








生物技术通报 ›› 2024, Vol. 40 ›› Issue (11): 296-311.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0150
康晓博1( ), 张璟汐1, 卢甜甜1, 刘亚月1,2, 周龙建1,2, 张翼1,2,3(
), 张璟汐1, 卢甜甜1, 刘亚月1,2, 周龙建1,2, 张翼1,2,3( )
)
收稿日期:2024-02-11
出版日期:2024-11-26
发布日期:2024-12-19
通讯作者:
张翼,男,博士,教授,研究方向:海洋天然产物;E-mail: hubeizhangyi@163.com作者简介:康晓博,男,硕士研究生,研究方向:海洋生物活性物质;E-mail: k17701892041@126.com张璟汐为本文共同第一作者
基金资助:
KANG Xiao-bo1( ), ZHANG Jing-xi1, LU Tian-tian1, LIU Ya-yue1,2, ZHOU Long-jian1,2, ZHANG Yi1,2,3(
), ZHANG Jing-xi1, LU Tian-tian1, LIU Ya-yue1,2, ZHOU Long-jian1,2, ZHANG Yi1,2,3( )
)
Received:2024-02-11
Published:2024-11-26
Online:2024-12-19
摘要:
【目的】 以一株海洋真菌爪曲霉Aspergillus unguis DLEP2008001为对象,研究不同盐度对其生物活性和次生代谢产物的影响。【方法】 对不同盐度培养下该菌株第14天和第28天的固体和液体培养基发酵产物提取物,分别采用薄层层析和抗氧化、乙酰胆碱酯酶抑制及抗菌生物活性自显影手段,分析其活性次生代谢产物差异,将样品进一步采用高效液相色谱以及基于液相色谱串联质谱的“多途径辅助的特征分子网络”代谢组学分析手段,系统地研究不同盐度培养下A. unguis DLEP2008001次生代谢产物的多样性变化。【结果】 盐度可以调节抗氧化、抑制乙酰胆碱酯酶以及抗菌活性成分的产量与多样性,采用海水马铃薯蔗糖液体培养基28 d培养时在35 g/L盐度下出现最为丰富的特征代谢产物,而大米固体培养基在5 g/L盐度下特征代谢产物最为丰富、35 g/L盐度下也较丰富,固体发酵优势代谢产物的平均分子量随着盐度上升而总体呈现下降趋势,该菌株在高盐条件下的优势代谢产物多为含多羟基、多氨基的小分子化合物,推测可能参与渗透压调节。【结论】 提取物盐度对该爪曲霉菌株的生物活性及次生代谢产物有显著的影响。
康晓博, 张璟汐, 卢甜甜, 刘亚月, 周龙建, 张翼. 不同盐度培养下海洋真菌Aspergillus unguis DLEP2008001生物活性及次生代谢组变化[J]. 生物技术通报, 2024, 40(11): 296-311.
KANG Xiao-bo, ZHANG Jing-xi, LU Tian-tian, LIU Ya-yue, ZHOU Long-jian, ZHANG Yi. Variation of Bioactivities and Secondary Metabolomics of Marine Fungus Aspergillus unguis DLEP2008001 Cultured under Different Salinities[J]. Biotechnology Bulletin, 2024, 40(11): 296-311.

图1 菌株液体和固体培养第14天和第28天提取物薄层层析分析及抗氧化与乙酰胆碱酯酶抑制活性自显影结果 A1-A6:菌株提取物254 nm紫外图像; B1-B6:菌株提取物365 nm荧光图像;C1-C6:菌株提取物DPPH自由基清除活性自显影图像;D1-D6:菌株提取物AChE抑制活性自显影图像;E1-E6:菌株提取物茴香醛-浓硫酸显色图像;F1-F6:菌株提取物三氯化铁-铁氰化钾显色图像。图片下的数字表示盐度(单位:g/L),下同
Fig. 1 Thin layer chromatography analysis of extracts from the liquid culture and solid culture of the strain on the day 14 and 28 and bioautography images of anti-oxidative and acetylcholinesterase's inhibitory activity A1-A6: 254 nm UV images of strain extract; B1-B6: 365 nm fluorescence images of strain extract; C1-C6: bioautography images of DPPH radical scavenging activity; D1-D6: bioautography images of strain extract AChE inhibitory activity; E1-E6: colorized images of strain extract anisaldehyde-sulfuric acid; F1-F6: colorized images strain extract iron trichloride-potassium ferrocyanide. The number under the images indicate different salinities(unit: g/L). The same below
| 培养类型Culture type | 培养时间Culture time/d | 提取物 Extracts | 不同手段展示的主要差异斑点(比移值)及其对应盐度 The main different spots(shown by Rf values)displayed by different approaches and the corresponding salinities | |||||
