mediate the vacuolar accumulation of crocins in saffron stigmas[J]. Plant Cell, 2019, 31(11): 2789-2804. |
| [28] |
Jasiński M, Stukkens Y, Degand H, et al. A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion[J]. Plant Cell, 2001, 13(5): 1095-1107.
pmid: 11340184 |
| [29] |
Pierman B, Toussaint F, Bertin A, et al. Activity of the purified plant ABC transporter NtPDR1 is stimulated by diterpenes and sesquiterpenes involved in constitutive and induced defenses[J]. J Biol Chem, 2017, 292(47): 19491-19502.
doi: 10.1074/jbc.M117.811935 pmid: 28972149 |
| [30] |
Shibata Y, Ojika M, Sugiyama A, et al. The full-size ABCG transporters Nb-ABCG1 and Nb-ABCG2 function in pre- and postinvasion defense against Phytophthora infestans in Nicotiana benthamiana[J]. Plant Cell, 2016, 28(5): 1163-1181.
doi: 10.1105/tpc.15.00721 URL |
| [31] |
Fu XQ, Shi P, He Q, et al. AaPDR3, a PDR transporter 3, is involved in sesquiterpene β-caryophyllene transport in Artemisia annua[J]. Front Plant Sci, 2017, 8: 723.
doi: 10.3389/fpls.2017.00723 URL |
| [32] |
Chang YL, Huang LM, Kuo XZ, et al. PbABCG1 and PbABCG2 transporters are required for the emission of floral monoterpenes in Phalaenopsis bellina[J]. Plant J, 2023, 114(2): 279-292.
doi: 10.1111/tpj.v114.2 URL |
| [33] |
Adebesin F, Widhalm JR, Boachon B, et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter[J]. Science, 2017, 356(6345): 1386-1388.
doi: 10.1126/science.aan0826 pmid: 28663500 |
| [34] |
Yu F, De Luca V. ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus[J]. Proc Natl Acad Sci USA, 2013, 110(39): 15830-15835.
doi: 10.1073/pnas.1307504110 URL |
| [35] | Khare D, Choi H, Huh SU, et al. Arabidopsis ABCG34 contributes to defense against necrotrophic pathogens by mediating the secretion of camalexin[J]. Proc Natl Acad Sci USA, 2017, 114(28): E5712-E5720. |
| [36] |
Fourcroy P, Sisó-Terraza P, Sudre D, et al. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency[J]. New Phytol, 2014, 201(1): 155-167.
doi: 10.1111/nph.12471 pmid: 24015802 |
| [37] |
Lefèvre F, Fourmeau J, Pottier M, et al. The Nicotiana tabacum ABC transporter NtPDR3 secretes O-methylated coumarins in response to iron deficiency[J]. J Exp Bot, 2018, 69(18): 4419-4431.
doi: 10.1093/jxb/ery221 URL |
| [38] |
Biala W, Banasiak J, Jarzyniak K, et al. Medicago truncatula ABCG10 is a transporter of 4-coumarate and liquiritigenin in the medicarpin biosynthetic pathway[J]. J Exp Bot, 2017, 68(12): 3231-3241.
doi: 10.1093/jxb/erx059 URL |
| [39] |
Yu Q, Li JY, Qin GH, et al. Characterization of the ABC transporter G subfamily in pomegranate and function analysis of PgrABCG14[J]. Int J Mol Sci, 2022, 23(19): 11661.
doi: 10.3390/ijms231911661 URL |
| [40] |
Fabre G, Garroum I, Mazurek S, et al. The ABCG transporter PEC1/ABCG32 is required for the formation of the developing leaf cuticle in Arabidopsis[J]. New Phytol, 2016, 209(1): 192-201.
doi: 10.1111/nph.2016.209.issue-1 URL |
| [41] |
Elejalde-Palmett C, Martinez San Segundo I, Garroum I, et al. ABCG transporters export cutin precursors for the formation of the plant cuticle[J]. Curr Biol, 2021, 31(10): 2111-2123.e9.
doi: 10.1016/j.cub.2021.02.056 URL |
| [42] | Liu LB, Bao AK, Li HJ, et al. Overexpression of ZxABCG11 from Zygophyllum xanthoxylum enhances tolerance to drought and heat in alfalfa by increasing cuticular wax deposition[J]. Crop J, 2022 |
| [43] |
Kang J, Hwang JU, Lee M, et al. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid[J]. Proc Natl Acad Sci USA, 2010, 107(5): 2355-2360.
doi: 10.1073/pnas.0909222107 pmid: 20133880 |
| [44] |
Shoji T, Inai K, Yazaki Y, et al. Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots[J]. Plant Physiol, 2009, 149(2): 708-718.
