








生物技术通报 ›› 2024, Vol. 40 ›› Issue (9): 181-189.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0080
收稿日期:2024-01-19
出版日期:2024-09-26
发布日期:2024-10-12
通讯作者:
李志敏,女,博士,研究方向:合成生物学;E-mail: lizhimin1119@163.com作者简介:杜薇,女,硕士研究生,研究方向:应用微生物;E-mail: 604003648@qq.com
基金资助:
DU Wei( ), LI Zhi-min(
), LI Zhi-min( ), XING Yan-ming, LIU Pu-lin, MIAO Li-hong
), XING Yan-ming, LIU Pu-lin, MIAO Li-hong
Received:2024-01-19
Published:2024-09-26
Online:2024-10-12
摘要:
【目的】地衣芽孢杆菌(Bacillus licheniformis)是发酵工业中表达异源蛋白的重要底盘细胞,筛选易转化和高生物量的B. licheniformis菌株,为有效提升宿主的改造效率和蛋白表达水平提供菌种资源。【方法】经过富集、gyrB扩增与16S rDNA序列分析从土壤中筛选与地衣芽孢杆菌同源性较高的菌株,将所筛菌株的电转效率和生物量与工业常用底盘细胞B. licheniformis 2709进行比较分析。对筛选出的易电转和高生物量菌株进行形态学与生理生化鉴定,并进行抗生素敏感性测定和高温碱性环境生长情况测定。【结果】筛选出10株与地衣芽孢杆菌同源性较高的菌株,其中菌株1-33电转化效率为6 700 CFU/μg DNA,是B. licheniformis 2709的13.7倍。在豆粕玉米粉半固体培养基和SR液体培养基中培养,菌株1-33最大生物量达到5.2×1010 CFU/mL和6.8 g/L,分别为B. licheniformis 2709的1.71倍和1.3倍。进一步经过形态学、生理生化特性实验将菌株1-33最终鉴定为B. licheniformis,并表现出多种常用抗生素的敏感性和良好的耐碱与耐高温的能力。【结论】筛选出一株易电转和高生物量的B. licheniformis 1-33,为开发成高效表达外源蛋白的宿主菌奠定基础。
杜薇, 李志敏, 邢晏铭, 刘蒲临, 缪礼鸿. 一株易转化、高生物量地衣芽孢杆菌的筛选与鉴定[J]. 生物技术通报, 2024, 40(9): 181-189.
DU Wei, LI Zhi-min, XING Yan-ming, LIU Pu-lin, MIAO Li-hong. Screening and Identification of a Bacillus licheniformis Strain with High Electro-transfection Efficiency and Elevated Biomass[J]. Biotechnology Bulletin, 2024, 40(9): 181-189.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| gyrB-F[ | GCCGGCTTCATGGGTTCCG |
| gyrB-R[ | GCGTCGGTGCTTCTGTTG |
| 27f[ | AGAGTTTGATCCTGGCTCAG |
| 1492r[ | GGTTACCTTGTTACGACTT |
| GFP-F | CTGCGGCCGGTGCACATATGATGGTGAGCAAGGGCGAG |
| GFP-R | CTGCAGGTCGACAAGCTTCTACTTGTACAGCTCGTCCATG |
| pBES-F | AAGCTTGTCGACCTGCAGTC |
| pBES-R | CATATGCACCGGCCGC |