|---|---|---|---|---|---|---|---|---|
| 254 nm紫外光 254 nm ultraviolet | 365 nm紫外光 365 nm ultraviolet | 抗氧化活性自显影 Antioxidant bioautography | AChE抑制活性自显影 AChE inhibitory bioautography | 茴香醛显色 Anisaldehyde coloration | 铁氰化钾-三氯化铁显色Potassium ferricyanide-FeCl3 coloration | |||
| 液体培养Liquid culture | 14 | 菌丝体提取物 Mycelial extract | — | — | — | Rf(0.6, 0.8): 10, 15 g/L | Rf(0.2, 1.0): 2, 5, 10 g/L | — |
| 发酵液提取物 Broth extract | — | Rf(0.6, 1.0): 5 g/L | Rf(0.4, 0.8): 0.5, 2, 5 g/L | Rf(0.8, 1.0): 0, 0.5, 2, 5 g/L | — | — | ||
| 28 | 菌丝体提取物 Mycelial extract | — | — | — | — | Rf(0.0, 1.0): 5 g/L | — | |
| 发酵液提取物 Broth extract | Rf(0.8, 1.0): 0, 0.5 g/L | — | Rf(0.5): 0, 0.5 g/L | — | — | — | ||
| 固体培养Solid culture | 14 | 总提取物 Total extract | Rf(0.4, 1.0): 5, 10 g/L | — | Rf(0.4, 1.0): 5, 10 g/L | Rf(0.4, 0.6): 0, 0.5, 2 g/L Rf(0.6, 0.8): 5, 10 g/L | Rf(0.0, 1.0): 5 g/L | Rf(0.4, 1.0): 5, 10 g/L |
| 28 | 总提取物 Total extract | Rf(0.6, 1.0): 5, 10 g/L | — | Rf(0.2, 0.5): 5, 10 g/L | Rf(0.8, 1.0): 5 g/L | Rf(0.0, 1.0): 5 g/L | Rf(0.6, 0.8): 5, 10 g/L Rf(0.2, 0.4): 5 g/L | |
表1 菌株不同组别提取物薄层层析分析及抗氧化与乙酰胆碱酯酶抑制活性自显影中的主要差异斑点及其盐度条件
Table 1 Main different spots displayed in the thin layer chromatography analysis and anti-oxidative and acetylcholinesterase inhibitory bioautographies of different groups of fungal extracts and corresponding salinity conditions
| 培养类型Culture type | 培养时间Culture time/d | 提取物 Extracts | 不同手段展示的主要差异斑点(比移值)及其对应盐度 The main different spots(shown by Rf values)displayed by different approaches and the corresponding salinities | |||||
|---|---|---|---|---|---|---|---|---|
| 254 nm紫外光 254 nm ultraviolet | 365 nm紫外光 365 nm ultraviolet | 抗氧化活性自显影 Antioxidant bioautography | AChE抑制活性自显影 AChE inhibitory bioautography | 茴香醛显色 Anisaldehyde coloration | 铁氰化钾-三氯化铁显色Potassium ferricyanide-FeCl3 coloration | |||
| 液体培养Liquid culture | 14 | 菌丝体提取物 Mycelial extract | — | — | — | Rf(0.6, 0.8): 10, 15 g/L | Rf(0.2, 1.0): 2, 5, 10 g/L | — |
| 发酵液提取物 Broth extract | — | Rf(0.6, 1.0): 5 g/L | Rf(0.4, 0.8): 0.5, 2, 5 g/L | Rf(0.8, 1.0): 0, 0.5, 2, 5 g/L | — | — | ||
| 28 | 菌丝体提取物 Mycelial extract | — | — | — | — | Rf(0.0, 1.0): 5 g/L | — | |
| 发酵液提取物 Broth extract | Rf(0.8, 1.0): 0, 0.5 g/L | — | Rf(0.5): 0, 0.5 g/L | — | — | — | ||
| 固体培养Solid culture | 14 | 总提取物 Total extract | Rf(0.4, 1.0): 5, 10 g/L | — | Rf(0.4, 1.0): 5, 10 g/L | Rf(0.4, 0.6): 0, 0.5, 2 g/L Rf(0.6, 0.8): 5, 10 g/L | Rf(0.0, 1.0): 5 g/L | Rf(0.4, 1.0): 5, 10 g/L |
