








生物技术通报 ›› 2025, Vol. 41 ›› Issue (5): 255-266.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0994
• 研究报告 • 上一篇
陈永旗1,2( ), 李志文1,2, 李鑫1, 原若曦1, 王春旭1,2, 韩毅强1,2,3, 高亚梅1,2,4(
), 李志文1,2, 李鑫1, 原若曦1, 王春旭1,2, 韩毅强1,2,3, 高亚梅1,2,4( )
)
收稿日期:2024-10-12
出版日期:2025-05-26
发布日期:2025-06-05
通讯作者:
高亚梅,女,教授,研究方向 :微生物资源开发与功能研究;E-mail: gaoym800@126.com作者简介:陈永旗,女,硕士,研究方向 :寒区环境微生物与农业废弃物资源化利用;E-mail: 3065408202@qq.com
基金资助:
CHEN Yong-qi1,2( ), LI Zhi-wen1,2, LI Xin1, YUAN Ruo-xi1, WANG Chun-xu1,2, HAN Yi-qiang1,2,3, GAO Ya-mei1,2,4(
), LI Zhi-wen1,2, LI Xin1, YUAN Ruo-xi1, WANG Chun-xu1,2, HAN Yi-qiang1,2,3, GAO Ya-mei1,2,4( )
)
Received:2024-10-12
Published:2025-05-26
Online:2025-06-05
摘要:
目的 大豆疫霉根腐病是由大豆疫霉引起的严重土传病害,从黑土区大豆根际土壤中筛选并鉴定对大豆疫霉具有拮抗作用的放线菌资源。 方法 选择4种分离培养基,利用稀释划线法从健康和患病大豆植株根际土壤中进行菌株分离,通过平板对峙法筛选对大豆疫霉有抑制活性的菌株,并对候选菌株进行培养特征、生理生化特性测定和基于16S rRNA的分子鉴定。 结果 从健康植株根际土壤中分离了87株放线菌,患病植株根际土壤中分离了43株放线菌。通过平板对峙实验在130株菌中筛选出了11株对大豆疫霉有较好抑制效果的菌株。来自患病根际土的HV-HDN-12对大豆疫霉抑制率为45.48%,健康土壤中分离的LSV-JDN-1对大豆疫霉抑制率达45.84%。HV-HDN-12和LSV-JDN-1具有ACC脱氨酶活性和促进植物生长的吲哚乙酸(IAA),LSV-JDN-1可产铁载体。根据其形态和生理生化结果以及16S rRNA序列分析,HV-HDN-12鉴定为Streptomyces albidoflavus,LSV-JDN-1鉴定为Streptomyces cavourensis。 结论 HV-HDN-12和LSV-JDN-1来自黑土大豆种植区大豆根际,具有广谱抗真菌和植物促生的优良特性,是有较好生防潜力的放线菌资源。
陈永旗, 李志文, 李鑫, 原若曦, 王春旭, 韩毅强, 高亚梅. 黑土区大豆根际土壤放线菌的分离与功能研究[J]. 生物技术通报, 2025, 41(5): 255-266.
CHEN Yong-qi, LI Zhi-wen, LI Xin, YUAN Ruo-xi, WANG Chun-xu, HAN Yi-qiang, GAO Ya-mei. Isolation and Function Study of Actinomycetes from Rhizosphere Soil of Soybean in the Black Soil Region[J]. Biotechnology Bulletin, 2025, 41(5): 255-266.
