








生物技术通报 ›› 2024, Vol. 40 ›› Issue (3): 322-332.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0996
杨伟杰1( ), 杨周林1, 朱浩东1, 魏煜1, 刘君1,2(
), 杨周林1, 朱浩东1, 魏煜1, 刘君1,2( ), 刘训1(
), 刘训1( )
)
收稿日期:2023-10-23
出版日期:2024-03-26
发布日期:2024-04-08
通讯作者:
刘训,女,博士,讲师,研究方向:酒类风味物质代谢调控;E-mail: xunliu0123@suse.edu.cn;作者简介:杨伟杰,男,硕士研究生,研究方向:酿酒工程;E-mail: 18783502843@163.com
基金资助:
YANG Wei-jie1( ), YANG Zhou-lin1, ZHU Hao-dong1, WEI Yu1, LIU Jun1,2(
), YANG Zhou-lin1, ZHU Hao-dong1, WEI Yu1, LIU Jun1,2( ), LIU Xun1(
), LIU Xun1( )
)
Received:2023-10-23
Published:2024-03-26
Online:2024-04-08
摘要:
【目的】 地衣素(lichenysin)作为一种脂肽类物质,对白酒风味形成有促进作用。为研究地衣素合成酶中硫酯酶模块LchAD蛋白的性质和功能,采用传统分离纯化方法从酿酒大曲样品中,筛选产地衣素的芽孢杆菌菌株。【方法】 采用质谱串联的方式检测发酵产物,通过形态学、生理生化实验和构建系统发育树,分析菌株的种属关系。以筛选出的菌株基因组DNA为模板克隆LchAD基因,在大肠杆菌中异源表达LchAD蛋白并用镍柱纯化。利用在线软件对LchAD蛋白进行生物信息学分析,探究LchAD 蛋白的性质和功能。【结果】 在大曲中筛选获得的菌株YC7为地衣芽孢杆菌(Bacillus licheniformis),其发酵产物中含有地衣素A(D或G)的同系物,表明菌株YC7为地衣素产生菌。LchAD蛋白的可溶性表达研究发现,在温度为20℃,IPTG终浓度为100 μg/mL条件下可诱导LchAD蛋白异源表达,并形成可溶性蛋白,其分子量约为27.62 kD。镍柱纯化结果显示,在咪唑浓度为250 mmol/L时,可获得较高纯度的LchAD蛋白。生物信息学分析结果表明,LchAD蛋白为稳定的亲水性蛋白,无信号肽,理论等电点(pI)为7.23,含有一个GrsT保守结构域。LchAD蛋白的二级结构包含43.5%的α-螺旋,13.82%的β-折叠,5.69%的β-转角,其三级结构与表面活性素合成酶的硫脂酶模块相似。【结论】 筛选得到的地衣芽孢杆菌YC7菌株可产地衣素,其LchAD基因编码的蛋白为27.62 kD的亲水性蛋白,异源表达获得可溶性LchAD蛋白,该蛋白可能与合成表面活性素的硫酯酶模块功能相似。
杨伟杰, 杨周林, 朱浩东, 魏煜, 刘君, 刘训. 地衣素合成酶关键模块 LchAD 蛋白的性质和功能研究[J]. 生物技术通报, 2024, 40(3): 322-332.
YANG Wei-jie, YANG Zhou-lin, ZHU Hao-dong, WEI Yu, LIU Jun, LIU Xun. Study on the Properties and Functions of LchAD Protein, a Key Module of Lichenysin Synthase[J]. Biotechnology Bulletin, 2024, 40(3): 322-332.