| 28 | 总提取物 Total extract | Rf(0.6, 1.0): 5, 10 g/L | — | Rf(0.2, 0.5): 5, 10 g/L | Rf(0.8, 1.0): 5 g/L | Rf(0.0, 1.0): 5 g/L | Rf(0.6, 0.8): 5, 10 g/L Rf(0.2, 0.4): 5 g/L | |
| 培养方式及时间 Type and time of culture | 盐度Salinity/(g·L-1) | 对不同指示菌的抗菌活性强度 Antimicrobial intensity to different indicator strains | ||||
|---|---|---|---|---|---|---|
| BS | SA | EC | PA | CA | ||
| 液体培养14 d Liquid culture for 14 d | 0 | - | - | - | - | - |
| 0.5 | - | - | - | - | - | |
| 2 | ++ | - | - | + | + | |
| 5 | +++ | - | - | ++ | - | |
| 10 | +++ | - | - | + | - | |
| 15 | - | - | - | - | - | |
| 20 | - | - | - | - | - | |
| 35 | - | - | - | - | - | |
| 液体培养28 d Liquid culture for 28 d | 0 | - | - | - | - | - |
| 0.5 | - | - | - | - | - | |
| 2 | +++ | - | - | + | - | |
| 5 | +++ | - | - | ++ | - | |
| 10 | +++ | - | - | +++ | - | |
| 15 | - | - | - | - | - | |
| 20 | - | - | + | - | - | |
| 35 | - | - | + | - | - | |
| 固体培养14 d Solid culture for 14 d | 0 | +++ | - | - | ++ | ++ |
| 0.5 | +++ | - | - | ++ | # | |
| 2 | - | - | - | ++ | # | |
| 5 | - | - | - | - | + | |
| 10 | - | - | - | - | + | |
| 15 | - | - | - | - | +++ | |
| 20 | - | - | - | - | +++ | |
| 35 | +++ | - | - | ++ | ++ | |
| 固体培养28 d Solid culture for 28 d | 0 | +++ | - | - | - | +++ |
| 0.5 | ++ | - | + | + | +++ | |
| 2 | +++ | - | +++ | +++ | ++ | |
| 5 | ++ | - | ++ | ++ | # | |
| 10 | +++ | - | - | - | # | |
| 15 | - | - | - | - | # | |
| 20 | - | - | - | - | # | |
| 35 | - | - | - | - | +++ | |
表2 不同盐度培养菌株提取物抗菌活性初筛结果
Table 2 Preliminary antimicrobial screening results of the extracts of the strain cultured under different salinities
| 培养方式及时间 Type and time of culture | 盐度Salinity/(g·L-1) | 对不同指示菌的抗菌活性强度 Antimicrobial intensity to different indicator strains | ||||
|---|---|---|---|---|---|---|
| BS | SA | EC | PA | CA | ||
| 液体培养14 d Liquid culture for 14 d | 0 | - | - | - | - | - |
| 0.5 | - | - | - | - | - | |
| 2 | ++ | - | - | + | + | |
| 5 | +++ | - | - | ++ | - | |
| 10 | +++ | - | - | + | - | |
| 15 | - | - | - | - | - | |
| 20 | - | - | - | - | - | |
| 35 | - | - | - | - | - | |
| 液体培养28 d Liquid culture for 28 d | 0 | - | - | - | - | - |
| 0.5 | - | - | - | - | - | |
| 2 | +++ | - | - | + | - | |
| 5 | +++ | - | - | ++ | - | |
| 10 | +++ | - | - | +++ | - | |
| 15 | - | - | - | - | - | |
| 20 | - | - | + | - | - | |
| 35 | - | - | + | - | - | |
| 固体培养14 d Solid culture for 14 d | 0 | +++ | - | - | ++ | ++ |
| 0.5 | +++ | - | - | ++ | # | |
| 2 | - | - | - | ++ | # | |
| 5 | - | - | - | - | + | |
| 10 | - | - | - | - | + | |
| 15 | - | - | - | - | +++ | |
| 20 | - | - | - | - | +++ | |
| 35 | +++ | - | - | ++ | ++ | |
| 固体培养28 d Solid culture for 28 d | 0 | +++ | - | - | - | +++ |