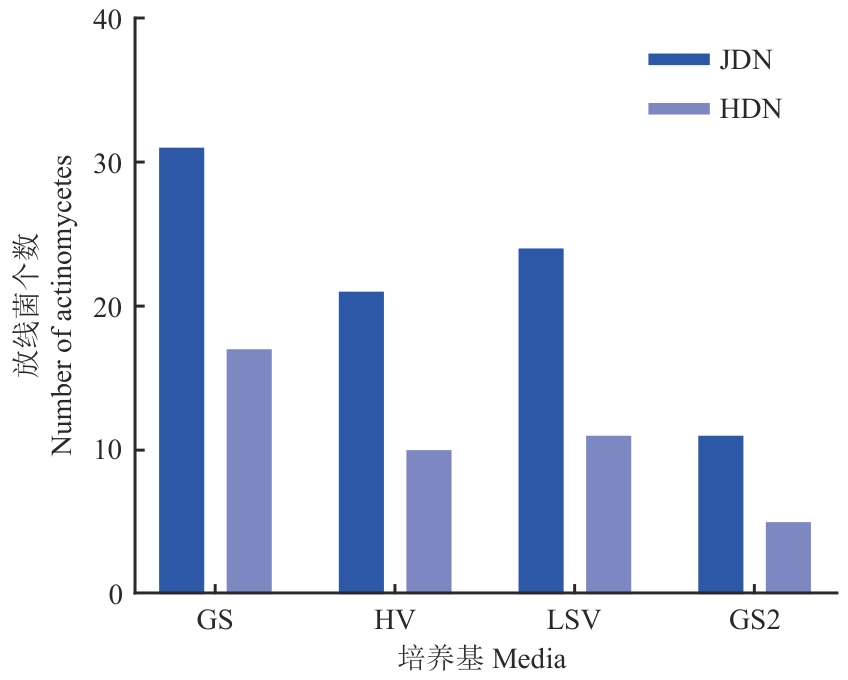
图1 患病植株和健康植株根际土不同培养基分离的放线菌数量对比LSV:黄豆饼粉培养基;GS:高氏一号培养基;HV:腐殖酸培养基;GS2:改良高氏一号培养基;JDN:菌株来源于健康大豆根际土壤;HDN:菌株来源于患病大豆的根际土壤。下同
Fig. 1 Comparison of the number of actinomycetes isolated from the rhizosphere soil of diseased and healthy plants using four different mediaLSV: Soybean cake medium; GS: Gauze's medium No.1; HV: humic acid medium; GS2: improved Gao's No.1; JDN: strain from the rhizosphere soil of healthy soybean; HDN: strain from the rhizosphere soil of diseased soybeans. The same below
菌株 Strain | 大豆疫霉 P. sojae | 尖孢镰刀菌 F. oxysporum | 小麦赤霉菌 G. triticum | 燕麦镰刀菌 F. oat | 玉米大斑病菌 J. spot | 核盘菌 S. sclerotiorum | 芹菜灰霉菌 Celery cinerine mold | 麦根镰刀菌 F. rhizome | 稻瘟病菌 M. oryzae |
|---|---|---|---|---|---|---|---|---|---|
| LSV-JDN-1 | 45.84 | 30.67 | 14.74 | 11.16 | 17.88 | 17.53 | 19.55 | 30.98 | 26.98 |
| LSV-JDN-3 | 44.67 | 30.79 | 27.99 | 20.48 | 20.78 | 19.35 | 17.46 | 17.69 | 27.38 |
| GS2-JDN-7 | 40.27 | 32.03 | 20.61 | 19.52 | 12.48 | 16.35 | 15.20 | 18.34 | 25.56 |
| LSV-JDN-8 | 42.56 | 27.80 | 15.05 | 14.62 | 14.45 | 18.26 | 24.55 | 27.38 | 35.95 |
| LSV-JDN-10 | 41.58 | 20.74 | 14.70 | 12.64 | 20.75 | 17.68 | 25.64 | 26.10 | 28.39 |
| GS-JDN-13 | 45.64 | 22.56 | 无 | 无 | 8.56 | 18.91 | 18.58 | 25.87 | 34.10 |
| HV-HDN-5-1 | 42.87 | 30.69 | 13.26 | 8.64 | 16.58 | 15.24 | 23.13 | 19.35 | 30.64 |
| LSV-HDN-6 | 40.77 | 20.14 | 12.84 | 18.76 | 13.26 | 19.21 | 20.68 | 25.67 | 31.49 |
| HV-HDN-12 | 45.48 | 34.59 | 10.19 | 21.15 | 20.18 | 18.18 | 22.87 | 24.87 | 28.76 |
| GS-HDN-12-1 | 43.44 | 34.98 | 20.69 | 25.05 | 22.14 | 16.54 | 15.74 | 11.43 | 23.98 |
| GS-HDN-12-2 | 43.64 | 36.39 | 无 | 12.38 | 23.67 | 12.36 | 21.35 | 18.64 | 29.86 |
表1 不同土壤中分离的放线菌对植物病原菌的抑菌活性 (%)
Table 1 Antifungal activity of actinomycetes isolated from different soils against plant pathogens
菌株 Strain | 大豆疫霉 P. sojae | 尖孢镰刀菌 F. oxysporum | 小麦赤霉菌 G. triticum | 燕麦镰刀菌 F. oat | 玉米大斑病菌 J. spot | 核盘菌 S. sclerotiorum | 芹菜灰霉菌 Celery cinerine mold | 麦根镰刀菌 F. rhizome | 稻瘟病菌 M. oryzae |
|---|---|---|---|---|---|---|---|---|---|
| LSV-JDN-1 | 45.84 | 30.67 | 14.74 | 11.16 | 17.88 | 17.53 | 19.55 | 30.98 | 26.98 |
| LSV-JDN-3 | 44.67 | 30.79 | 27.99 | 20.48 | 20.78 | 19.35 | 17.46 | 17.69 | 27.38 |
| GS2-JDN-7 | 40.27 | 32.03 | 20.61 | 19.52 | 12.48 | 16.35 | 15.20 | 18.34 | 25.56 |
| LSV-JDN-8 | 42.56 | 27.80 | 15.05 | 14.62 | 14.45 | 18.26 | 24.55 | 27.38 | 35.95 |
| LSV-JDN-10 | 41.58 | 20.74 | 14.70 | 12.64 | 20.75 | 17.68 | 25.64 | 26.10 | 28.39 |
| GS-JDN-13 | 45.64 | 22.56 | 无 | 无 | 8.56 | 18.91 | 18.58 | 25.87 | 34.10 |
| HV-HDN-5-1 | 42.87 | 30.69 | 13.26 | 8.64 | 16.58 | 15.24 | 23.13 | 19.35 | 30.64 |
