








生物技术通报 ›› 2025, Vol. 41 ›› Issue (3): 44-50.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0915
韩梦荞1( ), 吴疆2, 李丽华3, 王昭懿4, 邓习1, 韦凤杰5, 任民1, 孙洋洋1(
), 吴疆2, 李丽华3, 王昭懿4, 邓习1, 韦凤杰5, 任民1, 孙洋洋1( ), 李富欣5(
), 李富欣5( )
)
收稿日期:2024-09-20
出版日期:2025-03-26
发布日期:2025-03-20
通讯作者:
李富欣,男,博士,高级农艺师,研究方向 :烟叶生产管理;E-mail: lfx4413@163.com作者简介:韩梦荞,女,硕士研究生,研究方向 :作物科学;E-mail: hanmengqiao0502@163.com基金资助:
HAN Meng-qiao1( ), WU Jiang2, LI Li-hua3, WANG Zhao-yi4, DENG Xi1, WEI Feng-jie5, REN Min1, SUN Yang-yang1(
), WU Jiang2, LI Li-hua3, WANG Zhao-yi4, DENG Xi1, WEI Feng-jie5, REN Min1, SUN Yang-yang1( ), LI Fu-xin5(
), LI Fu-xin5( )
)
Received:2024-09-20
Published:2025-03-26
Online:2025-03-20
摘要:
目的 开发一套适合烟草的染色体制备体系,为推进烟草染色体工程以及远缘杂交研究的系统开展奠定基础。 方法 以普通烟草为供试材料,利用酶解法进行染色体制备体系的构建。试验针对根尖组织8个时间段进行采样;利用4种方法进行预处理,包括对照(前期无任何处理)、20%风油精室温处理2 h、冰水混合物0℃处理48 h以及N2O处理,其中,N2O处理又分为20‒70 min每间隔10 min取一次样;根尖样品分别在35、45和55 min不同时间下进行酶解。对每种处理方法或时长均进行了染色体制备并观察,根据染色体长度、清晰度及分散程度鉴定烟草染色体制备最佳方法。整合优化后的处理方法和时长,形成烟草染色体制备方法,并通过制备不同类型烟草品种的染色体样品进行体系的适用性验证。 结果 10∶30‒12∶30为烟草根尖取样的理想时间,N2O预处理30‒40 min,酶解45 min较为适宜,并利用该优化体系应用于普通烟草祖先种、普通烟草、远缘杂交衍生后代3个不同种质资源,均获得高质量染色体样品。 结论 建立了适用于烟草染色体的制备体系。利用该体系可在3 h内完成从烟草根尖取样到制备出合格的染色体样品。
韩梦荞, 吴疆, 李丽华, 王昭懿, 邓习, 韦凤杰, 任民, 孙洋洋, 李富欣. 烟草染色体制备体系的建立与优化[J]. 生物技术通报, 2025, 41(3): 44-50.
HAN Meng-qiao, WU Jiang, LI Li-hua, WANG Zhao-yi, DENG Xi, WEI Feng-jie, REN Min, SUN Yang-yang, LI Fu-xin. Establishment and Optimization of a Tobacco Chromosome Preparation System[J]. Biotechnology Bulletin, 2025, 41(3): 44-50.