| 0.5 | ++ | - | + | + | +++ | |
| 2 | +++ | - | +++ | +++ | ++ | |
| 5 | ++ | - | ++ | ++ | # | |
| 10 | +++ | - | - | - | # | |
| 15 | - | - | - | - | # | |
| 20 | - | - | - | - | # | |
| 35 | - | - | - | - | +++ | |

图3 菌株不同培养条件提取物抗菌活性层析自显影复筛结果 A1-A4:第14天液体培养提取物、第28天液体培养提取物、第14天固体培养提取物、第28天固体培养提取物抑制铜绿假单胞菌活性层析自显影结果;B1-B2:第28天液体培养提取物、第28天固体培养提取物抑制大肠杆菌活性层析自显影结果。C1-C4:第14天液体培养提取物、第28天液体培养提取物、第14天固体培养提取物、第28天固体培养提取物抑制枯草芽孢杆菌活性层析自显影结果。D1-D3:第14天液体培养提取物、第14天固体培养提取物、第28天固体培养提取物抑制白色假丝酵母活性层析自显影结果
Fig. 3 Bioautography re-screening results of the anti-microbial activities of the strain extracts via thin layer chromatography analysis A1-A4: The anti-Bacillus subtilis bioautography results of culture extracts of 14 d liquid culture, 28 d liquid culture, 14 d solid culture, and 28 d solid culture; B1-B2: the anti-Eschrichia coli bioautography results of culture extracts of 28 d liquid culture and 28 d solid culture; C1-C4: the anti-Pseudomonas aeruginosa bioautography results of culture extracts of 14 d liquid culture, 28 d liquid culture, 14 d solid culture, and 28 d solid culture; D1-D3: the anti-Candida albicans bioautography results of culture extracts of 14 d liquid culture, 14 d solid culture, and 28 d solid culture

图4 菌株不同盐度培养提取物的高效液相色谱分析(二极管阵列检测器检测的全波长等高线图)
Fig. 4 High performance liquid chromatography analysis of the strain extracts from different salinity cultures(The results are the full wavelength contour chromatograms detected by the diode array detector)

图5 菌株不同盐度液体和固体培养第28天提取物的液相色谱联用质谱分析
Fig. 5 Liquid chromatography-tandem mass spectrometry analysis of the strain extracts from different salinity liquid and solid cultures on day 28
| 培养类型Culture type | 盐度 Salinity /(g·L-1) | 代谢物编号 No.of metabolite | 离子质荷比Mass-to-charge ratio of the precursor ion(m/z) | 缀合离子形式Form of adduct ions | 分子式 Molecular formula | 化合物名称 Compound name | 已有报道真菌来源 Previously reported fungal origin | 真菌来源文献 Literature for fungal origin |
|---|---|---|---|---|---|---|---|---|
| 液体培养基 Liquid culture | 0.5 | 化合物1 Compound 1 | 417.1435 | [M+Na]+ | C21H27ClO5 | Isochromophilones IV | Penicillium multicolor | [ |
| 2 | 化合物2 Compound 2 | 477.3148 | [M+H-H2O]+ | C28H46O7 | 20-Hydroxyecdysone | Tapinella panuoides | [ | |
| 固体培养基 Solid culture | 5 | 化合物3 Compound 3 | 304.1362 | [M+H]+ | C13H21NO7 | 麦角菌素类 Mycosporin | Gnomonia leptostyla | [ |
| 化合物4 Compound 4 | 152.0088 | [M+H]+ | C7H5NOS | 2-苯并噻唑酮 2- Benzothiazolone | Dipodascus sp. | [ | ||
| 化合物5 Compound 5 | 233.1478 | [M+H-H2O]+ | C15H22O3 | 倍半萜类 Sesquiterpenoid | Drechslera gigantea | [ | ||