表1 本实验用引物序列
Table 1 Primer sequences used in this experiment
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| gyrB-F[ | GCCGGCTTCATGGGTTCCG |
| gyrB-R[ | GCGTCGGTGCTTCTGTTG |
| 27f[ | AGAGTTTGATCCTGGCTCAG |
| 1492r[ | GGTTACCTTGTTACGACTT |
| GFP-F | CTGCGGCCGGTGCACATATGATGGTGAGCAAGGGCGAG |
| GFP-R | CTGCAGGTCGACAAGCTTCTACTTGTACAGCTCGTCCATG |
| pBES-F | AAGCTTGTCGACCTGCAGTC |
| pBES-R | CATATGCACCGGCCGC |
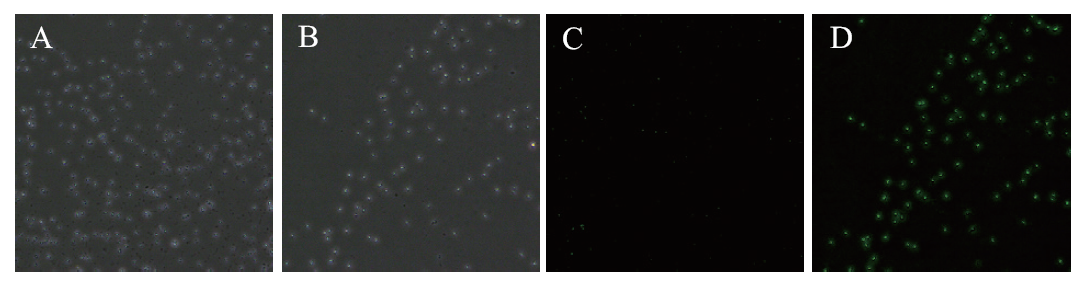
图2 荧光显微镜观察菌体绿色荧光 A:明场观察野生菌株1-33;B:明场观察转入pBES-P43-egfp的菌株1-33;C:荧光激发观察野生菌株1-33;D:荧光激发观察转入pBES-P43-egfp的菌株1-33
Fig. 2 Fluorescence microscope observation of green fluorescence of bacteriophage A: Bright field observation of wild strains 1-33. B: Bright field observation of strains 1-33 transfected with pBES-P43-egfp. C: Fluorescence-excited observation of wild strains 1-33. D: Fluorescence-excited observation of strains 1-33 transferred to pBES-P43-egfp
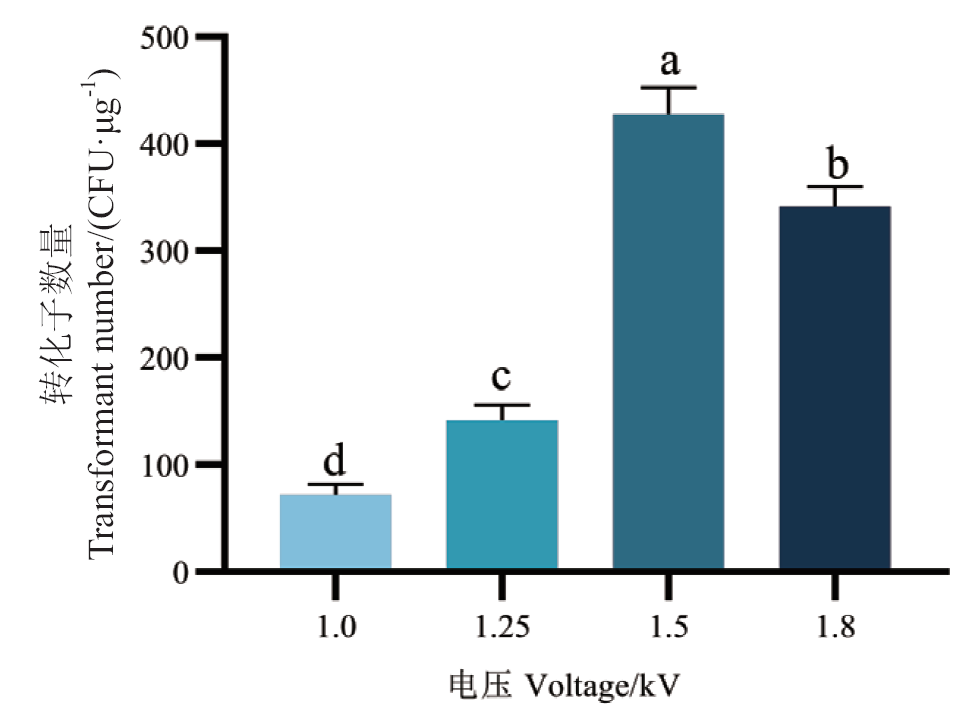
图3 不同电压对B. licheniformis 2709转化效率的影响 不同小写字母表示差异显著(P < 0.05),下同
Fig. 3 Effects of different voltages on the electro-transfection efficiency of B. licheniformis 2709 Different lowercase letters indicate significant differences(P < 0.05), the same below
| 菌株编号 Strain No. | 转化效率Electro-transfection efficiency/ (CFU·μg-1 DNA) | 菌株编号 Strain No. | 转化效率Electro-transfection efficiency/(CFU·μg-1 DNA) | |
|---|---|---|---|---|
| 1-4 | 1 420±20 | 1-25 | 1 570±81 | |
| 1-5 | 3 650±61 | 1-27 | 855±56 | |
| 1-8 | 0±0 | 1-33 | 6 700±89 | |
| 1-16 | 270±22 | 1-37 | 260±35 | |
| 1-17 | 118±9 | 2709 | 490±29 | |
| 1-19 | 416±46 |
表2 不同菌株的转化效率
Table 2 Electro-transfection efficiency of different strains