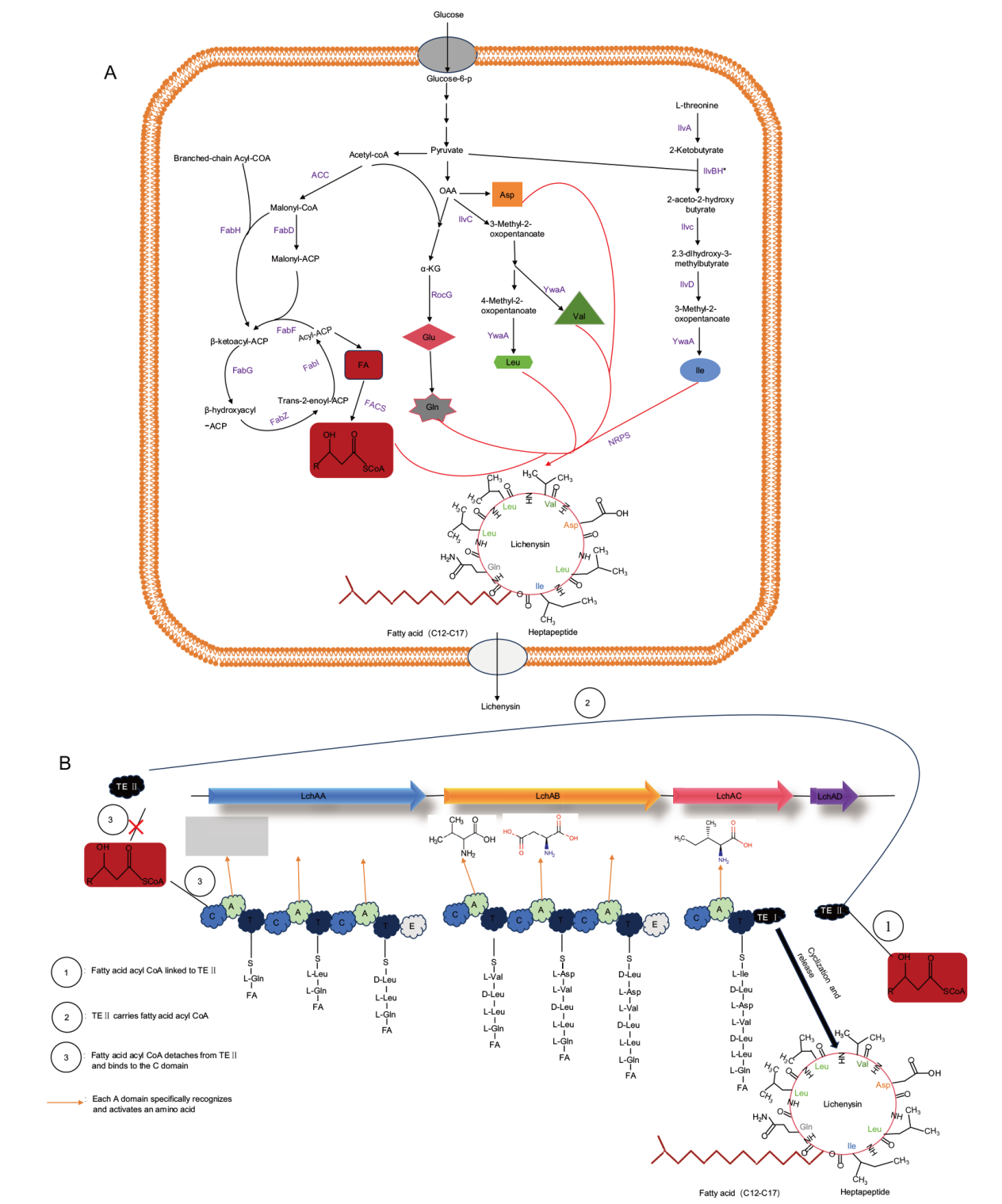
图1 地衣素生物合成示意图 A:地衣素产生和分泌(ACC:乙酰辅酶A羧化酶复合物;FabD:丙二酰辅酶A -ACP转酰基酶;FabH:3-酮酰基载体蛋白合成酶;FabF:3-氧酰基- ACP合成酶II;FabG:β-酮酰基ACP还原酶;FabI:酰基载体蛋白还原酶;FabZ:3-羟基酰基ACP脱水酶;IlvC:乙酰羟基酸异构酶;RocG:谷氨酸脱氢酶;YwaA:支链氨基酸转氨酶;IlvA:苏氨酸脱水酶;IlvBH*:工程乙酰羟基酸合成酶调控亚基;IlvD:二羟基酸脱水酶;FACS:脂肪酸酰基辅酶A连接酶);B:地衣素的合成;地衣素合成酶由4个模块组成,其中与模块3相连的是TE I,即TE结构域;模块4是TE II,即LchAD
Fig. 1 Schematic diagram of lichenysin biosynthesis A: Production and secretion of lichenysin(ACC: acetyl CoA carboxylase complex; FabD: malonyl-CoA-ACP transacylase; FabH: 3-keto-acyl carrier protein synthase III; FabF: 3-oxoacyl-ACP-synthase II;FabG: β-ketoacyl-ACP reductase; FabI: enoyl-ACP reductases; FabZ: 3-hydroxyacyl-ACP dehydratase; IlvC: acetohydroxy-acid isomeroreductase; RocG: glutamic dehydrogenase; YwaA: branched chain amino acid transaminase; IlvA: L-threonine dehydratase; IlvBH*: regulatory subunits of engineering acetyl hydroxy acid synthase; IlvD: dihydroxyacid dehydratase; FACS: fatty acyl CoA ligases). B: Synthesis of lichenysin. Lichenysin synthase, consists of four modules, with TE I, the TE domain, connected to module 3. Module 4 is TE II, i.e. LchAD
| 菌株 Strain | 菌落形态 Colony morphology |
|---|---|