| LSV-HDN-6 | 40.77 | 20.14 | 12.84 | 18.76 | 13.26 | 19.21 | 20.68 | 25.67 | 31.49 |
| HV-HDN-12 | 45.48 | 34.59 | 10.19 | 21.15 | 20.18 | 18.18 | 22.87 | 24.87 | 28.76 |
| GS-HDN-12-1 | 43.44 | 34.98 | 20.69 | 25.05 | 22.14 | 16.54 | 15.74 | 11.43 | 23.98 |
| GS-HDN-12-2 | 43.64 | 36.39 | 无 | 12.38 | 23.67 | 12.36 | 21.35 | 18.64 | 29.86 |
菌株 Strain | IAA产量 IAA yield (μg/mL) | ACC脱氨酶 ACC deaminase | 铁载体 Siderophore |
|---|---|---|---|
| LSV-JDN-1 | 13.47 | + | + |
| HV-HDN-12 | 49.6 | ++ | - |
表 2 LSV-JDN-1和HV-HDN-12促生活性结果
Table 2 Growth-promoting results of LSV-JDN-1 and HV-HDN-12
菌株 Strain | IAA产量 IAA yield (μg/mL) | ACC脱氨酶 ACC deaminase | 铁载体 Siderophore |
|---|---|---|---|
| LSV-JDN-1 | 13.47 | + | + |
| HV-HDN-12 | 49.6 | ++ | - |

图2 菌株HV-HDN-12 (A, B) 和LSV-JDN-1(C, D)的生长形态及光镜下菌丝形态(100×)
Fig. 2 Morphology and mycelial morphology of strain HV-HDN-12 (A, B) and LSV-JDN-1(C, D) under light microscope (100×)
培养基 Medium | HV-HDN-12 | LSV-JDN-1 | ||||||
|---|---|---|---|---|---|---|---|---|
气生菌丝 Aerial mycelium | 基内菌丝 Intracellular myceliumm | 生长状况 Growth state | 可溶性色素Soluble pigments | 气生菌丝 Aerial mycelium | 基内菌丝 Intracellular myceliumm | 生长状况 Growth state | 可溶性色素Soluble pigments | |
| ISP2 | 白色 White | 浅黄色 Yellowish | 一般 Poor | 无 None | 白色 White | 浅黄色 Yellowish | 良好 Good | 无 None |
| ISP3 | 白色 White | 浅黄色 Yellowish | 良好 Good | 无 None | 白色 White | 白色 White | 良好 Good | 无 None |
| ISP4 | 白色 White | 浅黄色 Yellowish | 良好 Good | 无 None | 白色 White | 黑灰色 Dark Gray | 良好 Good | 无 None |
| ISP5 | 浅黄色 Yellowish | 浅黄色 Yellowish | 一般 Poor | 无 None | 白色 White | 白色 White | 一般 Poor | 无 None |
| ISP6 | 灰色 Gray | 灰色 Gray | 一般 Poor | 无 None | 白色 White | 浅黄色 Yellowish | 良好 Good | 无 None |
| ISP7 | 白色 White | 灰色 Gray | 良好 Good | 无 None | 灰色 Gray | 黄棕色 Yellowish brown | 良好 Good | 无 None |
| 察氏Czapek’s | 白色 White | 白色 White | 一般 Poor | 无 None | 白色 White | 白色 White | 一般 Poor | 无 None |
表3 菌株HV-HDN-12和LSV-JDN-1在不同培养基上生长情况
Table 3 Growth feature of strain HV-HDN-12 and LSV-JDN-1 on different media
培养基 Medium | HV-HDN-12 | LSV-JDN-1 | ||||||
|---|---|---|---|---|---|---|---|---|
气生菌丝 Aerial mycelium | 基内菌丝 Intracellular myceliumm | 生长状况 Growth state | 可溶性色素Soluble pigments | 气生菌丝 Aerial mycelium | 基内菌丝 Intracellular myceliumm | 生长状况 Growth state | 可溶性色素Soluble pigments | |
| ISP2 | 白色 White | 浅黄色 Yellowish | 一般 Poor | 无 None | 白色 White | 浅黄色 Yellowish | 良好 Good | 无 None |
| ISP3 | 白色 White | 浅黄色 Yellowish | 良好 Good | 无 None | 白色 White | 白色 White | 良好 Good | 无 None |
| ISP4 | 白色 White | 浅黄色 Yellowish | 良好 Good | 无 None | 白色 White | 黑灰色 Dark Gray | 良好 Good | 无 None |
| ISP5 | 浅黄色 Yellowish | 浅黄色 Yellowish | 一般 Poor | 无 None | 白色 White | 白色 White | 一般 Poor | 无 None |