| 菌株编号 Strain No. | 转化效率Electro-transfection efficiency/ (CFU·μg-1 DNA) | 菌株编号 Strain No. | 转化效率Electro-transfection efficiency/(CFU·μg-1 DNA) | |
|---|---|---|---|---|
| 1-4 | 1 420±20 | 1-25 | 1 570±81 | |
| 1-5 | 3 650±61 | 1-27 | 855±56 | |
| 1-8 | 0±0 | 1-33 | 6 700±89 | |
| 1-16 | 270±22 | 1-37 | 260±35 | |
| 1-17 | 118±9 | 2709 | 490±29 | |
| 1-19 | 416±46 |
| 菌株 Strain | 湿重 Wet weight/(g·L-1) | 干重 Dry weight/(g·L-1) |
|---|---|---|
| 1-33 | 42.8±1.05 | 6.8±0.74 |
| B. licheniformis 2709 | 33.1±0.42 | 5.2±0.11 |
表3 菌株1-33与B. licheniformis 2709干湿重对比
Table 3 Comparison of wet and dry weights of strain 1-33 and B. licheniformis 2709
| 菌株 Strain | 湿重 Wet weight/(g·L-1) | 干重 Dry weight/(g·L-1) |
|---|---|---|
| 1-33 | 42.8±1.05 | 6.8±0.74 |
| B. licheniformis 2709 | 33.1±0.42 | 5.2±0.11 |

图5 菌株1-33形态学特征 A:1-33的菌落形态;B:1-33的革兰氏染色菌体特征
Fig. 5 Morphological characteristics of strains 1-33 A: Colony morphology of 1-33. B: Gram-stained bacterial characteristics of 1-33(10×100)
| 实验项目 Experimental project | 结果 Result | 实验项目 Experimental project | 结果 Result | |
|---|---|---|---|---|
| 利用木糖 | + | 厌氧生长 | + | |
| 利用阿拉伯糖 | + | V-P实验 | + | |
| 甘露醇 | + | pH=5.7生长 | + | |
| 明胶液化 | + | 柠檬酸盐 | + | |
| 7% NaCl生长 | + | 丙酸盐 | - | |
| 淀粉水解 | + | 硝酸盐还原 | + |
表4 菌株1-33生理生化特征
Table 4 Physiological and biochemical characterization of strain 1-33
| 实验项目 Experimental project | 结果 Result | 实验项目 Experimental project | 结果 Result | |
|---|---|---|---|---|
| 利用木糖 | + | 厌氧生长 | + | |
| 利用阿拉伯糖 | + | V-P实验 | + | |
| 甘露醇 | + | pH=5.7生长 | + | |
| 明胶液化 | + | 柠檬酸盐 | + | |
| 7% NaCl生长 | + | 丙酸盐 | - | |
| 淀粉水解 | + | 硝酸盐还原 | + |
| 抗生素种类 Antibiotic type | 抑菌浓度 Inhibitory concentration/(μg·mL-1) |
|---|---|
| 氨苄青霉素Benzylpenicillin | 10 |
| 四环素Tetracycline | 25 |
| 卡那霉素Kanamycin | 5 |
