








生物技术通报 ›› 2024, Vol. 40 ›› Issue (4): 179-188.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1069
收稿日期:2023-11-14
出版日期:2024-04-26
发布日期:2024-04-30
通讯作者:
李火根,男,博士,教授,研究方向:林木遗传育种;E-mail: hgli@njfu.edu.cn作者简介:刘换换,女,博士,讲师,研究方向:园林植物遗传育种;E-mail: lhh91@jsafc.edu.cn
基金资助:
LIU Huan-huan1,2( ), YANG Li-chun1, LI Huo-gen1(
), YANG Li-chun1, LI Huo-gen1( )
)
Received:2023-11-14
Published:2024-04-26
Online:2024-04-30
摘要:
【目的】MYB305作为MYB家族R2R3亚族成员,对植物花蜜腺发育、花蜜蛋白表达、淀粉积累和水解、类黄酮合成发挥重要作用。因此,研究MYB305的表达模式及功能对于北美鹅掌楸花蜜腺调控分子机理研究具有重要意义。【方法】本文以蜜源植物北美鹅掌楸的花蜜腺为材料,通过分光光度计法测定5个时期花蜜腺的花青素含量,采用RACE技术克隆LtMYB305基因,利用RT-qPCR技术检测了该基因在北美鹅掌楸不同组织间相对表达量,以烟草叶片为材料验证LtMYB305蛋白定位,并以野生型拟南芥为材料进行遗传转化实验,研究LtMYB305基因功能。【结果】北美鹅掌楸花蜜腺的花青素从膨大后期开始积累,初放期含量急剧增加,败花期含量最高,达27.14 μg/g,与花蜜分泌、着色过程相一致。LtMYB305基因全长为931 bp,编码了198个氨基酸,其蛋白为亲水性蛋白和非跨膜蛋白;LtMYB305仅在北美鹅掌楸盛花期花蜜腺中高水平表达,在其他组织中几乎不表达。LtMYB305蛋白定位于细胞核。过表达LtMYB305基因的拟南芥出现侧蜜腺的“蜜腺沟”消失和加深表型,与花蜜腺相关基因AtMYB305、AtPIN6和AtSWEET9表达量上调,AtCRC、AtBOP1/2、AtMYB21、AtSWEET3/4/7基因表达量下调。【结论】LtMYB305具有典型转录因子特征,并且参与调控拟南芥花蜜腺的发育。
刘换换, 杨立春, 李火根. 北美鹅掌楸LtMYB305基因的克隆及功能分析[J]. 生物技术通报, 2024, 40(4): 179-188.
LIU Huan-huan, YANG Li-chun, LI Huo-gen. Cloning and Functional Analysis of LtMYB305 in Liriodendron tulipifera[J]. Biotechnology Bulletin, 2024, 40(4): 179-188.
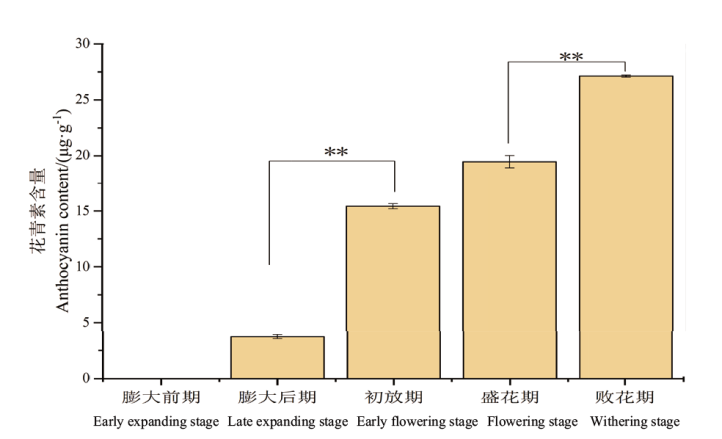
图1 北美鹅掌楸不同时期花蜜腺的花青素含量分析 *表示在P<0.05水平上差异显著,**表示在P<0.01水平上差异显著。下同
Fig. 1 Anthocyanidin content analysis during floral nectary growth of L. tulipifera * indicates significant difference at P<0.05 level; ** indicates significant difference at P<0.01 level. The same below