| ISP6 | 灰色 Gray | 灰色 Gray | 一般 Poor | 无 None | 白色 White | 浅黄色 Yellowish | 良好 Good | 无 None |
| ISP7 | 白色 White | 灰色 Gray | 良好 Good | 无 None | 灰色 Gray | 黄棕色 Yellowish brown | 良好 Good | 无 None |
| 察氏Czapek’s | 白色 White | 白色 White | 一般 Poor | 无 None | 白色 White | 白色 White | 一般 Poor | 无 None |
实验项目 Test | LSV-JDN-1 | HV-HDN-12 | 实验项目 Test item | LSV- JDN-1 | HV-HDN-12 |
|---|---|---|---|---|---|
乳糖 Lactose | + | + | 苏氨酸 Threonine | + | + |
鼠李糖 Rhamnose | + | - | 谷氨酰胺 Glutamine | + | + |
棉子糖 Marshmallow | + | - | 酪氨酸 Tyrosine | - | + |
甘露醇 Mannitol | + | - | 精氨酸 Argnine | + | + |
葡萄糖 Glucose | + | + | 丙氨酸 Alanine | + | + |
木糖 Xylose | - | + | 牛奶凝固与胨化 Milk peptonization | + | + |
甘露糖 Mannose | + | + | 明胶液化 Gelatin Liquefaction | + | + |
阿拉伯糖 Arabinose | - | + | 淀粉水解 Starch hydrolysis | + | + |
肌醇 Inositol | + | - | 硫化氢产生 Hydrogen Sulfide Generation | - | - |
半乳糖 Galactose | + | + | 硝酸盐还原 Nitrate Reduction | + | + |
蔗糖 Fructose | + | - | 纤维素水解 Urease | - | - |
天冬氨酸 Aspartic acid | - | - | 脲酶 Lipase | - | - |
肌酸 Creatine | + | + |
表4 菌株的生理生化特征
Table 4 Physiological and biochemical characteristics of strain
实验项目 Test | LSV-JDN-1 | HV-HDN-12 | 实验项目 Test item | LSV- JDN-1 | HV-HDN-12 |
|---|---|---|---|---|---|
乳糖 Lactose | + | + | 苏氨酸 Threonine | + | + |
鼠李糖 Rhamnose | + | - | 谷氨酰胺 Glutamine | + | + |
棉子糖 Marshmallow | + | - | 酪氨酸 Tyrosine | - | + |
甘露醇 Mannitol | + | - | 精氨酸 Argnine | + | + |
葡萄糖 Glucose | + | + | 丙氨酸 Alanine | + | + |
木糖 Xylose | - | + | 牛奶凝固与胨化 Milk peptonization | + | + |
甘露糖 Mannose | + | + | 明胶液化 Gelatin Liquefaction | + | + |
阿拉伯糖 Arabinose | - | + | 淀粉水解 Starch hydrolysis | + | + |
肌醇 Inositol | + | - | 硫化氢产生 Hydrogen Sulfide Generation | - | - |
半乳糖 Galactose | + | + | 硝酸盐还原 Nitrate Reduction | + | + |
蔗糖 Fructose | + | - | 纤维素水解 Urease | - | - |
天冬氨酸 Aspartic acid | - | - | 脲酶 Lipase | - | - |
肌酸 Creatine | + | + |
项目 Item | HV-HDN-12/F4 | LSV-JND-1/F3 |
|---|---|---|
菌落直径 Colony diameter (mm) | 48.62±1.8 | 67.69±1.43 |
抑制率 Inhibition rate (%) | 42.79 | 20.34 |
表5 不同发酵培养基对发酵液抑菌活性的影响
Table 5 Effects of different fermentation media on the inhibitory activities of fermentation broths
项目 Item | HV-HDN-12/F4 | LSV-JND-1/F3 |
|---|---|---|
菌落直径 Colony diameter (mm) | 48.62±1.8 | 67.69±1.43 |
抑制率 Inhibition rate (%) | 42.79 | 20.34 |

图5 LSVJDN-1(A, B)和HV-HDN-12(C, D)孢子悬液对大豆疫霉侵染豆芽的抑制效果A:LSV-JND-1;B:对照;C:HV-HDN-12;D:对照
Fig. 5 Inhibition of LSV-JDN-1(A, B) and HV-HDN-12(C, D) on P. sojae infecting the soybean sproutsA: LSV-JND-1; B: control; C: HV-HDN-12; D: control
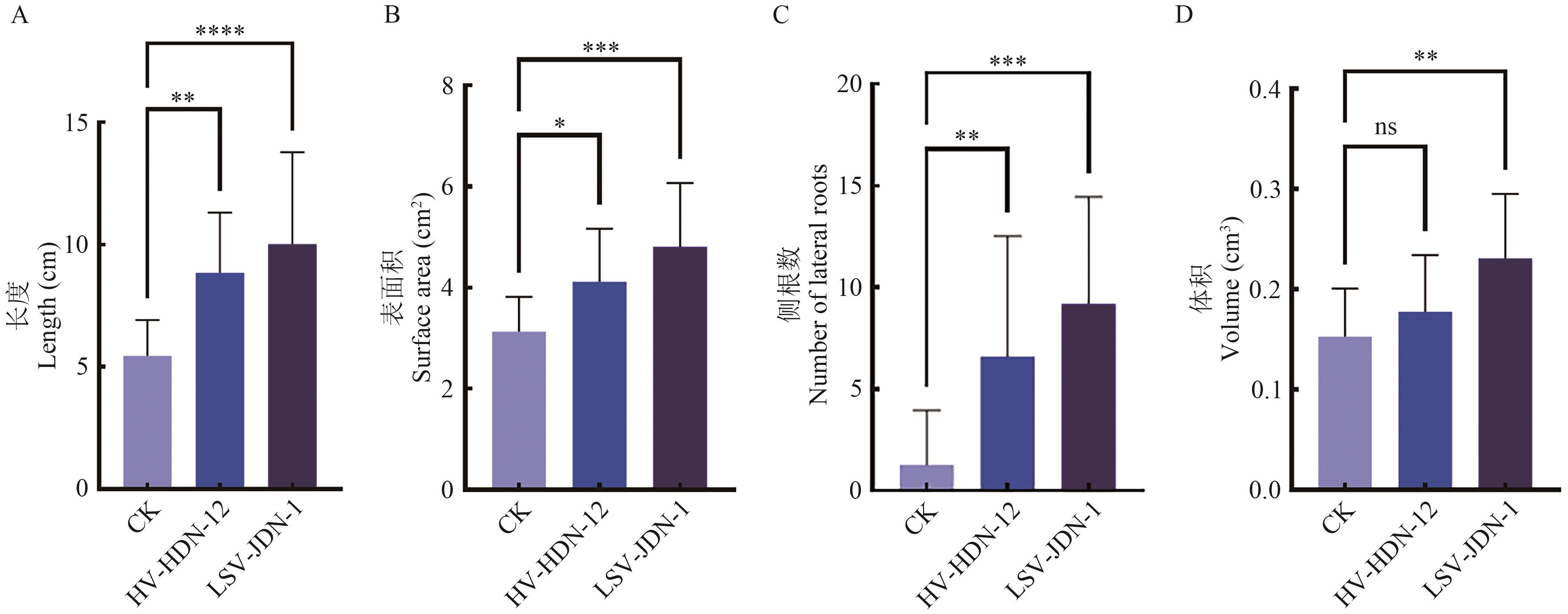
图6 HV-HDN-12和LSV-JDN-1孢子悬液对大豆种子的促生作用*P<0.1; **P<0.05; ***P<0.01; ns: no difference