取样时间 Sampling time | 根尖细胞观察情况 Observations on the chromosomes of root apical cells |
|---|---|
| 8∶30‒9∶30 | 分裂中期细胞数量最少,约占1.2% Minimum number of cells in mid-division, about 1.2% |
| 9∶30‒10∶30 | 分裂中期细胞数量上升,约占18.8% The number of cells rises in mid-division, about 18.8% |
| 10∶30‒11∶30 | 分裂中期细胞数量最多,约占35.2% Maximum number of cells in mid-division, about 35.2% |
| 11∶30‒12∶30 | 分裂中期细胞数量下降,约占27.8% Decrease in cell number during mid-division, about 27.8% |
| 12∶30‒13∶30 | 分裂中期细胞数量较少,约占1.6% Smaller number of cells in mid-division, about 1.6% |
| 13∶30‒14∶30 | 分裂中期细胞数量较少,约占10.8% Smaller number of cells in mid-division, about 10.8% |
| 14∶30‒15∶30 | 分裂中期细胞数量较少,约占6.4% Smaller number of cells in mid-division, about 6.4% |
| 15∶30‒16∶30 | 分裂中期细胞数量上升,约占19.2% The number of cells rises in mid-division, about 19.2% |
表1 不同取样时间下根尖细胞观察情况
Table 1 Observations on root tip cells at different sampling times
取样时间 Sampling time | 根尖细胞观察情况 Observations on the chromosomes of root apical cells |
|---|---|
| 8∶30‒9∶30 | 分裂中期细胞数量最少,约占1.2% Minimum number of cells in mid-division, about 1.2% |
| 9∶30‒10∶30 | 分裂中期细胞数量上升,约占18.8% The number of cells rises in mid-division, about 18.8% |
| 10∶30‒11∶30 | 分裂中期细胞数量最多,约占35.2% Maximum number of cells in mid-division, about 35.2% |
| 11∶30‒12∶30 | 分裂中期细胞数量下降,约占27.8% Decrease in cell number during mid-division, about 27.8% |
| 12∶30‒13∶30 | 分裂中期细胞数量较少,约占1.6% Smaller number of cells in mid-division, about 1.6% |
| 13∶30‒14∶30 | 分裂中期细胞数量较少,约占10.8% Smaller number of cells in mid-division, about 10.8% |
| 14∶30‒15∶30 | 分裂中期细胞数量较少,约占6.4% Smaller number of cells in mid-division, about 6.4% |
| 15∶30‒16∶30 | 分裂中期细胞数量上升,约占19.2% The number of cells rises in mid-division, about 19.2% |

图1 不同处理方式制备的烟草根尖染色体形态A:对照;B:20%风油精处理2 h;C:冰水混合物0℃处理48 h;D:N2O处理70 min;E:N2O处理60 min;F:N2O处理50 min;G:N2O处理40 min;H:N2O处理30 min;I:N2O处理20 min。bars=50 μm
Fig. 1 Different chromosomes morphologies of root tip cells in different treatmentsA: CK; B: 20% essential balm treatment for 2 h; C: ice-water mixture 0℃ treatment for 48 h; D: N2O treatment for 70 min; E: N2O treatment for 60 min; F: N2O treatment for 50 min; G: N2O treatment for 40 min; H: N2O treatment for 30 min; I: N2O treatment for 20 min. Scale bar=50 μm
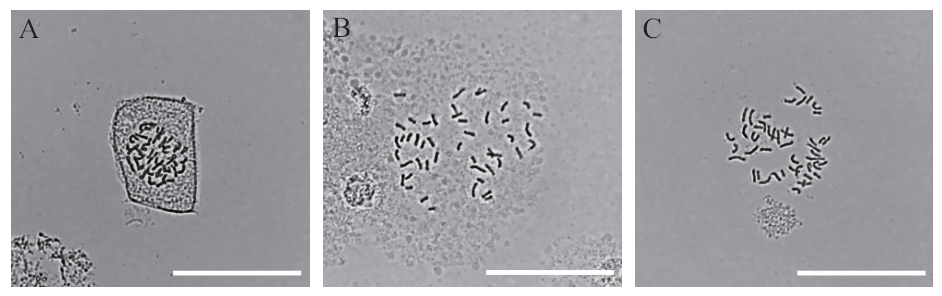
图2 不同酶解时间根尖细胞不同状态A:酶解35 min;B:酶解45 min;C:酶解55 min。bars=50 μm
Fig. 2 Different states of root tip cells at different enzymolysis timesA: Enzymolysis 35 min. B: Enzymolysis 45 min. C: Enzymolysis 55 min. Scale bar=50 μm

图4 酶解法制备的不同烟草种质资源染色体样品A:绒毛状烟草;B:中烟100;C:L8;D:绒毛状烟草DAPI图像;E:中烟100 DAPI图像;F:L8 DAPI图像(A‒C:bars=50 μm;D‒F:bars=5 μm)
Fig. 4 Chromosome samples from various tobacco germplasm resources prepared using an enzymatic digestion methodA: N. tomentosiformis; B: ZY100; C: L8; D: N. tomentosiformis DAPI image; E: ZY100 DAPI image; F: L8 DAPI image (A‒C: scale bar = 50 μm; D‒F: scale bar=5 μm)