| 蛋白 名称 Protein name | 可信度Reliability | |||
|---|---|---|---|---|
| 叶绿体转运肽Chloroplast transit peptide, cTP | 线粒体靶向肽Mitochondrial targeting peptide, mTP | 分泌通路信号肽Secretory pathway signal peptide, SP | 其他 Other | |
| LtMYB305 | 9.40 | 4.90 | 8.00 | 94.70 |
表1 北美鹅掌楸LtMYB305蛋白亚细胞定位SignalP-4.0 Server预测分析
Table 1 Prediction of LtMYB305 protein subcellular localization in L. tulipifera by SignalP-4.0 Server %
| 蛋白 名称 Protein name | 可信度Reliability | |||
|---|---|---|---|---|
| 叶绿体转运肽Chloroplast transit peptide, cTP | 线粒体靶向肽Mitochondrial targeting peptide, mTP | 分泌通路信号肽Secretory pathway signal peptide, SP | 其他 Other | |
| LtMYB305 | 9.40 | 4.90 | 8.00 | 94.70 |
| 蛋白名称 Protein name | 亚细胞结构Subcellular structure | |||
|---|---|---|---|---|
| 细胞核 Nuclear | 过氧化物酶体Peroxisome | 叶绿体Chloroplast | 液泡 Vacuole | |
| MYB305 | 13 | 1 | - | - |
表2 北美鹅掌楸LtMYB305蛋白亚细胞定位WOLF PSORT预测分析
Table 2 Prediction of LtMYB305 protein subcellular localization in L. tulipifera by WOLF PSORT
| 蛋白名称 Protein name | 亚细胞结构Subcellular structure | |||
|---|---|---|---|---|
| 细胞核 Nuclear | 过氧化物酶体Peroxisome | 叶绿体Chloroplast | 液泡 Vacuole | |
| MYB305 | 13 | 1 | - | - |

图2 北美鹅掌楸MYB305蛋白的氨基酸序列比对(A)和进化树构建(B)
Fig. 2 Multiple alignment of the amino acid(A)sequences and phylogenetic tree analysis(B)of LtMYB305 in L. tulipifera with other MYB proteins
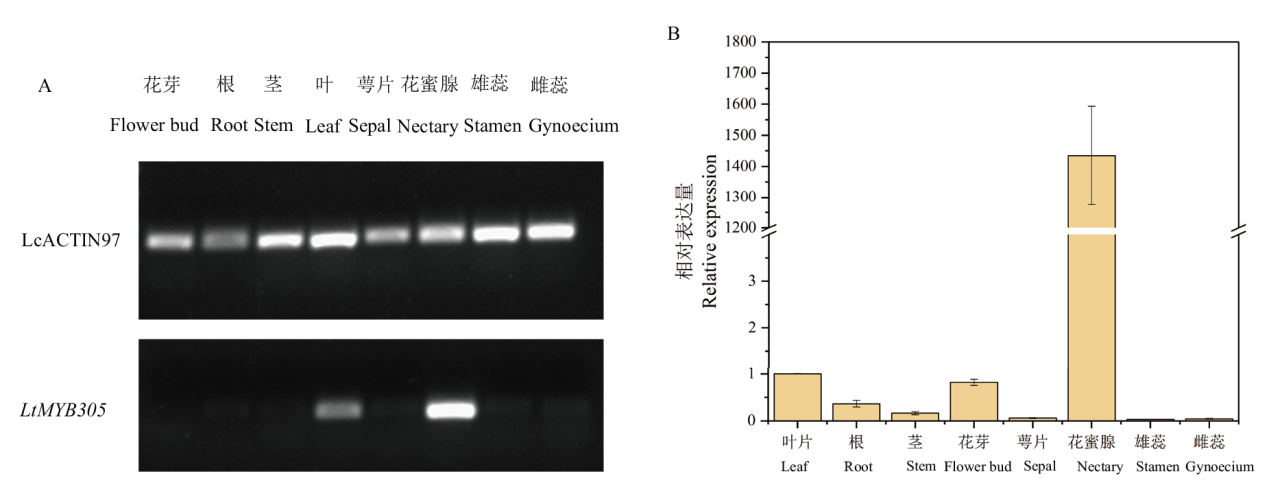
图3 半定量PCR(A)和实时荧光定量PCR(B)检测北美鹅掌楸MYB305基因在不同组织中的表达模式
Fig. 3 Expression profiles of LtMYB305 genes in different tissues of L. tulipifera by semi-quantitative PCR(A)andRT-qPCR assays(B)