doi: 10.1104/pp.108.132811 pmid: 19098091 |
| [45] |
Morita M, Shitan N, Sawada K, et al. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion(MATE)transporter in Nicotiana tabacum[J]. Proc Natl Acad Sci USA, 2009, 106(7): 2447-2452.
doi: 10.1073/pnas.0812512106 URL |
| [46] |
Shitan N, Minami S, Morita M, et al. Involvement of the leaf-specific multidrug and toxic compound extrusion(MATE)transporter Nt-JAT2 in vacuolar sequestration of nicotine in Nicotiana tabacum[J]. PLoS One, 2014, 9(9): e108789.
doi: 10.1371/journal.pone.0108789 URL |
| [47] |
Takanashi K, Yamada Y, Sasaki T, et al. A multidrug and toxic compound extrusion transporter mediates berberine accumulation into vacuoles in Coptis japonica[J]. Phytochemistry, 2017, 138: 76-82.
doi: S0031-9422(17)30103-6 pmid: 28318534 |
| [48] |
Thompson EP, Wilkins C, Demidchik V, et al. An Arabidopsis flavonoid transporter is required for anther dehiscence and pollen development[J]. J Exp Bot, 2010, 61(2): 439-451.
doi: 10.1093/jxb/erp312 pmid: 19995827 |
| [49] |
Marinova K, Pourcel L, Weder B, et al. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat[J]. Plant Cell, 2007, 19(6): 2023-2038.
doi: 10.1105/tpc.106.046029 URL |
| [50] |
Zhao J, Dixon RA. MATE transporters facilitate vacuolar uptake of epicatechin 3'-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis[J]. Plant Cell, 2009, 21(8): 2323-2340.
doi: 10.1105/tpc.109.067819 URL |
| [51] |
Zhao J, Huhman D, Shadle G, et al. MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula[J]. Plant Cell, 2011, 23(4): 1536-1555.
doi: 10.1105/tpc.110.080804 URL |
| [52] |
Frank S, Keck M, Sagasser M, et al. Two differentially expressed MATE factor genes from apple complement the Arabidopsis transparent testa12 mutant[J]. Plant Biol, 2011, 13(1): 42-50.
doi: 10.1111/plb.2010.13.issue-1 URL |
| [53] |
Gao JS, Wu N, Shen ZL, et al. Molecular cloning, expression analysis and subcellular localization of a Transparent Testa 12 ortholog in brown cotton(Gossypium hirsutum L.)[J]. Gene, 2016, 576(2 Pt 2): 763-769.
doi: 10.1016/j.gene.2015.11.002 URL |
| [54] |
Chen SY, Tang YM, Hu YY, et al. FaTT12-1, a multidrug and toxin extrusion(MATE)member involved in proanthocyanidin transport in strawberry fruits[J]. Sci Hortic, 2018, 231: 158-165.
doi: 10.1016/j.scienta.2017.12.032 URL |
| [55] |
Gomez C, Terrier N, Torregrosa L, et al. Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters[J]. Plant Physiol, 2009, 150(1): 402-415.
doi: 10.1104/pp.109.135624 pmid: 19297587 |
| [56] |
Ng MS, Ku YS, Yung WS, et al. MATE-type proteins are responsible for isoflavone transportation and accumulation in soybean seeds[J]. Int J Mol Sci, 2021, 22(21): 12017.
doi: 10.3390/ijms222112017 URL |
| [57] |
Ku YS, Cheng SS, Cheung MY, et al. The poly-glutamate motif of GmMATE4 regulates its isoflavone transport activity[J]. Membranes, 2022, 12(2): 206.
doi: 10.3390/membranes12020206 URL |
| [58] |
Dobritzsch M, Lübken T, Eschen-Lippold L, et al. MATE transporter-dependent export of hydroxycinnamic acid amides[J]. Plant Cell, 2016, 28(2): 583-596.
doi: 10.1105/tpc.15.00706 URL |
| [59] |
Gani U, Nautiyal AK, Kundan M, et al. Two homeologous MATE transporter genes, NtMATE21 and NtMATE22, are involved in the modulation of plant growth and flavonol transport in Nicotiana tabacum[J]. J Exp Bot, 2022, 73(18): 6186-6206.
doi: 10.1093/jxb/erac249 URL |
| [60] |
Biała-Leonhard W, Zanin L, Gottardi S, et al. Identification of an isoflavonoid transporter required for the nodule establishment of the Rhizobium- Fabaceae symbiotic interaction[J]. Front Plant Sci, 2021, 12: 758213.