| YC1 | 奶白色,菌落表面干燥,边缘规则,扁平 |
| YC2 | 半透明、微黄色,菌落表面湿润,边缘不规则,扁平 |
| YC7 | 微红色、菌落表面不光滑但湿润,边缘不规则,扁平 |
表1 菌落形态
Table 1 Colony morphology
| 菌株 Strain | 菌落形态 Colony morphology |
|---|---|
| YC1 | 奶白色,菌落表面干燥,边缘规则,扁平 |
| YC2 | 半透明、微黄色,菌落表面湿润,边缘不规则,扁平 |
| YC7 | 微红色、菌落表面不光滑但湿润,边缘不规则,扁平 |
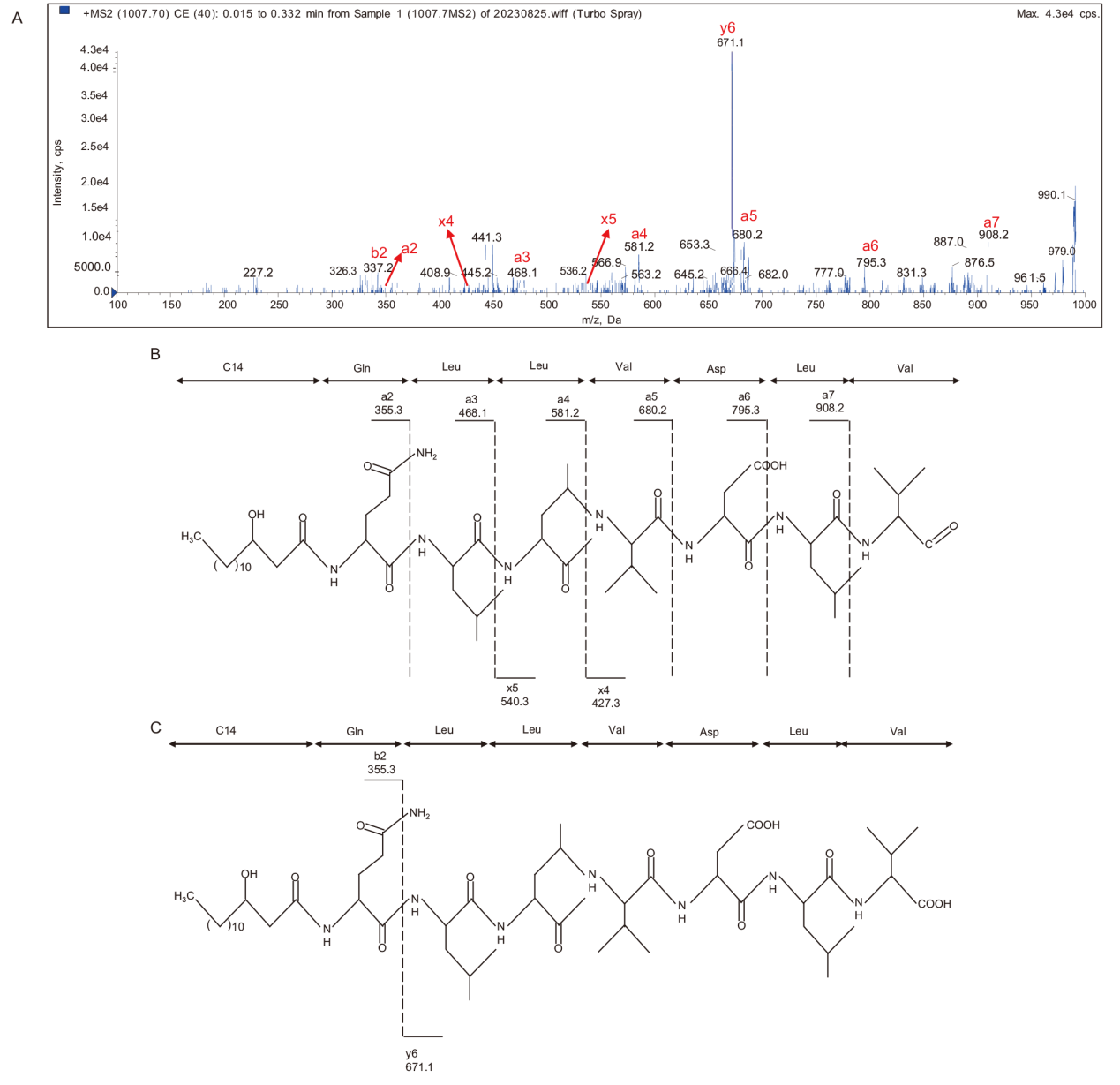
图3 m/z为1 007.4离子峰的二级质谱图(A)及该离子峰发生简单断裂(B)和发生双氢转移断裂(C)产生的特征离子碎片理论值
Fig. 3 Secondary mass spectrometry of ion peak with m/z of 1 007.4(A)and theoretical values of characteristic ion fragments generated by simple cleavage(B)and double hydrogen transfer cleavage(C)of this ion peak
| 实验项目Experiment item | 实验结果Experimental result |
|---|---|
| 革兰氏染色 | + |
| 芽孢染色 | + |
| 接触酶实验 | + |
| 厌氧生长实验 | + |
| 葡萄糖产酸实验 | + |
| 葡萄糖产气实验 | - |
| 硝酸盐还原实验 | + |
| 淀粉分解实验 | + |
| V-P实验 | + |
| 明胶分解实验 | + |
表2 生理生化结果
Table 2 Physiological and biochemical results
| 实验项目Experiment item | 实验结果Experimental result |
|---|---|
| 革兰氏染色 | + |
| 芽孢染色 | + |
| 接触酶实验 | + |
| 厌氧生长实验 | + |
| 葡萄糖产酸实验 | + |
| 葡萄糖产气实验 | - |
| 硝酸盐还原实验 | + |
| 淀粉分解实验 | + |
| V-P实验 | + |
| 明胶分解实验 | + |
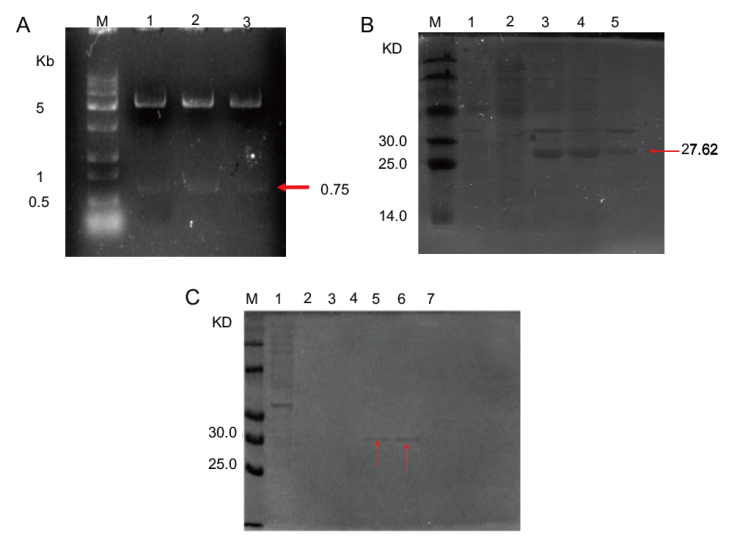