| 1 | Bai D, Reeleder R, Brandie JE. Identification of two RAPD markers tightly linked with the Nicotiana debneyi gene for resistance to black root rot of tobacco [J]. Theor Appl Genet, 1995, 91(8): 1184-1189. |
| 2 | Valleau WD, Stokes GW, Johnson EM. Nine years' experience with the Nicotiana longiflora factor for resistance to Phytophthora parasitica var. nicotianae in the control of black shank [J]. Tobacco, 1960, 4: 92-94. |
| 3 | 陈晶鑫. 梅花染色体制片技术优化及基于荧光原位杂交的核型分析 [D]. 北京: 北京林业大学, 2013. |
| Chen JX. Optimization of plum blossom chromosome slicing technique and karyotype analysis based on fluorescence in situ hybridization [D]. Beijing: Beijing Forestry University, 2013. | |
| 4 | 钱双. 基于荧光原位杂交技术的野菊核型分析 [D]. 重庆: 西南大学, 2016. |
| Qian S. Karyotype analysis of Chrysanthemum indicum based on fluorescence in situ hybridization [D]. Chongqing: Southwest University, 2016. | |
| 5 | 杨亚飞, 刘沈徽, 黄东益, 等. 适于荧光原位杂交的大薯叶片染色体制片技术 [J]. 热带生物学报, 2020, 11(1): 100-104. |
| Yang YF, Liu SH, Huang DY, et al. Chromosome preparation of leaf cells of Dioscorea alata L. for FISH [J]. J Trop Biol, 2020, 11(1): 100-104. | |
| 6 | 杨垚, 张艳, 党江波, 等. 以子房为材料制备烟草染色体标本的方法 [J]. 中国烟草科学, 2019, 40(4): 56-61. |
| Yang Y, Zhang Y, Dang JB, et al. Study on Nicotiana chromosome specimen preparation using ovary [J]. Chin Tob Sci, 2019, 40(4): 56-61. | |
| 7 | Braz GT, Yu F, Zhao HN, et al. Preferential meiotic chromosome pairing among homologous chromosomes with cryptic sequence variation in tetraploid maize [J]. New Phytol, 2021, 229(6): 3294-3302. |
| 8 | McCaw M, Swyers N, Graham N, et al. Preparation of chromosomes from Zea mays [J]. Curr Protoc Plant Biol, 2016, 1(3): 501-509. |
| 9 | Wang K, Yu WC. Chromosome preparation in rice (Oryza sativa) [J]. Curr Protoc Plant Biol, 2016, 1(1): 67-77. |
| 10 | Schwarzacher T, Liu Q, Pat Heslop-Harrison JS. Plant cytogenetics: from chromosomes to cytogenomics [J]. Methods Mol Biol, 2023, 2672: 3-21. |
| 11 | Vrána J, Kubaláková M, Číhalíková J, et al. Preparation of sub-genomic fractions enriched for particular chromosomes in polyploid wheat [J]. Biol Plant, 2015, 59(3): 445-455. |
| 12 | 姚启伦, 李玉洁, 陈发波, 等. 玉米染色体滴片关键技术及其优化 [J]. 江苏农业学报, 2019, 35(2): 255-261. |
| Yao QL, Li YJ, Chen FB, et al. Key techniques and their selective preference of maize chromosomal dropping-slides [J]. Jiangsu J Agric Sci, 2019, 35(2): 255-261. | |
| 13 | 张小娟, 覃建兵. 小麦染色体标本制备方法的优化 [J]. 山西师范大学学报: 自然科学版, 2013, 27(2): 47-50. |
| Zhang XJ, Tan JB. The optimization of preparation method for wheat chromosome specimen [J]. J Shanxi Norm Univ Nat Sci Ed, 2013, 27(2): 47-50. | |