| 化合物6 Compound 6 | 169.0424 | [M+H]+ | C8H8O4 | 苔色酸 Orsellinic acid | Aspergillus sp. | [ | ||
| 化合物7 Compound 7 | 237.1435 | [M+H]+ | C14H22O4 | Gliocladic acid | Trichoderma virens | [ | ||
| 10 | 化合物8 Compound 8 | 277.0707 | [M+H]+ | C14H12O6 | Talaroflavone | Talaromyces flavus et al | [ | |
| 35 | 化合物9 Compound 9 | 214.0542 | [M+H]+ | C6H13N3O3 | 瓜氨酸 Citrulline | Schizosaccharomyces pombe | [ | |
| 化合物10 Compound 10 | 327.1181 | [M+H]+ | C19H18O5 | Unguinol | Aspergillus unguis | [ | ||
| 化合物11 Compound 11 | 380.1190 | [M+H]+ | C13H21N3O8S | S-D-乳酰谷胱甘肽 S-Lactoylglutathione | Saccharomyces cerevisiae | [ |
表3 液体和固体培养28天提取物中的盐度特征代谢产物的详细注释
Table 3 Detailed annotation of the salinity featured metabolites in the day 28 extracts of the liquid and solid medium cultures
| 培养类型Culture type | 盐度 Salinity /(g·L-1) | 代谢物编号 No.of metabolite | 离子质荷比Mass-to-charge ratio of the precursor ion(m/z) | 缀合离子形式Form of adduct ions | 分子式 Molecular formula | 化合物名称 Compound name | 已有报道真菌来源 Previously reported fungal origin | 真菌来源文献 Literature for fungal origin |
|---|---|---|---|---|---|---|---|---|
| 液体培养基 Liquid culture | 0.5 | 化合物1 Compound 1 | 417.1435 | [M+Na]+ | C21H27ClO5 | Isochromophilones IV | Penicillium multicolor | [ |
| 2 | 化合物2 Compound 2 | 477.3148 | [M+H-H2O]+ | C28H46O7 | 20-Hydroxyecdysone | Tapinella panuoides | [ | |
| 固体培养基 Solid culture | 5 | 化合物3 Compound 3 | 304.1362 | [M+H]+ | C13H21NO7 | 麦角菌素类 Mycosporin | Gnomonia leptostyla | [ |
| 化合物4 Compound 4 | 152.0088 | [M+H]+ | C7H5NOS | 2-苯并噻唑酮 2- Benzothiazolone | Dipodascus sp. | [ | ||
| 化合物5 Compound 5 | 233.1478 | [M+H-H2O]+ | C15H22O3 | 倍半萜类 Sesquiterpenoid | Drechslera gigantea | [ | ||
| 化合物6 Compound 6 | 169.0424 | [M+H]+ | C8H8O4 | 苔色酸 Orsellinic acid | Aspergillus sp. | [ | ||
| 化合物7 Compound 7 | 237.1435 | [M+H]+ | C14H22O4 | Gliocladic acid | Trichoderma virens | [ | ||
| 10 | 化合物8 Compound 8 | 277.0707 | [M+H]+ | C14H12O6 | Talaroflavone | Talaromyces flavus et al | [ | |
| 35 | 化合物9 Compound 9 | 214.0542 | [M+H]+ | C6H13N3O3 | 瓜氨酸 Citrulline | Schizosaccharomyces pombe | [ | |
| 化合物10 Compound 10 | 327.1181 | [M+H]+ | C19H18O5 | Unguinol | Aspergillus unguis | [ | ||
| 化合物11 Compound 11 | 380.1190 | [M+H]+ | C13H21N3O8S | S-D-乳酰谷胱甘肽 S-Lactoylglutathione | Saccharomyces cerevisiae | [ |
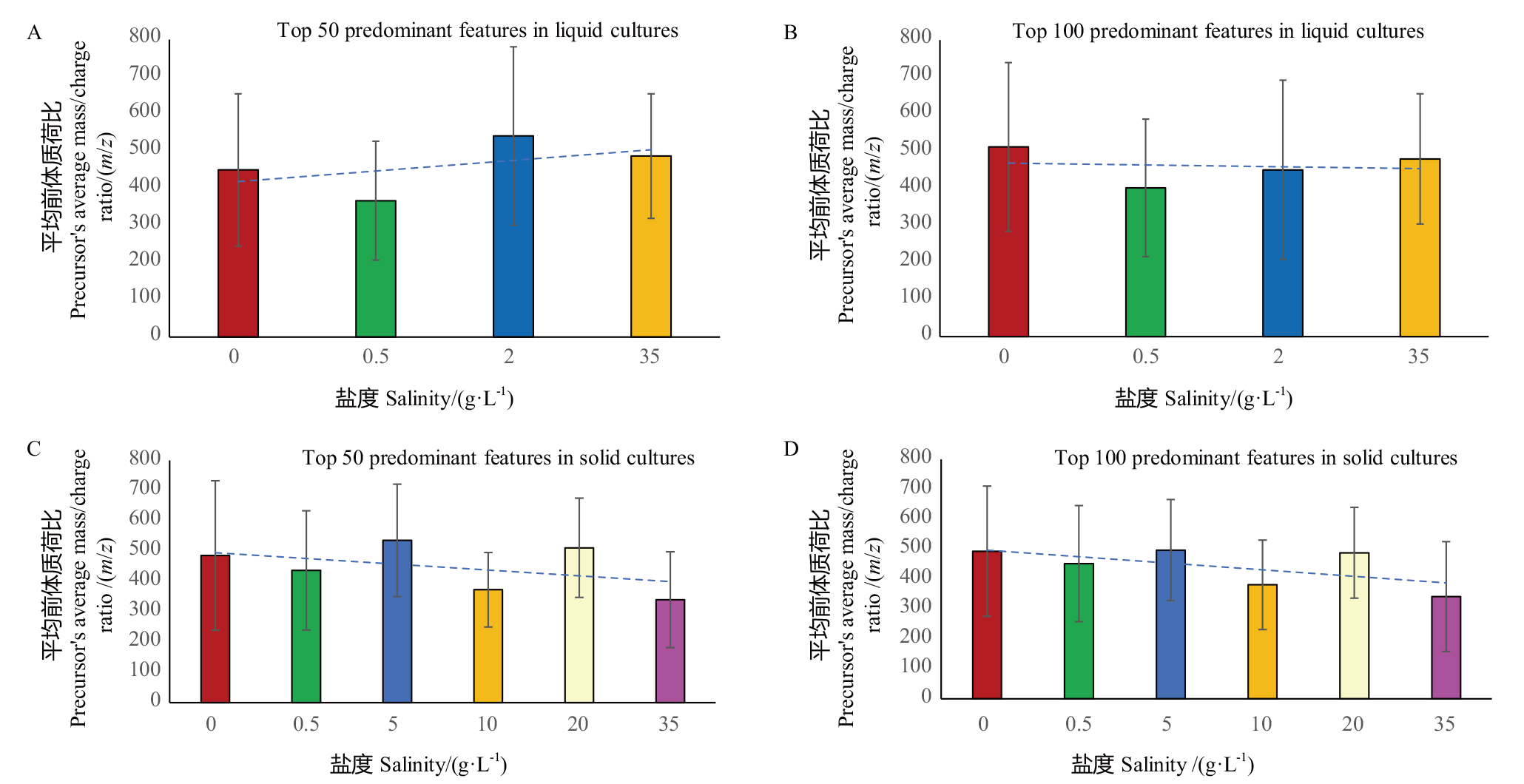
图8 菌株液体和固体培养不同盐度下优势代谢产物的前体离子质荷比分布
Fig. 8 Distribution of precursor m/z values of the predominant metabolites for the liquid and solid cultures of the strain under different salinities
| [1] |
马丽丽, 田新朋, 李桂菊, 等. 海洋微生物来源天然产物研究现状与态势[J]. 热带海洋学报, 2021, 40(5): 134-146.