doi: 10.3389/fpls.2021.758213 URL |
| [61] |
Ma YS, Li DW, Zhong Y, et al. Vacuolar MATE/DTX protein-mediated cucurbitacin C transport is co-regulated with bitterness biosynthesis in cucumber[J]. New Phytol, 2023, 238(3): 995-1003.
doi: 10.1111/nph.18786 pmid: 36732026 |
| [62] |
Serrano M, Wang BJ, Aryal B, et al. Export of salicylic acid from the chloroplast requires the multidrug and toxin extrusion-like transporter EDS5[J]. Plant Physiol, 2013, 162(4): 1815-1821.
doi: 10.1104/pp.113.218156 pmid: 23757404 |
| [63] |
Hildreth SB, Gehman EA, Yang HB, et al. Tobacco nicotine uptake permease(NUP1)affects alkaloid metabolism[J]. Proc Natl Acad Sci USA, 2011, 108(44): 18179-18184.
doi: 10.1073/pnas.1108620108 pmid: 22006310 |
| [64] |
Dastmalchi M, Chang LM, Chen RJ, et al. Purine permease-type benzylisoquinoline alkaloid transporters in opium poppy[J]. Plant Physiol, 2019, 181(3): 916-933.
doi: 10.1104/pp.19.00565 pmid: 31467164 |
| [65] |
Zhang YZ, Wei K, Guo LL, et al. Functional identification of purine permeases reveals their roles in caffeine transport in tea plants(Camellia sinensis)[J]. Front Plant Sci, 2022, 13: 1033316.
doi: 10.3389/fpls.2022.1033316 URL |
| [66] |
Jørgensen ME, Xu DY, Crocoll C, et al. Origin and evolution of transporter substrate specificity within the NPF family[J]. eLife, 2017, 6: e19466.
doi: 10.7554/eLife.19466 URL |
| [67] |
Nour-Eldin HH, Andersen TG, Burow M, et al. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds[J]. Nature, 2012, 488(7412): 531-534.
doi: 10.1038/nature11285 |
| [68] |
Grunewald S, Marillonnet S, Hause G, et al. The tapetal major facilitator NPF2.8 is required for accumulation of flavonol glycosides on the pollen surface in Arabidopsis thaliana[J]. Plant Cell, 2020, 32(5): 1727-1748.
doi: 10.1105/tpc.19.00801 URL |
| [69] |
Payne RME, Xu DY, Foureau E, et al. An NPF transporter exports a central monoterpene indole alkaloid intermediate from the vacuole[J]. Nat Plants, 2017, 3: 16208.
doi: 10.1038/nplants.2016.208 pmid: 28085153 |
| [70] |
Larsen B, Fuller VL, Pollier J, et al. Identification of iridoid glucoside transporters in Catharanthus roseus[J]. Plant Cell Physiol, 2017, 58(9): 1507-1518.
doi: 10.1093/pcp/pcx097 pmid: 28922750 |
| [71] |
Kazachkova Y, Zemach I, Panda S, et al. The GORKY glycoalkaloid transporter is indispensable for preventing tomato bitterness[J]. Nat Plants, 2021, 7(4): 468-480.
doi: 10.1038/s41477-021-00865-6 pmid: 33707737 |
| [72] |
Alam MT, Olin-Sandoval V, Stincone A, et al. The self-inhibitory nature of metabolic networks and its alleviation through compartmentalization[J]. Nat Commun, 2017, 8: 16018.
doi: 10.1038/ncomms16018 pmid: 28691704 |
| [73] |
Sirikantaramas S, Yamazaki M, Saito K. Mechanisms of resistance to self-produced toxic secondary metabolites in plants[J]. Phytochem Rev, 2008, 7(3): 467-477.
doi: 10.1007/s11101-007-9080-2 URL |
| [74] |
Roze LV, Chanda A, Linz JE. Compartmentalization and molecular traffic in secondary metabolism: a new understanding of established cellular processes[J]. Fungal Genet Biol, 2011, 48(1): 35-48.
doi: 10.1016/j.fgb.2010.05.006 pmid: 20519149 |
| [75] |
Knudsen C, Gallage NJ, Hansen CC, et al. Dynamic metabolic solutions to the sessile life style of plants[J]. Nat Prod Rep, 2018, 35(11): 1140-1155.
doi: 10.1039/c8np00037a pmid: 30324199 |
| [76] |
Wu SQ, Schalk M, Clark A, et al. Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants[J]. Nat Biotechnol, 2006, 24(11): 1441-1447.
