








生物技术通报 ›› 2025, Vol. 41 ›› Issue (8): 300-310.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0135
• 研究报告 • 上一篇
石艳华1( ), 李朔1, 高玉珠1, 郑保坤1, 朱杰华1,2, 张岱1,2(
), 李朔1, 高玉珠1, 郑保坤1, 朱杰华1,2, 张岱1,2( ), 杨志辉1,2(
), 杨志辉1,2( )
)
收稿日期:2025-02-12
出版日期:2025-08-26
发布日期:2025-08-14
通讯作者:
张岱,女,博士,副教授,研究方向 :植物保护;E-mail: adaiadai.1987@163.com作者简介:石艳华,女,硕士研究生,研究方向 :植物病理学;E-mail: 460722976@qq.com
基金资助:
SHI Yan-hua1( ), LI Shuo1, GAO Yu-zhu1, ZHENG Bao-kun1, ZHU Jie-hua1,2, ZHANG Dai1,2(
), LI Shuo1, GAO Yu-zhu1, ZHENG Bao-kun1, ZHU Jie-hua1,2, ZHANG Dai1,2( ), YANG Zhi-hui1,2(
), YANG Zhi-hui1,2( )
)
Received:2025-02-12
Published:2025-08-26
Online:2025-08-14
摘要:
目的 实验室前期筛选得到能够分泌具有抑菌作用挥发性物质的5株芽胞杆菌菌株,进一步明确生防芽胞杆菌挥发性物质的促生功能,并鉴定其挥发性活性组分,以期获得具有抑菌和促生双重功能的优良菌株,研发具有促生作用的挥发性天然产物。 方法 采用盆栽熏蒸法、选择培养基测定和熵值计算法综合评定出最佳促生菌株。通过高效液相色谱技术检测其分泌的挥发性物质对马铃薯中细胞分裂素、赤霉素和生长素含量的影响,并采用实时荧光定量PCR分析挥发性物质对马铃薯内源激素相关合成基因(StCKX1、StLAX1和StGA2ox1)表达量的影响。通过全二维气相色谱-质谱(two-dimensional gas chromatography mass spectrometry, GC×GC-MS)对菌株挥发性成分进行检测,利用NIST/EPA/NIH数据库鉴定成分。根据峰面积比例,购买相对含量最高的3种纯品,体外检测其对植株的促生作用。 结果 贝莱斯芽胞杆菌NZ-4分泌的挥发性物质熏蒸后马铃薯促生作用最为显著,植株株高和根长分别增加了40.01%和15.15%;NZ-4具有溶磷、固氮和产生铁载体的能力;熵值法综合评价结果中NZ-4菌株得分最高,为0.947 6。因此,确定NZ-4为最优促生菌株。经贝莱斯芽胞杆菌NZ-4挥发性物质熏蒸后,马铃薯叶片中细胞分裂素、赤霉素和生长素的含量较对照组分别增加了1.09倍、0.87倍和1.89倍,StGA20ox1和StLAX1基因的表达量较对照提升了3.61倍和4.71倍。GC×GC-MS结果表明,2-庚酮、3-乙基甲苯和2-壬酮相对含量最高;浓度为1 μg/μL的2-庚酮和100 ng/μL的3-乙基甲苯能够显著促进植株的生长,2-壬酮对植株的生长没有明显作用。 结论 贝莱斯芽胞杆菌NZ-4产生的挥发性物质对马铃薯具有良好的促生作用,明确了2-庚酮和3-乙基甲苯为挥发性活性成分,NZ-4菌株产生的挥发性有机物具有开发新型天然产物的潜力。
石艳华, 李朔, 高玉珠, 郑保坤, 朱杰华, 张岱, 杨志辉. 贝莱斯芽胞杆菌NZ-4挥发性有机物的促生作用及其活性成分分析[J]. 生物技术通报, 2025, 41(8): 300-310.
SHI Yan-hua, LI Shuo, GAO Yu-zhu, ZHENG Bao-kun, ZHU Jie-hua, ZHANG Dai, YANG Zhi-hui. Analysis of the Growth-promoting Effects and Active Components of Volatile Organic Compounds Produced by Bacillus velezensis NZ-4[J]. Biotechnology Bulletin, 2025, 41(8): 300-310.