| 红霉素Erythromycin | 2 |
表5 四种抗生素对B. licheniformis 1-33的抑菌浓度
Table 5 Inhibitory concentrations of four antibiotics against B. licheniformis 1-33
| 抗生素种类 Antibiotic type | 抑菌浓度 Inhibitory concentration/(μg·mL-1) |
|---|---|
| 氨苄青霉素Benzylpenicillin | 10 |
| 四环素Tetracycline | 25 |
| 卡那霉素Kanamycin | 5 |
| 红霉素Erythromycin | 2 |
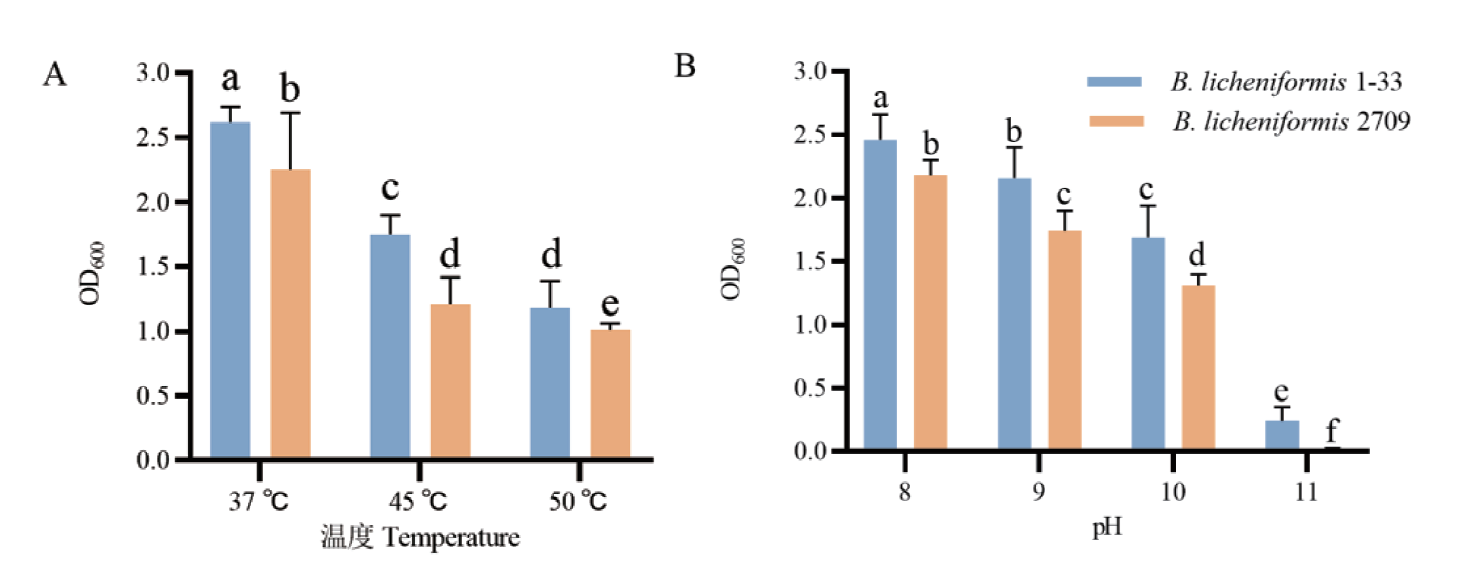
图6 高温(A)和碱性条件(B)下B. licheniformis 1-33与B. licheniformis 2709的生长情况比较
Fig. 6 Comparison of the growth of B. licheniformis 1-33 and B. licheniformis 2709 under high temperature(A)and alkaline conditions(B)
| [1] | Chen JQ, Zhu YM, Fu G, et al. High-level intra- and extra-cellular production of D-psicose 3-epimerase via a modified xylose-inducible expression system in Bacillus subtilis[J]. J Ind Microbiol Biotechnol, 2016, 43(11): 1577-1591. |
| [2] | 张莹, 韩晓静, 蔡逸安, 等. 解淀粉芽孢杆菌内源启动子的筛选及表达碱性果胶酶的应用研究[J]. 微生物学报, 2023, 63(4): 1575-1586. |
| Zhang Y, Han XJ, Cai YA, et al. Screening of endogenous promoters of Bacillus amyloliquefaciens and application of them in the expression of alkaline pectinase[J]. Acta Microbiol Sin, 2023, 63(4): 1575-1586. | |
| [3] | Shrestha S, Chio C, Khatiwada JR, et al. Optimization of multiple enzymes production by fermentation using lipid-producing Bacillus sp[J]. Front Microbiol, 2022, 13: 1049692. |
| [4] | Elemosho R, Suwanto A, Thenawidjaja M. Extracellular expression in Bacillus subtilis of a thermostable Geobacillus stearothermophilus lipase[J]. Electron J Biotechnol, 2021, 53: 71-79. |
| [5] |
Wang Q, Zheng H, Wan X, et al. Optimization of inexpensive agricultural by-products as raw materials for bacitracin production in Bacillus licheniformis DW2[J]. Appl Biochem Biotechnol, 2017, 183(4): 1146-1157.