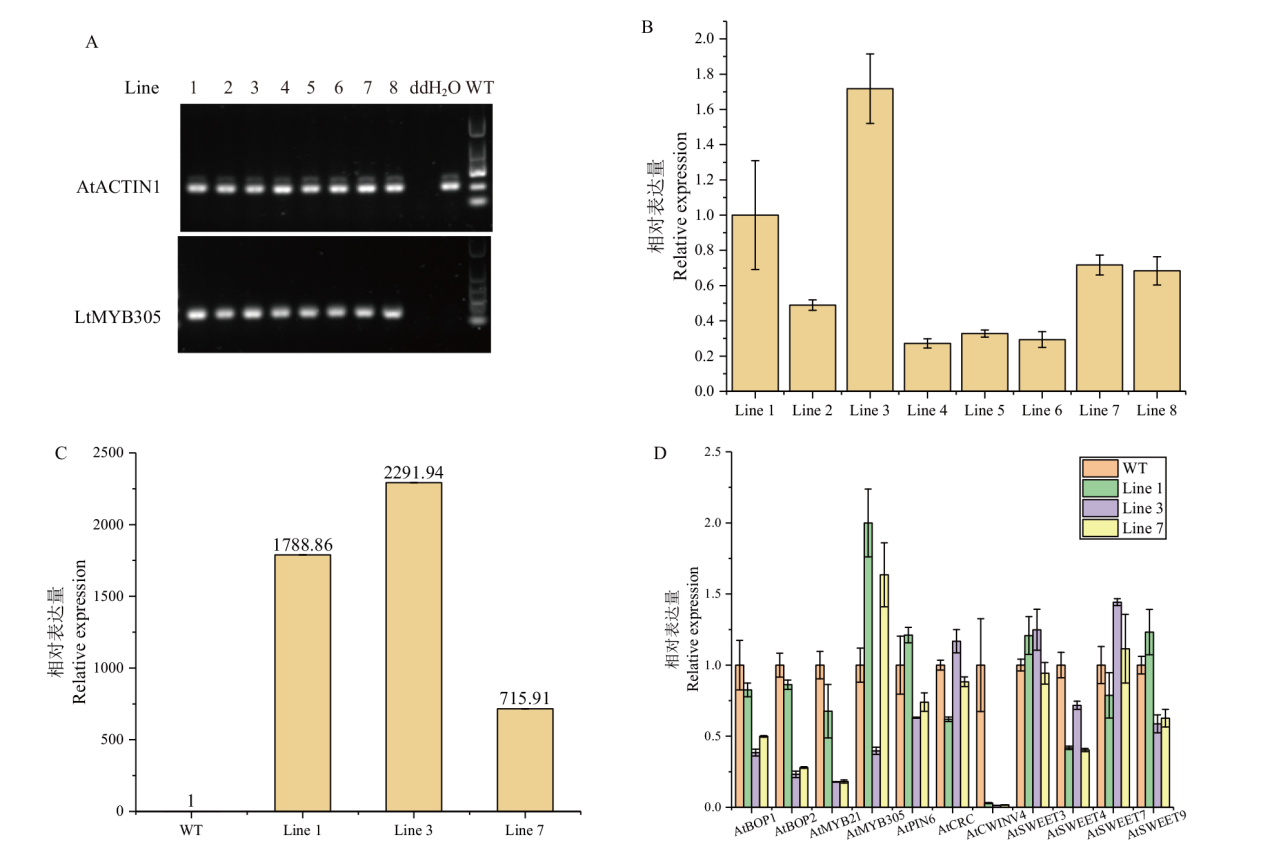
图5 超表达35S::LtMYB305拟南芥阳性植株T1代表达量分析 A:阳性植株T1代8个株系半定量PCR检测;B:阳性植株T1代8个株系实时荧光定量PCR检测;C:阳性植株T1代3个高表达株系实时荧光定量PCR检测;D:阳性植株T1代3个高表达株系的蜜腺发育相关基因实时荧光定量PCR检测
Fig. 5 Expression analysis of T1 generation of positive 35S::LtMYB305-overexpressed A. thaliana plants A: Semi-quantitative PCR analysis in eight lines of T1 generation positive plants; B: RT-qPCR analysis in eight lines of T1 generation positive plants; C: RT-qPCR analysis in three high-expressed lines of T1 generation positive plants; D: RT-qPCR analysis of eleven genes related to nectary development in three high-expressed lines of T1 generation positive plants

图6 过表达35S::LtMYB305拟南芥阳性植株筛选 A:过表达LtMYB305 T2-T3代阳性植株检测;B:野生型拟南芥和过表达植株的根系;C:野生型拟南芥和过表达植株幼苗筛选
Fig. 6 Screening of positive 35S:: LtMYB305-overexpressed A. thaliana plants A: Detection of positive T2-T3 generation 35S::LtMYB305-overexpressed A. thaliana plants. B: Root observation of wild type and 35S::LtMYB305-overexpressed A. thaliana plants. C: Screening of seedling of wild type and 35S::LtMYB305-overexpressed A. thaliana plants

图7 过表达35S::LtMYB305拟南芥表型观察 A:野生型拟南芥(col)花蜜腺;B:过表达35S::LtMYB305拟南芥花蜜腺;C:过表达LtMYB305拟南芥T1-T3代植株;LN:侧蜜腺
Fig. 7 Phenotype observation of 35S::LtMYB305-overexpressed A. thaliana A: Floral nectaries of wild type A. thaliana(col)plants. B: Floral nectaries 35S::LtMYB305-overexpressed A. thaliana plants. C: T1-T3 generation of 35S::LtMYB305 -overexpressed A. thaliana plants. LN: Lateral nectary
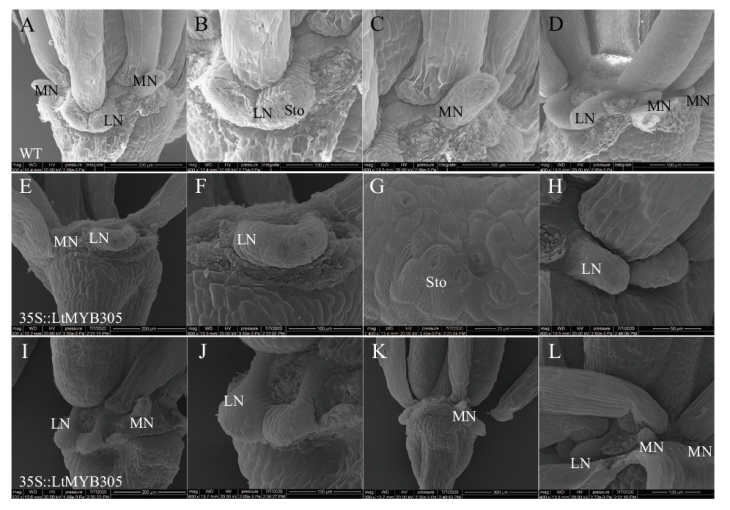