doi: 10.11978/2020104 |
| Ma LL, Tian XP, Li GJ, et al. Research status and development trends of natural products from marine microorganisms[J]. J Trop Oceanogr, 2021, 40(5): 134-146. | |
| [2] | 付逸群, 于颖敏, 马瑞遥, 等. 海洋来源真菌生物活性物质研究进展[J]. 山东化工, 2019, 48(22): 63-65, 67. |
| Fu YQ, Yu YM, Ma RY, et al. Advances in research on marine fungi bioactive substances[J]. Shandong Chem Ind, 2019, 48(22): 63-65, 67. | |
| [3] | 陈宁, 喻圣凯, 刘冰, 等. 海洋真菌次级代谢产物及其活性研究进展[J]. 中国公共卫生管理, 2019, 35(1): 44-47. |
| Chen N, Yu SK, Liu B, et al. Advances in research on secondary metabolites and activities of marine fungi[J]. Chin J Public Health Manag, 2019, 35(1): 44-47. | |
| [4] | 胡靖瑶, 袁瑞瑛, 王广明, 等. 海洋真菌Aspergillus jensenii SS5中化学成分研究[J]. 天然产物研究与开发, 2023, 35(11): 1902-1906. |
| Hu JY, Yuan RY, Wang GM, et al. Chemical constituents of marine fungus Aspergillus jensenii SS5[J]. Nat Prod Res Dev, 2023, 35(11): 1902-1906. | |
| [5] | Yang WC, Bao HY, Liu YY, et al. Depsidone derivatives and a cyclopeptide produced by marine fungus Aspergillus unguis under chemical induction and by its plasma induced mutant[J]. Molecules, 2018, 23(9): 2245. |
| [6] | Wang Y, Glukhov E, He YF, et al. Secondary metabolite variation and bioactivities of two marine Aspergillus strains in static co-culture investigated by molecular network analysis and multiple database mining based on LC-PDA-MS/MS[J]. Antibiotics, 2022, 11(4): 513. |
| [7] |
马小翔, 刘亚月, 聂影影, 等. 基于质谱的分子网络分析化学调控对土曲霉C23-3次生代谢产物及生物活性的影响[J]. 生物技术通报, 2021, 37(8): 95-110.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-1398 |
| Ma XX, Liu YY, Nie YY, et al. LC-MS/MS based molecular network analysis of the effects of chemical regulation on the secondary metabolites and biological activities of a fungal strain Aspergillus terreus C23-3[J]. Biotechnol Bull, 2021, 37(8): 95-110. | |
| [8] |
Wang Y, Lu ZY, Sun KL, et al. Effects of high salt stress on secondary metabolite production in the marine-derived fungus Spicaria elegans[J]. Mar Drugs, 2011, 9(4): 535-542.
doi: 10.3390/md9040535 pmid: 21731548 |
| [9] |
Wang Y, Zheng JK, Liu PP, et al. Three new compounds from Aspergillus terreus PT06-2 grown in a high salt medium[J]. Mar Drugs, 2011, 9(8): 1368-1378.
doi: 10.3390/md9081368 pmid: 21892351 |
| [10] | 王治维, 窦莹颖, 祝兴伟, 等. 盐度和pH对红树根际土壤来源的曲霉属真菌F3的生长及分泌活性物质的影响[J]. 微生物学通报, 2008, 35(12): 1873-1878. |
| Wang ZW, Dou YY, Zhu XW, et al. Effects of salinity and pH on the growth and active products-secreting of Aspergillus sp. F3 from the mangrove rhizosphere[J]. Microbiology, 2008, 35(12): 1873-1878. | |
| [11] | Overy D, Correa H, Roullier C, et al. Does osmotic stress affect natural product expression in fungi?[J]. Mar Drugs, 2017, 15(8): 254. |
| [12] | 吴振龙, 王英, 叶文才. 天然生物活性分子高效发现的新策略和方法研究进展[J]. 药学进展, 2022, 46(3): 163-172. |
| Wu ZL, Wang Y, Ye WC. Advances in research on novel strategies and approaches for the efficient discovery of bioactive natural molecules[J]. Prog Pharm Sci, 2022, 46(3): 163-172. | |
| [13] | 覃舒然, 刘海翠, 李大山, 等. 质谱分子网络在天然产物结构研究中的应用[J]. 天然产物研究与开发, 2022, 34(11): 1978-1987. |
|
Qin SR, Liu HC, Li DS, et al. Application of mass spectrometry molecular networking in the study of natural product structure[J]. Nat Prod Res Dev, 2022, 34(11): 1978-1987.
doi: 10.16333/j.1001-6880.2022.11.019 |
|
| [14] | Nothias LF, Petras D, Schmid R, et al. Feature-based molecular networking in the GNPS analysis environment[J]. Nat Methods, 2020, 17(9): 905-908. |
| [15] | Lu TT, Liu YY, Zhou LJ, et al. The screening for marine fungal strains with high potential in alkaloids production by in situ colony assay and LC-MS/MS based secondary metabolic profiling[J]. Front Microbiol, 2023, 14: 1144328. |
| [16] |
Zhang Y, Mu J, Feng Y, et al. Four chlorinated depsidones from a seaweed-derived strain of Aspergillus unguis and their new biological activities[J]. Nat Prod Res, 2014, 28(7): 503-506.
doi: 10.1080/14786419.2013.879305 pmid: 24479775 |
| [17] | 张翼, 鲍海燕, 冯妍, 等. 一种海洋真菌爪曲霉溴代缩酚环酸醚类化合物及其制备方法和应用: CN106632230B[P]. 2019-09-10. |
| Zhang Y, Bao H, Feng Y, et al. A marine fungal brominated depsidone-type compound and its preparation method and application: CN106632230B[P]. 2019-09-10. | |
| [18] | 张翼, 杨文聪, 鲍海燕, 等. 一种缩酚酸环醚类化合物及其制备方法和应用: CN108640841B[P]. 2021-04-02. |
| Zhang Y, Yang W, Bao H, et al. A depsidone-type compound and its preparation method and application: CN108640841B[P]. 2021-04-02. | |
| [19] | 张翼, 杨文聪, 鲍海燕, 等. 一种缩酚酸环醚类化合物的应用: CN108925565B[P]. 2021-05-07. |
| Zhang Y, Yang W, Bao H, et al. The application of a depsidone-type compound: CN108925565B[P]. 2021-05-07. | |
| [20] | 张翼, 杨文聪, 聂影影, 等. 化合物Aspergillusidone G在制备神经保护药物中的应用: CN110604731B[P]. 2023-03-17. |
| Zhang Y, Yang W, Nie Y, et al. The application of the compound Aspergillusidone G in the preparation of neuroprotective drugs: CN110604731B[P]. 2023-03-17. | |
| [21] |
Arai N, Shiomi K, Tomoda H, et al. Isochromophilones III-VI, inhibitors of acyl-CoA: cholesterol acyltransferase produced by Penicillium multicolor FO-3216[J]. J Antibiot, 1995, 48(7): 696-702.