图5 重组质粒pET-28a-LchAD双酶切(A)和 LchAD蛋白表达(B)及纯化(C)结果 (A)M:DNA maker;1、2、3是重组质粒双酶切验证;(B)M:蛋白maker;1:载体pET-28a的上清液;2:重组质粒在20℃未加IPTG诱导的上清液;3:重组质粒在20℃诱导表达的全细胞液;4:重组质粒在20℃诱导表达的上清液;5:重组质粒在20℃诱导表达的沉淀;(C)M:蛋白maker;1:流穿液;2-7:浓度分别为10、25、50、100、250、500 mmol/L的咪唑洗脱
Fig. 5 Results of double digestion of recombinant plasmid pET-28a-LchAD(A)and LchAD protein expression(B)and purification(C) (A)M: DNA maker; 1, 2 and 3 are double restriction endonuclease digestion validation of recombinant plasmids.(B)M: Protein maker. 1: The supernatant of carrier pET-28a. 2: Recombinant plasmid supernatant without IPTG induction at 20℃. 3: Recombinant plasmid induced expression in whole cell fluid at 20℃. 4: The supernatant of the recombinant plasmid induced expression at 20℃.5: Precipitation of recombinant plasmid induced expression at 20℃.(C)M: Protein maker. 1: Flow through fluid. 2-7: Imidazole elution with a concentration of 10, 25, 50, 100, 250 and 500 mmol/L, respectively

图6 LchAD蛋白的保守结构域(A)、亲疏水性(B)和信号肽(C)预测
Fig. 6 Prediction of conserved domain(A), hydrophilicity and hydrophobicity(B)and signal peptide(C)of LchAD protein
| [1] |
Inoda S, Hirohashi Y, Torigoe T, et al. Cytotoxic T lymphocytes efficiently recognize human colon cancer stem-like cells[J]. Am J Pathol, 2011, 178(4): 1805-1813.