Fig. 6 Growth-promoting effect of spore suspensions of HV-HDN-12 and LSV-JDN-1 on soybean seed
| 1 | 张鸿雁, 高擎, 张琳园, 等. 大豆疫病拮抗菌的筛选及促生抗病作用研究 [J]. 生物技术通报, 2020, 36(10): 25-31. |
| Zhang HY, Gao Q, Zhang LY, et al. Screening of actinomycetes against Phytophthora root rot of soybean and its growth promotion and disease control [J]. Biotechnol Bull, 2020, 36(10): 25-31. | |
| 2 | 马淑梅, 李宝英. 大豆疫霉根腐病菌生理小种鉴定结果初报 [J]. 大豆科学, 1999, 18(2): 58-60. |
| Ma SM, Li BY. A prelminary report on the identification ofthe physiological races of Phytophthora megasperma [J]. Soybean Sci, 1999, 18(2): 58-60. | |
| 3 | Tyler BM. Phytophthora sojae: root rot pathogen of soybean and model oomycete [J]. Mol Plant Pathol, 2007, 8(1): 1-8. |
| 4 | Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health [J]. Trends Plant Sci, 2012, 17(8): 478-486. |
| 5 | Khamna S, Yokota A, Lumyong S. Actinomycetes isolated from medicinal plant rhizosphere soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production [J]. World J Microbiol Biotechnol, 2009, 25(4): 649-655. |
| 6 | Jiménez-Esquilín AE, Roane TM. Antifungal activities of actinomycete strains associated with high-altitude sagebrush rhizosphere [J]. J Ind Microbiol Biotechnol, 2005, 32(8): 378-381. |
| 7 | 蒋莲秀, 吴越, 陈建宏, 等. 6株红树林根际土壤放线菌的分离鉴定及活性测定 [J]. 中国病原生物学杂志, 2017, 12(6): 513-518. |
| Jiang LX, Wu Y, Chen JH, et al. Identification of six Actinomycetes strains isolated from mangrove rhizosphere soil and determination of their activity [J]. J Pathog Biol, 2017, 12(6): 513-518. | |
| 8 | 陈龙池, 廖利平, 汪思龙, 等. 根系分泌物生态学研究 [J]. 生态学杂志, 2002, 21(6): 57-62, 28. |
| Chen LC, Liao LP, Wang SL, et al. A review for research of root exudates ecology [J]. Chin J Ecol, 2002, 21(6): 57-62, 28. | |
| 9 | AbdElgawad H, Abuelsoud W, Madany MMY, et al. Actinomycetes enrich soil rhizosphere and improve seed quality as well as productivity of legumes by boosting nitrogen availability and metabolism [J]. Biomolecules, 2020, 10(12): 1675. |
| 10 | Poomthongdee N, Duangmal K, Pathom-aree W. Acidophilic actinomycetes from rhizosphere soil: diversity and properties beneficial to plants [J]. J Antibiot, 2015, 68(2): 106-114. |
| 11 | Genilloud O. Actinomycetes: still a source of novel antibiotics [J]. Nat Prod Rep, 2017, 34(10): 1203-1232. |
| 12 | Kunova A, Bonaldi M, Saracchi M, et al. Selection of Streptomyces against soil borne fungal pathogens by a standardized dual culture assay and evaluation of their effects on seed germination and plant growth [J]. BMC Microbiol, 2016, 16(1): 272. |
| 13 | Arcamone F, Camerino B, Cotta E, et al. New carotenoids from Streptomyces mediolani n. sp [J]. Experientia, 1969, 25(3): 241-242. |