doi: 10.1007/s12010-017-2489-1 pmid: 28593603 |
| [6] | Li YX, Li ZR, Yamanaka K, et al. Directed natural product biosynthesis gene cluster capture and expression in the model bacterium Bacillus subtilis[J]. Sci Rep, 2015, 5: 9383. |
| [7] | He FM, Gao BJ, Cheng X, et al. High-level production of poly-γ-glutamic acid by a newly isolated Bacillus sp. YJY-8 and potential use in increasing the production of tomato[J]. Prep Biochem Biotechnol, 2024, 54(5): 637-646. |
| [8] |
Cai D, Rao Y, Zhan Y, et al. Engineering Bacillus for efficient production of heterologous protein: current progress, challenge and prospect[J]. J Appl Microbiol, 2019, 126(6): 1632-1642.
doi: 10.1111/jam.14192 pmid: 30609144 |
| [9] | Souza CC, Guimarães JM, Pereira SDS, et al. The multifunctionality of expression systems in Bacillus subtilis: emerging devices for the production of recombinant proteins[J]. Exp Biol Med, 2021, 246(23): 2443-2453. |
| [10] | Liu HL, Wang S, Song LX, et al. Trehalose production using recombinant trehalose synthase in Bacillus subtilis by integrating fermentation and biocatalysis[J]. J Agric Food Chem, 2019, 67(33): 9314-9324. |
| [11] |
Zhang K, Su LQ, Wu J. Enhanced extracellular pullulanase production in Bacillus subtilis using protease-deficient strains and optimal feeding[J]. Appl Microbiol Biotechnol, 2018, 102(12): 5089-5103.
doi: 10.1007/s00253-018-8965-x pmid: 29675805 |
| [12] |
Wang Y, Chen ZM, Zhao RL, et al. Deleting multiple lytic genes enhances biomass yield and production of recombinant proteins by Bacillus subtilis[J]. Microb Cell Fact, 2014, 13: 129.
doi: 10.1186/s12934-014-0129-9 pmid: 25176138 |
| [13] | Wang Q, Yu HM, Wang MM, et al. Enhanced biosynthesis and characterization of surfactin isoforms with engineered Bacillus subtilis through promoter replacement and Vitreoscilla hemoglobin co-expression[J]. Process Biochemistry, 2018, 70:36-44. |
| [14] | Muras A, Romero M, Mayer C, et al. Biotechnological applications of Bacillus licheniformis[J]. Crit Rev Biotechnol, 2021, 41(4): 609-627. |
| [15] | Zhou CX, Zhou HY, Zhang HT, et al. Optimization of alkaline protease production by rational deletion of sporulation related genes in Bacillus licheniformis[J]. Microb Cell Fact, 2019, 18(1): 127. |
| [16] | Shen PL, Niu DD, Liu XL, et al. High-efficiency chromosomal integrative amplification strategy for overexpressing α-amylase in Bacillus licheniformis[J]. J Ind Microbiol Biotechnol, 2022, 49(3): kuac009. |
| [17] | 陈坤. 2709碱性蛋白酶的高产工程菌株构建及应用性能分析[D]. 天津: 天津科技大学, 2018. |
| Chen K. Construction of the engineering bacteria with high yield of alkaline protease 2709 and its application performance[D]. Tianjin:Tianjin University of Science & Technology, 2018. | |
| [18] | Xue GP, Johnson JS, Dalrymple BP. High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacillus licheniformis[J]. J Microbiol Meth, 1999, 34(3): 183-191. |
| [19] | Huang CH, Chang MT, Huang LN, et al. Development of a novel PCR assay based on the gyrase B gene for species identification of Bacillus licheniformis[J]. Mol Cell Probes, 2012, 26(5): 215-217. |
| [20] | 马凯, 刘光全, 程池. 地衣芽孢杆菌16S rRNA基因的TD-PCR扩增及系统发育分析[J]. 微生物学通报, 2007, 34(4): 709-711. |
| Ma K, Liu GQ, Cheng C. The TD-PCR and phylogenetic analysis of Bacillus licheniformis 16S rDNA[J]. Microbiology, 2007, 34(4): 709-711. | |
| [21] | 东秀珠, 蔡妙英. 常见细菌系统鉴定手册[M]. 北京: 科学出版社, 2001. |
| Dong XZ, Cai MY. Handbook of identification of common bacterial systems[M]. Beijing: Science Press, 2001. | |
| [22] |
Brockmeier U, Caspers M, Freudl R, et al. Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in Gram-positive bacteria[J]. J Mol Biol, 2006, 362(3): 393-402.