doi: 10.1016/j.ajpath.2011.01.004 pmid: 21435460 |
| [2] |
Wang XQ, Hu WW, Zhu LQ, et al. Bacillus subtilis and surfactin inhibit the transmissible gastroenteritis virus from entering the intestinal epithelial cells[J]. Biosci Rep, 2017, 37(2): BSR20170082.
doi: 10.1042/BSR20170082 URL |
| [3] |
Zhang YY, Liu C, Dong B, et al. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages[J]. Inflammation, 2015, 38(2): 756-764.
doi: 10.1007/s10753-014-9986-y pmid: 25331175 |
| [4] |
Chauhan V, Kanwar SS. Lipopeptide(s)associated with human microbiome as potent cancer drug[J]. Semin Cancer Biol, 2021, 70: 128-133.
doi: 10.1016/j.semcancer.2020.06.012 pmid: 32574814 |
| [5] |
Zhang R, Wu Q, Xu Y. Lichenysin, a cyclooctapeptide occurring in Chinese liquor Jiannanchun reduced the headspace concentration of phenolic off-flavors via hydrogen-bond interactions[J]. J Agric Food Chem, 2014, 62(33): 8302-8307.
doi: 10.1021/jf502053g URL |
| [6] | 郅岩, 吴群, 徐岩. 传统白酒中生物活性物质脂肽类化合物及其产生机制[J]. 酿酒科技, 2017(10): 17-23. |
| Zhi Y, Wu Q, Xu Y. Identification and formation mechanism of lipopeptides in Baijiu[J]. Liquor Mak Sci Technol, 2017(10): 17-23. | |
| [7] |
Madslien EH, Rønning HT, Lindbäck T, et al. Lichenysin is produced by most Bacillus licheniformis strains[J]. J Appl Microbiol, 2013, 115(4): 1068-1080.
doi: 10.1111/jam.12299 pmid: 23844764 |
| [8] |
Süssmuth RD, Mainz A. Nonribosomal peptide synthesis-principles and prospects[J]. Angew Chem Int Ed Engl, 2017, 56(14): 3770-3821.
doi: 10.1002/anie.v56.14 URL |
| [9] |
Koglin A, Löhr F, Bernhard F, et al. Structural basis for the selectivity of the external thioesterase of the surfactin synthetase[J]. Nature, 2008, 454(7206): 907-911.
doi: 10.1038/nature07161 |
| [10] |
Du LC, Lou LL. PKS and NRPS release mechanisms[J]. Nat Prod Rep, 2010, 27(2): 255-278.
doi: 10.1039/b912037h pmid: 20111804 |
| [11] |
乔龙亮, 朱鹏, 庞建虎. II型硫脂酶与次生代谢产物合成[J]. 中国生物化学与分子生物学报, 2017, 33(11): 1115-1124.
doi: 10.13865/j.cnki.cjbmb.2017.11.06 |
| Qiao LL, Zhu P, Pang JH. Type II thioesterases and their role in the synthesis of secondary metabolites[J]. Chin J Biochem Mol Biol, 2017, 33(11): 1115-1124. | |
| [12] |
Peschke M, Brieke C, Heimes M, et al. The thioesterase domain in glycopeptide antibiotic biosynthesis is selective for cross-linked aglycones[J]. ACS Chem Biol, 2018, 13(1): 110-120.
doi: 10.1021/acschembio.7b00943 pmid: 29192758 |
| [13] |
Schneider A, Marahiel MA. Genetic evidence for a role of thioesterase domains, integrated in or associated with peptide synthetases, in non-ribosomal peptide biosynthesis in Bacillus subtilis[J]. Arch Microbiol, 1998, 169(5): 404-410.