| 14 | 虢国成, 向洋. 大麦和玉米染色体去壁低渗法制片及其核型分析 [J]. 大麦与谷类科学, 2008, 25(2): 8-11. |
| Guo GC, Xiang Y. Method of chromosome flaking for barleyand & maize and its' karyotype analysis [J]. Barley Cereal Sci, 2008, 25(2): 8-11. | |
| 15 | 刘继琳, 刘彩霞, 郭佳静, 等. 甜玉米与糯玉米的染色体核型分析 [J]. 华南师范大学学报: 自然科学版, 2014, 46(6): 98-103. |
| Liu JL, Liu CX, Guojia J, et al. Chromosomal karyotype analysis of sweet corn and waxy corn [J]. J South China Norm Univ Nat Sci Ed, 2014, 46(6): 98-103. | |
| 16 | 丁鸿, 邱东萍, 陈少雄. 植物染色体标本的制备和染色体核型分析研究进展 [J]. 南方农业学报, 2012, 43(12): 1958-1962. |
| Ding H, Qiu DP, Chen SX. Research progress in plant chromosome samples preparation and karyotype analysis [J]. J South Agric, 2012, 43(12): 1958-1962. | |
| 17 | 杨清淮. 植物染色体倍性鉴定方法概述 [J]. 现代园艺, 2014(3): 12-13. |
| Yang QH. Summary of identification methods of plant chromosome ploidy [J]. Xiandai Hortic, 2014(3): 12-13. | |
| 18 | Han FP, Gao Z, Yu WC, et al. Minichromosome analysis of chromosome pairing, disjunction, and sister chromatid cohesion in maize [J]. Plant Cell, 2007, 19(12): 3853-3863. |
| 19 | Kato A, Lamb JC, Birchler JA. Chromosome painting using repetitive DNA sequences as probes for somatic chromosome identification in maize [J]. Proc Natl Acad Sci USA, 2004, 101(37): 13554-13559. |
| 20 | Wang J, Liu YL, Su HD, et al. Centromere structure and function analysis in wheat-rye translocation lines [J]. Plant J, 2017, 91(2): 199-207. |
| 21 | 李玉玺, 轩淑欣, 王彦华, 等. 大白菜适于FISH的染色体制片技术研究 [J]. 中国农学通报, 2011, 27(10): 284-288. |
| Li YX, Xuan SX, Wang YH, et al. Studies on the chromosome preparation for FISH in Chinese cabbage [J]. Chin Agric Sci Bull, 2011, 27(10): 284-288. | |
| 22 | Ren TH, Sun ZX, Ren ZL, et al. Development and molecular cytogenetic characterization of a novel wheat-rye T6RS.6AL translocation line from Secale cereale L. Qinling with resistance to stripe rust and powdery mildew [J]. Int J Mol Sci, 2022, 23(18): 10495. |
| 23 | Singh AK, Zhang P, Dong CM, et al. Development and molecular cytogenetic characterization of Thinopyrum bessarabicum introgression lines in hexaploid and tetraploid wheats [J]. Theor Appl Genet, 2020, 133(7): 2117-2130. |
| 24 | Idziak-Helmcke D, Warzecha T, Sowa M, et al. 3-D nucleus architecture in oat×maize addition lines [J]. Int J Mol Sci, 2020, 21(12): 4280. |
| 25 | 刘丹, 夏雪, 吴益梅, 等. 植物染色体制片效果影响因素的解析 [J]. 浙江农业科学, 2015, 56(10): 1654-1657. |
| Liu D, Xia X, Wu YM, et al. Analysis of factors affecting the effect of plant chromosome production [J]. J Zhejiang Agric Sci, 2015, 56(10): 1654-1657. | |
| 26 | 韩杰, 孔德仪, 彭方仁. 薄壳山核桃染色体制片技术的优化与核型分析 [J]. 分子植物育种, 2018, 16(16): 5240-5246. |
| Han J, Kong DY, Peng FR. Optimization of chromosome mounting technique and karyotype analysis of Carya illinoinensis [J]. Mol Plant Breed, 2018, 16(16): 5240-5246. | |