pmid: 16930615 |
| [23] | Zhan YY, Xu Y, Zheng PL, et al. Establishment and application of multiplexed CRISPR interference system in Bacillus licheniformis[J]. Appl Microbiol Biotechnol, 2020, 104(1): 391-403. |
| [24] |
Zakataeva NP, Nikitina OV, Gronskiy SV, et al. A simple method to introduce marker-free genetic modifications into the chromosome of naturally nontransformable Bacillus amyloliquefaciens strains[J]. Appl Microbiol Biotechnol, 2010, 85(4): 1201-1209.
doi: 10.1007/s00253-009-2276-1 pmid: 19820923 |
| [25] | He HH, Zhang YP, Shi GY, et al. Recent biotechnological advances and future prospective of Bacillus licheniformis as microbial cell factories[J]. Syst Microbiol Biomanuf, 2023, 3(4): 521-532. |
| [26] | Ahn S, Jun SM, Ro HJ, et al. Complete genome of Bacillus subtilis subsp. subtilis KCTC 3135T and variation in cell wall genes of B. subtilis strains[J]. J Microbiol Biotechnol, 2018, 28(10): 1760-1768. |
| [27] | Kunst F, Ogasawara N, Moszer I, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis[J]. Nature, 1997, 390(6657): 249-256. |
| [28] | Rahimnahal S, Meimandipour A, Fayazi J, et al. Biochemical and molecular characterization of novel keratinolytic protease from Bacillus licheniformis(KRLr1)[J]. Front Microbiol, 2023, 14: 1132760. |
| [29] |
Priya I, Dhar MK, Bajaj BK, et al. Cellulolytic activity of thermophilic bacilli isolated from tattapani hot spring sediment in North West Himalayas[J]. Indian J Microbiol, 2016, 56(2): 228-231.
doi: 10.1007/s12088-016-0578-4 pmid: 27570317 |
| [30] | 全爽, 陈涛, 毛宗林, 等. 一株土壤源地衣芽孢杆菌的鉴定及生物学特性研究[J]. 中国畜牧杂志, 2023, 59(1): 217-222. |
| Quan S, Chen T, Mao ZL, et al. Identification and biological characterization of a soil-derived Bacillus licheniformis strain[J]. Chin J Anim Sci, 2023, 59(1): 217-222. | |
| [31] | Li YR, Wang HR, Zhang L, et al. Efficient genome editing in Bacillus licheniformis mediated by a conditional CRISPR/Cas9 system[J]. Microorganisms, 2020, 8(5): 754. |
| [32] | Hoffmann K, Wollherr A, Larsen M, et al. Facilitation of direct conditional knockout of essential genes in Bacillus licheniformis DSM13 by comparative genetic analysis and manipulation of genetic competence[J]. Appl Environ Microbiol, 2010, 76(15): 5046-5057. |