| 14 | 纠敏, 李晶晶, 李伟山, 等. 大豆疫霉病病菌生防放线菌的筛选、鉴定及生防效果 [J]. 江苏农业学报, 2021, 37(5): 1137-1142. |
| Jiu M, Li JJ, Li WS, et al. Screening, identification and biocontrol effect of actinomycetes against Phytophthora sojae [J]. Jiangsu J Agric Sci, 2021, 37(5): 1137-1142. | |
| 15 | 李菲, 杨玲, 王巧贞, 等. 红树林放线菌抗沃柑病原真菌的研究 [J]. 中国抗生素杂志, 2022, 47(7): 638-646. |
| Li F, Yang L, Wang QZ, et al. Study on mangrove actinomycetes against pathogenetic fungi of Orah [J]. Chin J Antibiot, 2022, 47(7): 638-646. | |
| 16 | Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores [J]. Anal Biochem, 1987, 160(1): 47-56. |
| 17 | 徐丽华, 李文均, 刘志恒, 等. 放线菌系统学: 原理、方法及实践 [M]. 北京: 科学出版社, 2007. |
| Xu LH, Li WJ, Liu ZH, et al. Actinomycete systematic [M]. Beijing: Science Press, 2007. | |
| 18 | 张璐. 产铁载体细菌强化甜高粱修复土壤重金属污染 [J]. 环境科学与技术, 2014, 37(4): 74-79. |
| Zhang L. Bioaugmentation with siderophore-producing bacteria to enhance phytoremediation of heavy metal polluted soil by sweet Sorghum [J]. Environ Sci Technol, 2014, 37(4): 74-79. | |
| 19 | 谢远国, 王雅楠, 刘畅, 等. 盐生植物碱蓬二型果实表生细菌的群落组成及促生属性 [J]. 微生物学通报, 2018, 45(7): 1426-1437. |
| Xie YG, Wang YN, Liu C, et al. The community composition and growth-promoting activities of the cultivable epiphytic bacteria from the dimorphic fruits of Suaeda glauca Bunge [J]. Microbiol China, 2018, 45(7): 1426-1437. | |
| 20 | Shin SH, Lim Y, Lee SE, et al. CAS agar diffusion assay for the measurement of siderophores in biological fluids [J]. J Microbiol Methods, 2001, 44(1): 89-95. |
| 21 | 邵嘉朱, 吕雯, 廖鑫琳, 等. 大豆根际促生菌的分离、鉴定及其耐盐促生作用 [J]. 中国农业科学, 2024, 57(21): 4248-4263. |
| Shao JZ, Lü W, Liao XL, et al. Isolation and identification of soybean rhizosphere growth-promoting bacteria and their salt tolerance and growth-promoting effects [J]. Sci Agric Sin, 2024, 57(21): 4248-4263. | |
| 22 | 周雪, 崔俊涛, 李明堂, 等. 土壤微生物助力东北黑土区农业资源与环境保护与发展 [J]. 吉林农业大学学报, 2022, 44(6): 679-687. |
| Zhou X, Cui JT, Li MT, et al. Contribution of soil microorganisms to agricultural resources and environment development in black soil region of NorthEast China [J]. J Jilin Agric Univ, 2022, 44(6): 679-687. | |
| 23 | Lundberg DS, Lebeis SL, Paredes SH, et al. Defining the core Arabidopsis thaliana root microbiome [J]. Nature, 2012, 488(7409): 86-90. |
| 24 | Schreiter S, Ding GC, Heuer H, et al. Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce [J]. Front Microbiol, 2014, 5: 144. |
| 25 | Li H, Yang XR, Weng BS, et al. The phenological stage of rice growth determines anaerobic ammonium oxidation activity in rhizosphere soil [J]. Soil Biol Biochem, 2016, 100: 59-65. |
| 26 | 赵珊珊. 大豆菌核病生防放线菌的筛选及Micromonospora parathelypteridis新种的鉴定 [D]. 哈尔滨: 东北农业大学, 2017. |