pmid: 9560421 |
| [14] | Wu H, Liang JD, Gou LX, et al. Recycling of overactivated acyls by a type II thioesterase during calcimycin biosynthesis in Streptomyces chartreusis NRRL 3882[J]. Appl Environ Microbiol, 2018, 84(12): e00587-e00518. |
| [15] | Kotowska M, Ciekot J, Pawlik K. Type II thioesterase ScoT is required for coelimycin production by the modular polyketide synthase Cpk of Streptomyces coelicolor A3(2)[J]. Acta Biochim Pol, 2014, 61(1): 141-147. |
| [16] |
Claxton HB, Akey DL, Silver MK, et al. Structure and functional analysis of RifR, the type II thioesterase from the rifamycin biosynthetic pathway[J]. J Biol Chem, 2009, 284(8): 5021-5029.
doi: 10.1074/jbc.M808604200 pmid: 19103602 |
| [17] |
Whicher JR, Florova G, Sydor PK, et al. Structure and function of the RedJ protein, a thioesterase from the prodiginine biosynthetic pathway in Streptomyces coelicolor[J]. J Biol Chem, 2011, 286(25): 22558-22569.
doi: 10.1074/jbc.M110.213512 URL |
| [18] |
Kotowska M, Pawlik K. Roles of type II thioesterases and their application for secondary metabolite yield improvement[J]. Appl Microbiol Biotechnol, 2014, 98(18): 7735-7746.
doi: 10.1007/s00253-014-5952-8 pmid: 25081554 |
| [19] |
Yakimov MM, Fredrickson HL, Timmis KN. Effect of heterogeneity of hydrophobic moieties on surface activity of lichenysin A, a lipopeptide biosurfactant from Bacillus licheniformis BAS50[J]. Biotechnol Appl Biochem, 1996, 23(1): 13-18.
pmid: 8867891 |
| [20] | 钟桂芳, 张帆, 郭辉祥, 等. 高温大曲中产四甲基吡嗪细菌的筛选及鉴定[J]. 中国酿造, 2020, 39(8): 107-111. |
| Zhong GF, Zhang F, Guo HX, et al. Screening and identification of tetramethylpyrazine-producing strains from high-temperature Daqu[J]. China Brew, 2020, 39(8): 107-111. | |
| [21] | 梁慧珍, 卢延想, 刘正, 等. 高温大曲中高产吡嗪类物质芽孢杆菌的筛选与应用[J]. 中国酿造, 2022, 41(1): 116-122. |
| Liang HZ, Lu YX, Liu Z, et al. Screening and application of high-yield pyrazines Bacillus from high temperature Daqu[J]. China Brew, 2022, 41(1): 116-122. | |
| [22] |
Qiu YM, Xiao F, Wei XT, et al. Improvement of lichenysin production in Bacillus licheniformis by replacement of native promoter of lichenysin biosynthesis operon and medium optimization[J]. Appl Microbiol Biotechnol, 2014, 98(21): 8895-8903.
doi: 10.1007/s00253-014-5978-y URL |
| [23] | 王成俊, 李玲珊, 范梅, 等. 酱香型大曲中产4-乙基愈创木酚芽孢杆菌的筛选、鉴定及特性研究[J]. 酿酒科技, 2023(4): 45-52. |
| Wang CJ, Li LS, Fan M, et al. Screening, identification and characterization of 4-ethylguaiacol producing Bacillus strains from Jiangxiang daqu[J]. Liquor Mak Sci Technol, 2023(4): 45-52. | |
| [24] |
Ayyaz K, Zaheer A, Rasul G, et al. Isolation and identification by 16S rRNA sequence analysis of plant growth-promoting azospirilla from the rhizosphere of wheat[J]. Braz J Microbiol, 2016, 47(3): 542-550.
doi: 10.1016/j.bjm.2015.11.035 pmid: 27133558 |
| [25] |
Ngo G, Centola M, Krasnoselska G, et al. LptC from Anabaena sp. PCC 7120: expression, purification and crystallization[J]. Protein Expr Purif, 2020, 175: 105689.
doi: 10.1016/j.pep.2020.105689 URL |
| [26] |
Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold[J]. Nature, 2021, 596(7873): 583-589.
doi: 10.1038/s41586-021-03819-2 |
| [27] |
Wu Q, Zhang R, Peng SQ, et al. Transcriptional characteristics associated with lichenysin biosynthesis in Bacillus licheniformis from Chinese Maotai-flavor liquor making[J]. J Agric Food Chem, 2015, 63(3): 888-893.