| 27 | 喻雪莲, 李兴锋, 张丽霞. 茶树染色体制片技术优化 [J/OL].分子植物育种,2024. . |
| Yu XL, Li XF, Zhang LX. Technical optimization of chromosome preparation in Camellia sinensis [J/OL]. Mol Plant Breed, 2024. . | |
| 28 | Kirov I, Divashuk M, Van Laere K, et al. An easy “SteamDrop” method for high quality plant chromosome preparation [J]. Mol Cytogenet, 2014, 7(1): 21. |
| 29 | Findley SD, Birchler JA, Stacey G. Metaphase chromosome preparation from soybean (Glycine max) root tips [J]. Curr Protoc Plant Biol, 2017, 2(1): 78-88. |
| 30 | Song XY, Song RR, Zhou JW, et al. Development and application of oligonucleotide-based chromosome painting for chromosome 4D of Triticum aestivum L. [J]. Chromosome Res, 2020, 28(2): 171-182. |
| 31 | Zhang SY, Zhu MQ, Shang Y, et al. Physical organization of repetitive sequences and chromosome diversity of barley revealed by fluorescence in situ hybridization (FISH) [J]. Genome, 2019, 62(5): 329-339. |
| 32 | 孙音, 李兆鹏, 房义福, 等. 大叶女贞染色体制片及核型分析[J]. 山东林业科技, 2019, 49(4): 1-5. |
| Sun Y, Li ZP, Fang YF, et al. Chromosome preparation and karyotype analysis of Ligustrum compactum [J]. J Shandong For Sci Technol, 2019, 49(4): 1-5. |
| [1] | 马耀武, 张麒宇, 杨淼, 蒋诚, 张振宇, 张伊琳, 李梦莎, 许嘉阳, 张斌, 崔光周, 姜瑛. 烟草根际促生菌的筛选鉴定及促生性能研究[J]. 生物技术通报, 2025, 41(3): 271-281. |
| [2] | 张曼玉, 董嘉诚, 苟福凡, 弓朝晖, 刘倩, 孙文良, 孔臻, 郝捷, 王敏, 田朝光. 嗜热毁丝霉果胶酯酶MtCE12-1的克隆表达及其酶学性质和应用研究[J]. 生物技术通报, 2024, 40(9): 291-300. |
| [3] | 李亦君, 杨小贝, 夏琳, 罗朝鹏, 徐馨, 杨军, 宁黔冀, 武明珠. 烟草NtPRR37基因克隆及功能分析[J]. 生物技术通报, 2024, 40(8): 221-231. |
| [4] | 杜仲阳, 杨泽, 梁梦静, 刘义珍, 崔红利, 史达明, 薛金爱, 孙岩, 张春辉, 季春丽, 李润植. 纳米硒(SeNPs)缓解烟草幼苗铅胁迫和促生效应[J]. 生物技术通报, 2024, 40(7): 183-196. |
| [5] | 孔小平, 陈利文, 刘思思, 严湘萍. 胡萝卜抽薹相关性状全基因组关联分析[J]. 生物技术通报, 2024, 40(5): 120-130. |
| [6] | 王颢杰, 常栋, 李俊营, 孟颢光, 蒋士君, 周硕野, 崔江宽. 不同生境下烤烟三段式育苗微生物群落变化及抗逆酶活分析[J]. 生物技术通报, 2024, 40(4): 242-254. |
| [7] | 李灿, 蒋湘宁, 盖颖. 日本落叶松LkF3H2基因克隆及调控类黄酮代谢功能研究[J]. 生物技术通报, 2024, 40(2): 245-252. |
| [8] | 曾鸿运, 许林兵, 吴元立, 黄秉智. 基于ITS Sanger测序的香蕉杂交和自交后代的鉴定[J]. 生物技术通报, 2024, 40(12): 53-60. |
| [9] | 杨志晓, 侯骞, 刘国权, 卢志刚, 曹毅, 芶剑渝, 王轶, 林英超. 不同抗性烟草品系Rubisco及其活化酶对赤星病胁迫的响应[J]. 生物技术通报, 2023, 39(9): 202-212. |
| [10] | 刘珍银, 段郅臻, 彭婷, 王童欣, 王健. 基于三角梅的病毒诱导基因沉默体系的建立与优化[J]. 生物技术通报, 2023, 39(7): 123-130. |
| [11] | 李文辰, 刘鑫, 康越, 李伟, 齐泽铮, 于璐, 王芳. TRV病毒诱导大豆基因沉默体系优化及应用[J]. 生物技术通报, 2023, 39(7): 143-150. |
| [12] | 张路阳, 韩文龙, 徐晓雯, 姚健, 李芳芳, 田效园, 张智强. 烟草TCP基因家族的鉴定及表达分析[J]. 生物技术通报, 2023, 39(6): 248-258. |
| [13] | 王羽, 尹铭绅, 尹晓燕, 奚家勤, 杨建伟, 牛秋红. 烟草甲体内烟碱降解菌的筛选、鉴定及降解特性[J]. 生物技术通报, 2023, 39(6): 308-315. |
| [14] | 申云鑫, 施竹凤, 周旭东, 李铭刚, 张庆, 冯路遥, 陈齐斌, 杨佩文. 三株具生防功能芽孢杆菌的分离鉴定及其生物活性研究[J]. 生物技术通报, 2023, 39(3): 267-277. |
| [15] | 蔡梦鲜, 高作敏, 胡利娟, 冯群, 王洪程, 朱斌. 天然甘蓝型油菜C染色体组C1,C2缺体的创建及遗传分析[J]. 生物技术通报, 2023, 39(3): 81-88. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||