| Zhao SS. Screening of actinomycetes for biocontrol of soybean Sclerotinia sclerotiorum and identification of new species of Micromonospora parathylpteridis [D]. Harbin: Northeast Agricultural University, 2017. | |
| 27 | Nakagawa K, Sato K, Okazaki T, et al. Microbial conversion of milbemycins: 13 beta, 29-dihydroxylation of milbemycins by soil isolate Streptomyces cavourensis [J]. J Antibiot, 1991, 44(7): 803-805. |
| 28 | Sudha S, Masilamani SM. Characterization of cytotoxic compound from marine sediment derived actinomycete Streptomyces avidinii strain SU4 [J]. Asian Pac J Trop Biomed, 2012, 2(10): 770-773. |
| 29 | 周丽娜, 王莉莉, 张永娜, 等. 2株放线菌的抗菌活性及分类学地位 [J]. 中国农学通报, 2015, 31(11): 182-189. |
| Zhou LN, Wang LL, Zhang YN, et al. Antifungal activity and taxonomic status of two actinomycetes [J]. Chin Agric Sci Bull, 2015, 31(11): 182-189. | |
| 30 | Sheik GB, Alhumaidy AA, Abdel Raheim AIA, et al. Taxonomic characterizations of soil Streptomyces cavourensis DW102 and its activity against fungal pathogens [J]. J Pharm Bioallied Sci, 2020, 12(4): 462-467. |
| 31 | 杨少彬, 黄永春, 陈志永, 等. 卡伍尔链霉菌TJ430的分离鉴定及抗菌活性研究 [J]. 中国抗生素杂志, 2015, 40(7): 506-512. |
| Yang SB, Huang YC, Chen ZY, et al. Isolation, identification of Streptomyces cavourensis TJ430 strain and its antimicrobial activity [J]. Chin J Antibiot, 2015, 40(7): 506-512. | |
| 32 | Kaaniche F, Hamed A, Elleuch L, et al. Purification and characterization of seven bioactive compounds from the newly isolated Streptomyces cavourensis TN638 strain via solid-state fermentation [J]. Microb Pathog, 2020, 142: 104106. |
| 33 | Pan HQ, Yu SY, Song CF, et al. Identification and characterization of the antifungal substances of a novel Streptomyces cavourensis NA4 [J]. J Microbiol Biotechnol, 2015, 25(3): 353-357. |
| 34 | Tangwattanachuleeporn M, Ruangsuj P, Yamprayoonswat W, et al. Genome sequence of Streptomyces cavourensis BUU135, isolated from soil from a tropical fruit farm in Thailand [J]. Microbiol Resour Announc, 2021, 10(19): e01428-20. |
| 35 | Lai JH, Liu B, Xiong GH, et al. Inhibitory mechanism of 4-ethyl-1, 2-dimethoxybenzene produced by Streptomyces albidoflavus strain ML27 against Colletotrichum gloeosporioides [J]. Pestic Biochem Physiol, 2024, 204: 106086. |
| 36 | Pérez-Valero Á, Ye SH, Magadán-Corpas P, et al. Metabolic engineering in Streptomyces albidoflavus for the biosynthesis of the methylated flavonoids sakuranetin, acacetin, and genkwanin [J]. Microb Cell Fact, 2023, 22(1): 234. |
| 37 | Lee SY, Tindwa H, Lee YS, et al. Biocontrol of anthracnose in pepper using chitinase, beta-1, 3 glucanase, and 2-furancarboxaldehyde produced by Streptomyces cavourensis SY224 [J]. J Microbiol Biotechnol, 2012, 22(10): 1359-1366. |