图9 过表达35S::LtMYB305拟南芥阳性植株的花蜜腺扫描电镜观察 A-D:野生型拟南芥(col)花蜜腺;E-L:过表达LtMYB305拟南芥花蜜腺;MN:主蜜腺;LN:侧蜜腺;Sto:气孔
Fig. 9 SEM of floral nectary of 35S::LtMYB305-overexpressed A. thaliana plants A-D: Floral nectaries of wild type A. thaliana(col)plants. E-L: Floral nectaries of 35S::LtMYB305-overexpressed A. thaliana plants. MN: Medial nectary. LN: Lateral nectary. Sto: Stoma
| [1] | Parks CR, Miller NG, Wendel JF, et al. Genetic divergence within the genus Liriodendron(Magnoliaceae)[J]. Ann Mo Bot Gard, 1983, 70(4): 658. |
| [2] |
Hao ZD, Liu SQ, Hu LF, et al. Transcriptome analysis and metabolic profiling reveal the key role of carotenoids in the petal coloration of Liriodendron tulipifera[J]. Hortic Res, 2020, 7: 70.
doi: 10.1038/s41438-020-0287-3 |
| [3] |
Lee JY, Baum SF, Oh SH, et al. Recruitment of CRABS CLAW to promote nectary development within the eudicot clade[J]. Development, 2005, 132(22): 5021-5032.
doi: 10.1242/dev.02067 URL |
| [4] |
Hepworth SR, Zhang YL, McKim S, et al. BLADE-ON-PETIOLE-dependent signaling controls leaf and floral patterning in Arabidopsis[J]. Plant Cell, 2005, 17(5): 1434-1448.
doi: 10.1105/tpc.104.030536 pmid: 15805484 |
| [5] |
Nagpal P, Ellis CM, Weber H, et al. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation[J]. Development, 2005, 132(18): 4107-4118.
doi: 10.1242/dev.01955 pmid: 16107481 |
| [6] |
McKim SM, Stenvik GE, Butenko MA, et al. The BLADE-ON-PETIOLE genes are essential for abscission zone formation in Arabidopsis[J]. Development, 2008, 135(8): 1537-1546.