doi: 10.1038/nbt1251 pmid: 17057703 |
| [77] |
Nour-Eldin HH, Madsen SR, Engelen S, et al. Reduction of antinutritional glucosinolates in Brassica oilseeds by mutation of genes encoding transporters[J]. Nat Biotechnol, 2017, 35(4): 377-382.
doi: 10.1038/nbt.3823 pmid: 28288105 |
| [78] |
Yamada Y, Urui M, Oki H, et al. Transport engineering for improving the production and secretion of valuable alkaloids in Escherichia coli[J]. Metab Eng Commun, 2021, 13: e00184.
doi: 10.1016/j.mec.2021.e00184 URL |
| [79] |
Zhou K, Qiao KJ, Edgar S, et al. Distributing a metabolic pathway among a microbial consortium enhances production of natural products[J]. Nat Biotechnol, 2015, 33(4): 377-383.
doi: 10.1038/nbt.3095 pmid: 25558867 |
| [1] | 周会汶, 吴兰花, 韩德鹏, 郑伟, 余跑兰, 吴杨, 肖小军. 甘蓝型油菜种子硫苷含量全基因组关联分析[J]. 生物技术通报, 2024, 40(1): 222-230. |
| [2] | 宋志忠, 徐维华, 肖慧琳, 唐美玲, 陈景辉, 管雪强, 刘万好. 酿酒葡萄铁调节转运蛋白基因VvIRT1的克隆、表达与功能[J]. 生物技术通报, 2023, 39(8): 234-240. |
| [3] | 马芳芳, 刘冠闻, 庞冰, 蒋春美, 师俊玲. 强化细胞外排提高工程菌类黄酮产量的策略[J]. 生物技术通报, 2023, 39(5): 63-76. |
| [4] | 高凯月, 郭雨婷, 杜奕谋, 郑小梅, 马欣荣, 赵伟, 郑平, 孙际宾. 黑曲霉葡萄糖吸收定量检测的方法建立及其在MstC功能研究中的应用[J]. 生物技术通报, 2023, 39(12): 71-80. |
| [5] | 李昕悦, 周明海, 樊亚超, 廖莎, 张风丽, 刘晨光, 孙悦, 张霖, 赵心清. 基于转运蛋白工程提升微生物菌株耐受性和生物制造效率的研究进展[J]. 生物技术通报, 2023, 39(11): 123-136. |
| [6] | 位欣欣, 兰海燕. 植物MYB转录因子调控次生代谢及逆境响应的研究进展[J]. 生物技术通报, 2022, 38(8): 12-23. |
| [7] | 洪天澍, 海英, 恩和巴雅尔, 高峰. 甜瓜CmABCG8基因的表达特性分析[J]. 生物技术通报, 2022, 38(7): 178-185. |
| [8] | 周国彦, 银珊珊, 高佳鑫, 武春成, 闫立英, 谢洋. 黄瓜AHP基因家族的鉴定及其非生物胁迫表达分析[J]. 生物技术通报, 2022, 38(6): 112-119. |
| [9] | 陈福暖, 黄瑜, 蔡佳, 王忠良, 简纪常, 王蓓. ABC转运蛋白结构及其在细菌致病性中的研究进展[J]. 生物技术通报, 2022, 38(6): 43-52. |
| [10] | 薛欣月, 于雪然, 刘晓刚, 马嘉欣, 田蕾, 李培富. 水稻锌吸收、转运、累积机理研究进展[J]. 生物技术通报, 2022, 38(4): 29-43. |
| [11] | 赵婷婷, 王俊刚, 王文治, 冯翠莲, 冯小艳, 张树珍. 甘蔗单糖转运蛋白基因ShSTP7序列分析及组织表达特征测定[J]. 生物技术通报, 2022, 38(4): 72-78. |
| [12] | 曹映辉, 胡美娟, 童妍, 张燕萍, 赵凯, 彭东辉, 周育真. 建兰ABC基因家族鉴定及其在花发育过程中的表达模式分析[J]. 生物技术通报, 2022, 38(11): 162-174. |
| [13] | 梁振霆, 唐婷. 内生菌对植物次生代谢产物的生物合成影响和抗逆功能研究[J]. 生物技术通报, 2021, 37(8): 35-45. |
| [14] | 王洁, 蔡昱萌, 张楠, 张雅丽. 植物蔗糖转运蛋白表达的调控因素与分子机制[J]. 生物技术通报, 2021, 37(3): 115-124. |
| [15] | 谢伟, 郝志鹏, 郭兰萍, 张莘, 张淑彬, 王幼珊, 陈保冬. 丛枝菌根影响植物萜类化合物合成与积累研究进展[J]. 生物技术通报, 2020, 36(9): 49-63. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