| 38 | Broadway RM, Williams DL, Kain WC, et al. Partial characterization of chitinolytic enzymes from Streptomyces albidoflavus [J]. Lett Appl Microbiol, 1995, 20(5): 271-276. |
| 39 | Du YX, Wang TL, Jiang JY, et al. Biological control and plant growth promotion properties of Streptomyces albidoflavus St-220 isolated from Salvia miltiorrhiza rhizosphere [J]. Front Plant Sci, 2022, 13: 976813. |
| 40 | Carlucci A, Raimondo ML, Colucci D, et al. Streptomyces albidoflavus strain CARA17 as a biocontrol agent against fungal soil-borne pathogens of fennel plants [J]. Plants, 2022, 11(11): 1420. |
| 41 | Boukelloul I, Aouar L, Cherb N, et al. Actinobacteria isolated from soils of arid Saharan regions display simultaneous antifungal and plant growth promoting activities [J]. Curr Microbiol, 2024, 81(10): 327. |
| 42 | Hocinat A, Boudemagh A, Ali-Khodja H, et al. Aerobic degradation of BTEX compounds by Streptomyces species isolated from activated sludge and agricultural soils [J]. Arch Microbiol, 2020, 202(9): 2481-2492. |
| [1] | 刘丽, 王辉, 关天舒, 李柏宏, 于舒怡. 葡萄脱落酸受体VvPYL4互作蛋白的筛选及互作蛋白基因表达[J]. 生物技术通报, 2025, 41(4): 188-197. |
| [2] | 宋佳怡, 苏芸丽, 郑兴艳, 夏文念, 杨冬梅, 胡慧贞. 金鱼草Expansin基因家族鉴定及其抗核盘菌相关基因筛选[J]. 生物技术通报, 2025, 41(4): 227-242. |
| [3] | 刘爽, 江洲, 赵帅, 赵雷真, 黄峰, 周佳, 屈建航. 一株产蛋白酶细菌的筛选、鉴定及发酵工艺优化[J]. 生物技术通报, 2025, 41(4): 335-344. |
| [4] | 慕雪男, 吴桐, 郑子薇, 张越, 王志刚, 徐伟慧. 一株番茄青枯病生防细菌的筛选、鉴定及其生防潜力分析[J]. 生物技术通报, 2025, 41(1): 276-286. |
| [5] | 杜薇, 李志敏, 邢晏铭, 刘蒲临, 缪礼鸿. 一株易转化、高生物量地衣芽孢杆菌的筛选与鉴定[J]. 生物技术通报, 2024, 40(9): 181-189. |
| [6] | 邢丽南, 张艳芳, 葛明然, 赵令敏, 陈妍, 霍秀文. 山药DoWRKY40基因表达特征分析及互作蛋白筛选[J]. 生物技术通报, 2024, 40(8): 118-128. |
| [7] | 周江鸿, 夏菲, 仲丽, 仇兰芬, 李广, 刘倩, 张国锋, 邵金丽, 李娜, 车少臣. 黄栌枯萎病拮抗细菌CCBC3-3-1的全基因组测序及比较基因组分析[J]. 生物技术通报, 2024, 40(7): 235-246. |
| [8] | 邵长轩, 张少华, 邓浩然, 于伟康, 朱永杰, 单安山. 抗菌肽的数据库辅助设计[J]. 生物技术通报, 2024, 40(12): 12-19. |
| [9] | 赵正阳, 谢丙炎, 成新跃, 李惠霞. 昆虫相关放线菌资源挖掘利用研究进展[J]. 生物技术通报, 2024, 40(11): 113-124. |
| [10] | 叶柳健, 贺愉岚, 王小虎, 韦圣博, 何双, 朱绮霞, 卢洁, 周礼芹. 解淀粉芽孢杆菌YK3对沃柑溃疡病的防效及叶际细菌群落相关性的影响[J]. 生物技术通报, 2024, 40(11): 248-258. |
| [11] | 皮一飞, 宋新辉, 王淅琳, 李谨谨, 孙长斌, 徐炜. 基于R-loop靶向编辑技术的R-loop功能位点高通量筛选系统[J]. 生物技术通报, 2024, 40(10): 181-190. |
| [12] | 刘星雨, 李洁, 朱龙佼, 李相阳, 许文涛. 铜绿假单胞菌适配体的获得及应用[J]. 生物技术通报, 2024, 40(1): 186-193. |
| [13] | 温晓蕾, 李建嫄, 李娜, 张娜, 杨文香. 小麦叶锈菌与小麦互作的酵母双杂交cDNA文库构建与应用[J]. 生物技术通报, 2023, 39(9): 136-146. |
| [14] | 吴巧茵, 施友志, 李林林, 彭政, 谭再钰, 刘利平, 张娟, 潘勇. 类胡萝卜素降解菌株的原位筛选及其在雪茄提质增香中的应用[J]. 生物技术通报, 2023, 39(9): 192-201. |
| [15] | 江海溶, 崔若琪, 王玥, 白淼, 张明露, 任连海. NH3和H2S降解功能菌的分离鉴定及降解特性研究[J]. 生物技术通报, 2023, 39(9): 246-254. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||