doi: 10.1021/jf5036806 URL |
| [28] | 李海岩, 麻新妍, 薛永常. 非核糖体肽合成酶硫酯酶结构域研究进展[J]. 中国抗生素杂志, 2021, 46(12): 1073-1077. |
| Li HY, Ma XY, Xue YC. The research progress of thioesterase domain in non-ribosomal peptide synthetase[J]. Chin J Antibiot, 2021, 46(12): 1073-1077. | |
| [29] | 聂凌鸿, 樊璐, 季方. 大曲中细菌和酵母菌的分离及其Biolog微生物系统分析鉴定[J]. 安徽农业科学, 2012, 40(2): 1036-1038, 1122. |
| Nie LH, Fan L, Ji F. Study on color-protection technique of Tricholoma matsutake in flexible packages[J]. J Anhui Agric Sci, 2012, 40(2): 1036-1038, 1122. | |
| [30] | 周平, 罗惠波, 黄丹, 等. 中高温大曲中一株耐热细菌的分离鉴定及其风味代谢产物分析[J]. 食品工业科技, 2016, 37(24): 215-220. |
| Zhou P, Luo HB, Huang D, et al. Separation and identification of thermoduric bacteria strains in medium/high temperature Daqu and the analysis of the flavor metabolites[J]. Sci Technol Food Ind, 2016, 37(24): 215-220. | |
| [31] | 柏永昊, 张明春, 缪礼鸿. 芽孢杆菌对白酒发酵过程中正丙醇含量的影响[J]. 酿酒科技, 2013(11): 26-29. |
| Bai YH, Zhang MC, Miao LH. Effects of the addition of Bacillus NX3-5Y during liquor fermentation on the content of n-propan-ol[J]. Liquor Mak Sci Technol, 2013(11): 26-29. | |
| [32] | 明红梅, 董瑞丽, 许德富, 等. 浓香型大曲中优势菌的分离及初步鉴定[J]. 酿酒科技, 2013(12): 57-60. |
| Ming HM, Dong RL, Xu DF, et al. Separation and preliminary identification of dominant microbes in Nong-flavor daqu[J]. Liquor Mak Sci Technol, 2013(12): 57-60. | |
| [33] | Rønning HT, Madslien EH, Asp TN, et al. Identification and quantification of lichenysin - a possible source of food poisoning[J]. Food Addit Contam, 2015, 32(12): 2120-2130. |
| [34] |
Suharti, Mahardika G, Raissa, et al. Cloning, heterologous expression, and characterization of a novel thioesterase from natural sample[J]. Heliyon, 2021, 7(3): e06542.
doi: 10.1016/j.heliyon.2021.e06542 URL |
| [35] | 陈维斌. 恰塔努加链霉菌L10中II型硫酯酶生物学功能研究[D]. 杭州: 浙江大学, 2015. |
| Chen WB. The study of the functions of type II Thioesterases in Streptomyces chattanoosensis L10[D]. Hangzhou: Zhejiang University, 2015. | |
| [36] |
Swarbrick CMD, Nanson JD, Patterson EI, et al. Structure, function, and regulation of thioesterases[J]. Prog Lipid Res, 2020, 79: 101036.
doi: 10.1016/j.plipres.2020.101036 URL |
| [37] |
Pourmasoumi F, De S, Peng HY, et al. Proof-reading thioesterase boosts activity of engineered nonribosomal peptide synthetase[J]. ACS Chem Biol, 2022, 17(9): 2382-2388.
doi: 10.1021/acschembio.2c00341 pmid: 36044980 |
| [38] |
Krätzschmar J, Krause M, Marahiel MA. Gramicidin S biosynthesis operon containing the structural genes grsA and grsB has an open reading frame encoding a protein homologous to fatty acid thioesterases[J]. J Bacteriol, 1989, 171(10): 5422-5429.
pmid: 2477357 |
| [39] |
Berditsch M, Trapp M, Afonin S, et al. Antimicrobial peptide gramicidin S is accumulated in granules of producer cells for storage of bacterial phosphagens[J]. Sci Rep, 2017, 7: 44324.
doi: 10.1038/srep44324 pmid: 28295017 |
| [40] |
Coronel-León J, Marqués AM, Bastida J, et al. Optimizing the production of the biosurfactant lichenysin and its application in biofilm control[J]. J Appl Microbiol, 2016, 120(1): 99-111.