pmid: 7649870 |
| [22] | Vokáč K, Buděšńský M, Harmatha J, et al. Ecdysteroid constituents of the mushroom Tapinella panuoides[J]. Phytochemistry, 1998, 49: 2109-2114. |
| [23] | Fayret J, Bernillon J, Bouillant ML, et al. Open and ring forms of mycosporin-2 from the ascomycete Gnomonia leptostyla[J]. Phytochemistry, 1981, 20(12): 2709-2710. |
| [24] | Adriano R, Mara S, Jakub G, et al. LOTUS: Natural Products Online for 2-benzothiazolone[EB/OL].(2022-02-27). [2023-12-24]. https://lotus.naturalproducts.net/compound/lotus_id/LTS0063626. |
| [25] | Sugawara F, Hallock YF, Bunkers GD, et al. Phytoactive eremophilanes produced by the weed pathogen Drechslera gigantea[J]. Biosci Biotechnol Biochem, 1993, 57(2): 236-239. |
| [26] |
Packter NM. Studies on the biosynthesis of phenols in fungi. Conversion of[14C]orsellinic acid and[14C]orcinol into fumigatol by Aspergillus fumigatus I.M.I. 89353[J]. Biochem J, 1966, 98(2): 353-359.
pmid: 5296209 |
| [27] | Chen HQ, Daletos G, Abdel-Aziz MS, et al. Inducing secondary metabolite production by the soil-dwelling fungus Aspergillus terreus through bacterial co-culture[J]. Phytochem Lett, 2015, 12: 35-41. |
| [28] |
Itoh Y, Takahashi S, Arai M. Structure of gliocladic acid[J]. J Antibiot, 1982, 35(4): 541-542.
pmid: 7096211 |
| [29] | Ayer WA, Racok JS. The metabolites of Talaromycesflavus: part 2. Biological activity and biosynthetic studies[J]. Can J Chem, 1990, 68(11): 2095-2101. |
| [30] | Aly AH, Ebel R, Edrada RA, et al. Protein kinase inhibitors from the endophytic fungus Alternaria sp. isolated from Polygonum senegalense growing in Egypt[J]. Planta Med, 2009, 75(9): PE55. |
| [31] |
Chaleckis R, Ebe M, Pluskal T, et al. Unexpected similarities between the Schizosaccharomyces and human blood metabolomes, and novel human metabolites[J]. Mol Biosyst, 2014, 10(10): 2538-2551.
doi: 10.1039/c4mb00346b pmid: 25010571 |
| [32] | Stodola FH, Vesonder RF, Fennell DI, et al. A new depsidone from Aspergillus unguis[J]. Phytochemistry, 1972, 11(6): 2107-2108. |
| [33] | Xia JY, Sánchez BJ, Chen Y, et al. Proteome allocations change linearly with the specific growth rate of Saccharomyces cerevisiae under glucose limitation[J]. Nat Commun, 2022, 13(1): 2819. |
| [34] | National Center for Biotechnology Information. PubChem compound summary for CID 14543486[DB].(2007-02-09). [2023-12-24].. |
| [35] | National Center for Biotechnology Information. PubChem compound summary for CID 68072[DB].(2004-09-16). [2023-12-24].. |
| [36] | National Center for Biotechnology Information. PubChem compound summary for CID 26495249[DB].(2009-05-28). [2023-12-24].. |
| [37] | Huang JJ, Lu CH, Qian XM, et al. Effect of salinity on the growth, biological activity and secondary metabolites of some marine fungi[J]. Acta Oceanol Sin, 2011, 30(3): 118-123. |
| [38] | Li CJ, Zhao D, Yan JY, et al. Metabolomics integrated with transcriptomics: assessing the central metabolism of marine red yeast Sporobolomyces pararoseus under salinity stress[J]. Arch Microbiol, 2021, 203(3): 889-899. |
| [39] | Jiménez-Gómez I, Valdés-Muñoz G, Moreno-Ulloa A, et al. Surviving in the brine: a multi-omics approach for understanding the physiology of the halophile fungus Aspergillus sydowii at saturated NaCl concentration[J]. Front Microbiol, 2022, 13: 840408. |
| [40] | He B, Ma L, Hu ZH, et al. Deep sequencing analysis of transcriptomes in Aspergillus oryzae in response to salinity stress[J]. Appl Microbiol Biotechnol, 2018, 102(2): 897-906. |
| [41] | Jones EBG, Ramakrishna S, Vikineswary S, et al. How do fungi survive in the sea and respond to climate change?[J]. J Fungi, 2022, 8(3): 291. |
| [42] | Velez P, Alejandri-Ramírez ND, González MC, et al. Comparative transcriptome analysis of the cosmopolitan marine fungus Corollospora maritima under two physiological conditions[J]. G3, 2015, 5(9): 1805-1814. |
| [43] |
廖清楠, 周龙建, 杨志友, 等. 石珊瑚共附生真菌次级代谢产物的抗炎活性及化学多样性研究[J]. 生物技术通报, 2023, 39(12): 261-275.