doi: 10.1242/dev.012807 URL |
| [7] | Reeves PH, Ellis CM, Ploense SE, et al. A regulatory network for coordinated flower maturation[J]. PLoS Genet, 2012, 8(2): e1002506. |
| [8] |
Gross T, Broholm S, Becker A. CRABS CLAW acts as a bifunctional transcription factor in flower development[J]. Front Plant Sci, 2018, 9: 835.
doi: 10.3389/fpls.2018.00835 pmid: 29973943 |
| [9] |
Graf T. MYB: a transcriptional activator linking proliferation and differentiation in hematopoietic cells[J]. Curr Opin Genet Dev, 1992, 2(2): 249-255.
pmid: 1638119 |
| [10] |
Dubos C, Stracke R, Grotewold E, et al. MYB transcription factors in Arabidopsis[J]. Trends Plant Sci, 2010, 15(10): 573-581.
doi: 10.1016/j.tplants.2010.06.005 URL |
| [11] |
Stracke R, Ishihara H, Huep G, et al. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling[J]. Plant J, 2007, 50(4): 660-677.
doi: 10.1111/j.1365-313X.2007.03078.x pmid: 17419845 |
| [12] |
Jackson D, Culianez-Macia F, Prescott AG, et al. Expression patterns of MYB genes from Antirrhinum flowers[J]. Plant Cell, 1991, 3(2): 115-125.
pmid: 1840903 |
| [13] |
Moyano E, Martínez-Garcia JF, Martin C. Apparent redundancy in myb gene function provides gearing for the control of flavonoid biosynthesis in antirrhinum flowers[J]. Plant Cell, 1996, 8(9): 1519-1532.
doi: 10.1105/tpc.8.9.1519 pmid: 8837506 |
| [14] |
Liu GY, Ren G, Guirgis A, et al. The MYB305 transcription factor regulates expression of nectarin genes in the ornamental tobacco floral nectary[J]. Plant Cell, 2009, 21(9): 2672-2687.
doi: 10.1105/tpc.108.060079 URL |
| [15] |
Liu GY, Thornburg RW. Knockdown of MYB305 disrupts nectary starch metabolism and floral nectar production[J]. Plant J, 2012, 70(3): 377-388.
doi: 10.1111/tpj.2012.70.issue-3 URL |
| [16] | Radhika V, Kost C, Boland W, et al. The role of jasmonates in floral nectar secretion[J]. PLoS One, 2010, 5(2): e9265. |
| [17] |
Bender RL, Fekete ML, Klinkenberg PM, et al. PIN6 is required for nectary auxin response and short stamen development[J]. Plant J, 2013, 74(6): 893-904.
doi: 10.1111/tpj.2013.74.issue-6 URL |
| [18] |
Heil M. Induction of two indirect defences benefits Lima bean(Phaseolus Lunatus, Fabaceae)in nature[J]. J Ecol, 2004, 92(3): 527-536.
doi: 10.1111/jec.2004.92.issue-3 URL |
| [19] | Liu HH, Ma JK, Li HG. Transcriptomic and microstructural analyses in Liriodendron tulipifera Linn. reveal candidate genes involved in nectary development and nectar secretion[J]. BMC Plant Biol, 2019, 19(1): 531. |
| [20] | Zhou YW, Li MP, Zhao FF, et al. Floral nectary morphology and proteomic analysis of nectar of Liriodendron tulipifera Linn[J]. Front Plant Sci, 2016, 7: 826. |
| [21] |
Horner HT, Healy RA, Ren G, et al. Amyloplast to chromoplast conversion in developing ornamental tobacco floral nectaries provides sugar for nectar and antioxidants for protection[J]. Am J Bot, 2007, 94(1): 12-24.
doi: 10.3732/ajb.94.1.12 pmid: 21642203 |
| [22] |
Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids[J]. Plant J, 2008, 54(4): 733-749.
doi: 10.1111/tpj.2008.54.issue-4 URL |
| [23] |
Wu YQ, Guo J, Zhou Q, et al. De novo transcriptome analysis revealed genes involved in flavonoid biosynthesis, transport and regulation in Ginkgo biloba[J]. Ind Crops Prod, 2018, 124: 226-235.
doi: 10.1016/j.indcrop.2018.07.060 URL |
| [24] |
张月, 袁媛, 何弦, 等. 茉莉花JsMYB108和JsMYB305基因的克隆及其对TPS基因的激活作用[J]. 热带作物学报, 2021, 42(6): 1539-1548.