| [33] | 莫静燕, 陈献忠, 王正祥. 地衣芽孢杆菌原生质体的制备、再生及转化研究[J]. 生物技术, 2009, 19(5): 75-77. |
| Mo JY, Chen XZ, Wang ZX. Preparation, regeneration and genetic transformation of Bacillus licheniformis protoplasts[J]. Biotechnology, 2009, 19(5): 75-77. | |
| [34] | Zhang Y, Hu JM, Zhang Q, et al. Enhancement of alkaline protease production in recombinant Bacillus licheniformis by response surface methodology[J]. Bioresour Bioprocess, 2023, 10(1): 27. |
| [35] | 肖静, 张虎, 李子源, 等. 抑制菌体自溶的地衣芽孢杆菌工程菌及其构建方法和应用:CN109868253A[P]. 2019-06-11. |
| Xiao J, Zhang H, Li ZY, et al. Bacillus licheniformis engineered bacteria that inhibit bacterial autolysis and its construction method and application: CN109868253A[P]. 2019-06-11. |
| [1] | 张亚亚, 李盼盼, 高惠惠, 贾晨波, 徐春燕. 基于根表真菌群落与病原菌鉴定探究‘宁杞5号’枸杞根腐病的发生机制[J]. 生物技术通报, 2024, 40(9): 238-248. |
| [2] | 袁兰, 黄娅楠, 张贝妮, 熊雨萌, 王洪洋. 基于流式细胞仪鉴定马铃薯倍性的高通量样品制备方法[J]. 生物技术通报, 2024, 40(9): 141-147. |
| [3] | 吴慧琴, 王延宏, 刘涵, 司政, 刘雪晴, 王静, 阳宜, 成妍. 辣椒UGT基因家族的鉴定及表达分析[J]. 生物技术通报, 2024, 40(9): 198-211. |
| [4] | 谭博文, 张懿, 张鹏, 王振宇, 马秋香. 木薯镁离子转运蛋白家族基因的鉴定及生物信息学分析[J]. 生物技术通报, 2024, 40(9): 20-32. |
| [5] | 崔原瑗, 王昭懿, 白双宇, 任毓昭, 豆飞飞, 刘彩霞, 刘凤楼, 王掌军, 李清峰. 大麦非特异性磷脂酶C基因家族全基因组鉴定及苗期胁迫表达分析[J]. 生物技术通报, 2024, 40(8): 74-82. |
| [6] | 邢丽南, 张艳芳, 葛明然, 赵令敏, 陈妍, 霍秀文. 山药DoWRKY40基因表达特征分析及互作蛋白筛选[J]. 生物技术通报, 2024, 40(8): 118-128. |
| [7] | 臧文蕊, 马明, 砗根, 哈斯阿古拉. 甜瓜BZR转录因子家族基因的全基因组鉴定及表达模式分析[J]. 生物技术通报, 2024, 40(7): 163-171. |
| [8] | 王芳, 于璐, 齐泽铮, 周长军, 于吉东. 大豆镰刀菌根腐病拮抗菌的筛选及生防效果[J]. 生物技术通报, 2024, 40(7): 216-225. |
| [9] | 杨鹭, 袁源, 方志锴, 林如, 江红, 周剑. 一株链霉菌的鉴定及其产格尔德霉素的发酵工艺研究[J]. 生物技术通报, 2024, 40(6): 299-309. |
| [10] | 阿丽亚·外力, 陈永坤, 克拉热木·克里木江, 王宝庆, 陈凌娜. 核桃SPL基因家族的系统进化和表达分析[J]. 生物技术通报, 2024, 40(6): 180-189. |
| [11] | 徐伟芳, 李贺宇, 张慧, 何仔昂, 高文恒, 谢紫洋, 王传文, 尹登科. 生防细菌HX0037对栝楼炭疽病的防病能力及其机制[J]. 生物技术通报, 2024, 40(4): 228-241. |
| [12] | 饶永斌, 张君丽. 亚紫裸伞的生物学特性及驯化栽培[J]. 生物技术通报, 2024, 40(4): 264-270. |
| [13] | 杨伟成, 孙岩, 杨倩, 王壮琳, 马菊花, 薛金爱, 李润植. 陆地棉FAX家族的全基因组鉴定及GhFAX1的功能分析[J]. 生物技术通报, 2024, 40(3): 155-169. |
| [14] | 王璐, 刘梦雨, 张富源, 纪守坤, 王云, 张英杰, 段春辉, 刘月琴, 严慧. 瘤胃源粪臭素降解菌的分离鉴定及其降解特性研究[J]. 生物技术通报, 2024, 40(3): 305-311. |
| [15] | 杨伟杰, 杨周林, 朱浩东, 魏煜, 刘君, 刘训. 地衣素合成酶关键模块 LchAD 蛋白的性质和功能研究[J]. 生物技术通报, 2024, 40(3): 322-332. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||