doi: 10.13560/j.cnki.biotech.bull.1985.2023-0501 |
| Liao QN, Zhou LJ, Yang ZY, et al. Studies on anti-inflammatory activity and chemical diversity of secondary metabolites from symbiotic fungi in stony corals[J]. Biotechnol Bull, 2023, 39(12): 261-275. | |
| [44] | 李祥荣, 郑洪利, 张宗艺, 等. 抗副溶血弧菌海洋真菌HL-3菌株的鉴定及其活性物质的分离[J]. 微生物学通报, 2022, 49(6): 1999-2008. |
| Li XR, Zheng HL, Zhang ZY, et al. Identification of marine fungus HL-3 with activity against Vibrio parahaemolyticus and separation of its active substances[J]. Microbiol China, 2022, 49(6): 1999-2008. |
| [1] | 马小翔, 马泽源, 刘亚月, 周龙建, 和羿帆, 张翼. 仿突变生物合成调控对土曲霉C23-3次生代谢产物的影响[J]. 生物技术通报, 2024, 40(8): 275-287. |
| [2] | 蔡楠, 方静平, 陈必链, 何勇锦. 高值化等鞭金藻固碳研究进展[J]. 生物技术通报, 2024, 40(6): 68-80. |
| [3] | 苑海鹏, 叶云舒, 司皓, 纪秋研, 张玉红. 丛枝菌根真菌对植物逆境胁迫抗性及次生代谢产物合成的影响[J]. 生物技术通报, 2024, 40(6): 45-56. |
| [4] | 王楠, 廖永琴, 施竹凤, 申云鑫, 杨童雨, 冯路遥, 矣小鹏, 唐加菜, 陈齐斌, 杨佩文. 三株无量山森林土壤芽孢杆菌鉴定及其生物活性挖掘[J]. 生物技术通报, 2024, 40(2): 277-288. |
| [5] | 赵睿萌, 王梦雨, 吕国英, 宋婷婷, 张作法. 药用真菌桑黄中多酚类成分药用机理研究进展[J]. 生物技术通报, 2024, 40(11): 3-13. |
| [6] | 游子娟, 陈汉林, 邓辅财. 鱼皮生物活性肽的提取及功能活性研究进展[J]. 生物技术通报, 2023, 39(7): 91-104. |
| [7] | 汪巧菊, 胡雨萌, 温亚亚, 宋丽, 孟闯, 潘志明, 焦新安. 新型冠状病毒S1蛋白的表达及活性鉴定[J]. 生物技术通报, 2022, 38(3): 157-163. |
| [8] | 王楠, 苏誉, 刘文杰, 封明, 毛瑜, 张新国. 植物内生菌中抗耐药微生物活性成分的研究进展[J]. 生物技术通报, 2021, 37(8): 263-274. |
| [9] | 梁振霆, 唐婷. 内生菌对植物次生代谢产物的生物合成影响和抗逆功能研究[J]. 生物技术通报, 2021, 37(8): 35-45. |
| [10] | 马小翔, 刘亚月, 聂影影, 黎燕媚, 王远, 薛欣怡, 洪鹏志, 张翼. 基于质谱的分子网络分析化学调控对土曲霉C23-3次生代谢产物及生物活性的影响[J]. 生物技术通报, 2021, 37(8): 95-110. |
| [11] | 刘珊, 叶伟, 朱牧孜, 李赛妮, 邓张双, 章卫民. 一种新型酰基转移酶GPAT的克隆、表达与酶学性质研究[J]. 生物技术通报, 2021, 37(11): 257-266. |
| [12] | 薛帆正, 黄海辰, 吴福泉, 李晓敏, 吴小平, 傅俊生. 真菌黑色素研究现状与产业应用[J]. 生物技术通报, 2021, 37(11): 32-41. |
| [13] | 徐重新, 张存政, 刘媛, 张霄, 仲建锋, 刘贤金. 食源性致病微生物危害风险及其防控用抗菌生物活性肽研究进展[J]. 生物技术通报, 2019, 35(7): 202-212. |
| [14] | 赵祥杰, 杨文君, 杨荣玲, 吴婷婷, 王朝宇, 许宁宁, 何佳美. 花色苷生物转化修饰的研究进展[J]. 生物技术通报, 2019, 35(10): 205-211. |
| [15] | 黄自磊, 章卫民, 叶伟, 李赛妮, 李浩华, 朱牧孜. 深海真菌Dichotomomyces cejpii胶霉毒素生物合成基因启动子的克隆和功能鉴定[J]. 生物技术通报, 2018, 34(4): 144-150. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||