doi: 10.3969/j.issn.1000-2561.2021.06.005 |
| Zhang Y, Yuan Y, He X, et al. Cloning of JsMYB108 and JsMYB305 and analysis of their activation on TPS gene in Jasmi-num sambac[J]. Chin J Trop Crops, 2021, 42(6): 1539-1548. | |
| [25] |
Davis AR, Pylatuik JD, Paradis JC, et al. Nectar-carbohydrate production and composition vary in relation to nectary anatomy and location within individual flowers of several species of Brassicaceae[J]. Planta, 1998, 205(2): 305-318.
pmid: 9637073 |
| [26] |
Ruhlmann JM, Kram BW, Carter CJ. CELL WALL INVERTASE 4 is required for nectar production in Arabidopsis[J]. J Exp Bot, 2010, 61(2): 395-404.
doi: 10.1093/jxb/erp309 pmid: 19861655 |
| [1] | 杜泽光, 任少文, 张凤勤, 李梅兰, 李改珍, 齐仙惠. 大白菜BrMLP328的克隆、表达及功能验证[J]. 生物技术通报, 2024, 40(4): 122-129. |
| [2] | 钟匀, 林春, 刘正杰, 董陈文华, 毛自朝, 李兴玉. 芦笋皂苷合成相关糖基转移酶基因克隆及原核表达分析[J]. 生物技术通报, 2024, 40(4): 255-263. |
| [3] | 杨艳, 胡洋, 刘霓如, 殷璐, 杨锐, 王鹏飞, 穆霄鹏, 张帅, 程春振, 张建成. ‘红满堂’苹果MbbZIP43基因的克隆与功能研究[J]. 生物技术通报, 2024, 40(2): 146-159. |
| [4] | 任延靖, 张鲁刚, 赵孟良, 李江, 邵登魁. 白菜种子cDNA酵母文库的构建及BrTTG1互作蛋白的筛选及分析[J]. 生物技术通报, 2024, 40(2): 223-232. |
| [5] | 朱毅, 柳唐镜, 宫国义, 张洁, 王晋芳, 张海英. 西瓜ClPP2C3克隆及表达分析[J]. 生物技术通报, 2024, 40(1): 243-249. |
| [6] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [7] | 王佳蕊, 孙培媛, 柯瑾, 冉彬, 李洪有. 苦荞糖基转移酶基因FtUGT143的克隆及表达分析[J]. 生物技术通报, 2023, 39(8): 204-212. |
| [8] | 孙明慧, 吴琼, 刘丹丹, 焦小雨, 王文杰. 茶树CsTMFs的克隆与表达分析[J]. 生物技术通报, 2023, 39(7): 151-159. |
| [9] | 胡明月, 杨宇, 郭仰东, 张喜春. 低温胁迫下番茄SlMYB96的功能分析[J]. 生物技术通报, 2023, 39(4): 236-245. |
| [10] | 刘思佳, 王浩楠, 付宇辰, 闫文欣, 胡增辉, 冷平生. ‘西伯利亚’百合LiCMK基因克隆及功能分析[J]. 生物技术通报, 2023, 39(3): 196-205. |
| [11] | 杨旭妍, 赵爽, 马天意, 白玉, 王玉书. 三个甘蓝WRKY基因的克隆及其对非生物胁迫的表达[J]. 生物技术通报, 2023, 39(11): 261-269. |
| [12] | 张玉娟, 黎冬华, 宫慧慧, 崔新晓, 高春华, 张秀荣, 游均, 赵军胜. 芝麻NAC转录因子基因SiNAC77的克隆及耐盐功能分析[J]. 生物技术通报, 2023, 39(11): 308-317. |
| [13] | 侯瑞泽, 鲍悦, 陈启亮, 毛桂玲, 韦博霖, 侯雷平, 李梅兰. 普通白菜PRR5的克隆、表达及功能验证[J]. 生物技术通报, 2023, 39(10): 128-135. |
| [14] | 陈浩婷, 张玉静, 刘洁, 代泽敏, 刘伟, 石玉, 张毅, 李天来. 低磷胁迫下番茄转录因子WRKY6功能分析[J]. 生物技术通报, 2023, 39(10): 136-147. |
| [15] | 杨敏, 龙雨青, 曾娟, 曾梅, 周新茹, 王玲, 付学森, 周日宝, 刘湘丹. 灰毡毛忍冬UGTPg17、UGTPg36基因克隆及功能研究[J]. 生物技术通报, 2023, 39(10): 256-267. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||