doi: 10.1111/jam.12992 pmid: 26519210 |
| [41] | Jacques P. Surfactin and other lipopeptides from Bacillus spp.[M]// Biosurfactants. Berlin, Heidelberg: Springer, 2011: 57-91. |
| [42] |
Curran SC, Pereira JH, Baluyot MJ, et al. Structure and function of BorB, the type II thioesterase from the borrelidin biosynthetic gene cluster[J]. Biochemistry, 2020, 59(16): 1630-1639.
doi: 10.1021/acs.biochem.0c00126 pmid: 32250597 |
| [1] | 陈晓松, 刘超杰, 郑佳, 乔宗伟, 罗惠波, 邹伟. TMT定量蛋白质组学解析Rummeliibacillus suwonensis 3B-1 生长及己酸代谢机制[J]. 生物技术通报, 2024, 40(3): 135-145. |
| [2] | 龚丽丽, 余花, 杨杰, 陈天池, 赵双滢, 吴月燕. 葡萄CYP707A基因家族的鉴定及对果实成熟的功能验证[J]. 生物技术通报, 2024, 40(2): 160-171. |
| [3] | 张路阳, 韩文龙, 徐晓雯, 姚健, 李芳芳, 田效园, 张智强. 烟草TCP基因家族的鉴定及表达分析[J]. 生物技术通报, 2023, 39(6): 248-258. |
| [4] | 李敬蕊, 王育博, 解紫薇, 李畅, 吴晓蕾, 宫彬彬, 高洪波. 甜瓜PIN基因家族的鉴定及高温胁迫表达分析[J]. 生物技术通报, 2023, 39(5): 192-204. |
| [5] | 郭三保, 宋美玲, 李灵心, 尧子钊, 桂明明, 黄胜和. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156. |
| [6] | 王艺清, 王涛, 韦朝领, 戴浩民, 曹士先, 孙威江, 曾雯. 茶树SMAS基因家族的鉴定及互作分析[J]. 生物技术通报, 2023, 39(4): 246-258. |
| [7] | 杨岚, 张晨曦, 樊学伟, 王阳光, 王春秀, 李文婷. 鸡 BMP15 基因克隆、表达模式及启动子活性分析[J]. 生物技术通报, 2023, 39(4): 304-312. |
| [8] | 陈强, 邹明康, 宋家敏, 张冲, 吴隆坤. 甜瓜LBD基因家族的鉴定和果实发育进程中的表达分析[J]. 生物技术通报, 2023, 39(3): 176-183. |
| [9] | 平怀磊, 郭雪, 余潇, 宋静, 杜春, 王娟, 张怀璧. 滇牡丹PdANS的克隆、表达及与花青素含量的相关性[J]. 生物技术通报, 2023, 39(3): 206-217. |
| [10] | 邢媛, 宋健, 李俊怡, 郑婷婷, 刘思辰, 乔治军. 谷子AP基因家族鉴定及其对非生物胁迫的响应分析[J]. 生物技术通报, 2023, 39(11): 238-251. |
| [11] | 陈楚怡, 杨小梅, 陈胜艳, 陈斌, 岳莉然. ABA和干旱胁迫下菊花脑ZF-HD基因家族的表达分析[J]. 生物技术通报, 2023, 39(11): 270-282. |
| [12] | 杨敏, 龙雨青, 曾娟, 曾梅, 周新茹, 王玲, 付学森, 周日宝, 刘湘丹. 灰毡毛忍冬UGTPg17、UGTPg36基因克隆及功能研究[J]. 生物技术通报, 2023, 39(10): 256-267. |
| [13] | 郭志浩, 金泽鑫, 刘琦, 高利. 小麦矮腥黑粉菌效应蛋白g11335的生物信息学分析、亚细胞定位及毒性验证[J]. 生物技术通报, 2022, 38(8): 110-117. |
| [14] | 于秋琳, 马婧怡, 赵盼, 孙鹏芳, 何玉美, 刘世彪, 郭惠红. 绞股蓝GpMIR156a和GpMIR166b的克隆与功能分析[J]. 生物技术通报, 2022, 38(7): 186-193. |
| [15] | 陈佳敏, 刘永杰, 马锦绣, 李丹, 公杰, 赵昌平, 耿洪伟, 高世庆. 小麦组蛋白甲基化酶在杂交种中干旱胁迫表达模式分析[J]. 生物技术通报, 2022, 38(7): 51-61. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||