| 引物名称 Primer name | 序列 Sequence (5′-3′) |
|---|---|
| qStLAX1-F | GGTTTGGCATTTAATTGTAC |
| qStLAX1-R | CAGATTCGGTAGTTGTGA |
| qCKX1-F | AAGTTCAGTTCTGAAGGAAAAGT |
| qCKX1-R | AAAAACTTCTTGTGCAAAGTCATG |
| qStGA20ox1-F | CGGCCCAACAAGCATCTAAG |
| qStGA20ox1-R | AAGCCATGACTCCGACG |
| Stactin-F | GGTATTGTGCTGGATTCTGG |
| Stactin-R | CGTTCAGCACTAGTGGTGAA |
表1 RT-qPCR引物序列
Table 1 RT-qPCR primer sequence
| 引物名称 Primer name | 序列 Sequence (5′-3′) |
|---|---|
| qStLAX1-F | GGTTTGGCATTTAATTGTAC |
| qStLAX1-R | CAGATTCGGTAGTTGTGA |
| qCKX1-F | AAGTTCAGTTCTGAAGGAAAAGT |
| qCKX1-R | AAAAACTTCTTGTGCAAAGTCATG |
| qStGA20ox1-F | CGGCCCAACAAGCATCTAAG |
| qStGA20ox1-R | AAGCCATGACTCCGACG |
| Stactin-F | GGTATTGTGCTGGATTCTGG |
| Stactin-R | CGTTCAGCACTAGTGGTGAA |
菌株编号 Strain No. | 株高 Plant height (cm) | 茎粗 Stem diameter (cm) | 根长 Root length (cm) | 根体积 Root volume (cm³) | 地上鲜重 Aboveground fresh weight (g) | 地下鲜重 Belowground fresh weight (g) | 地上干重 Aboveground dry weight (g) |
|---|---|---|---|---|---|---|---|
| Control | 16.32±2.09d | 1.83±0.26b | 30.62±1.60b | 8.67±1.63b | 5.17±0.59b | 6.77±1.30b | 0.48±0.05b |
| L19 | 21.32±1.61c | 2.58±0.15a | 32.46±5.10ab | 10.00±2.53a | 8.53±1.29a | 7.66±0.46a | 0.54±0.07a |
| G32 | 21.38±1.07bc | 2.44±0.21a | 33.72±2.60ab | 12.00±3.35a | 8.51±0.81a | 7.33±0.94a | 0.54±0.06a |
| NZ-4 | 22.85±1.87a | 2.32±0.37a | 35.26±2.65a | 12.00±2.76a | 8.55±1.33a | 7.97±2.07a | 0.54±0.10a |
| HN-Q-8 | 21.04±1.06abc | 2.46±0.11a | 29.04±4.50ab | 10.67±2.73a | 8.01±0.69a | 6.98±0.99a | 0.53±0.05a |
| ZD01 | 22.81±1.52ab | 2.40±0.21a | 31.75±1.31ab | 9.33±2.07a | 8.75±1.16a | 7.33±1.16a | 0.55±0.09a |
表2 芽胞杆菌菌株挥发性物质熏蒸25 d后马铃薯各项生理指标
Table 2 Physiological indices of potato after 25 d of fumigation with volatile compounds from Bacillus strains
菌株编号 Strain No. | 株高 Plant height (cm) | 茎粗 Stem diameter (cm) | 根长 Root length (cm) | 根体积 Root volume (cm³) | 地上鲜重 Aboveground fresh weight (g) | 地下鲜重 Belowground fresh weight (g) | 地上干重 Aboveground dry weight (g) |
|---|---|---|---|---|---|---|---|
| Control | 16.32±2.09d | 1.83±0.26b | 30.62±1.60b | 8.67±1.63b | 5.17±0.59b | 6.77±1.30b | 0.48±0.05b |
| L19 | 21.32±1.61c | 2.58±0.15a | 32.46±5.10ab | 10.00±2.53a | 8.53±1.29a | 7.66±0.46a | 0.54±0.07a |
| G32 | 21.38±1.07bc | 2.44±0.21a | 33.72±2.60ab | 12.00±3.35a | 8.51±0.81a | 7.33±0.94a | 0.54±0.06a |
| NZ-4 | 22.85±1.87a | 2.32±0.37a | 35.26±2.65a | 12.00±2.76a | 8.55±1.33a | 7.97±2.07a | 0.54±0.10a |
| HN-Q-8 | 21.04±1.06abc | 2.46±0.11a | 29.04±4.50ab | 10.67±2.73a | 8.01±0.69a | 6.98±0.99a | 0.53±0.05a |
| ZD01 | 22.81±1.52ab | 2.40±0.21a | 31.75±1.31ab | 9.33±2.07a | 8.75±1.16a | 7.33±1.16a | 0.55±0.09a |
处理 Treatment | 溶解无机磷 Soluble inorganic phosphorus | 溶解有机磷 Dissolved organic phosphorus | 固氮 Nitrogen fixation | 铁载体 Siderophore |
|---|---|---|---|---|
| L19 | + | + | - | - |
| G32 | - | + | + | + |
| NZ-4 | + | + | + | + |
| HN-Q-8 | + | + | - | + |
| ZD01 | + | + | + | + |
表3 芽胞杆菌菌株的促生能力
Table 3 Growth-promoting ability of Bacillus strains
处理 Treatment | 溶解无机磷 Soluble inorganic phosphorus | 溶解有机磷 Dissolved organic phosphorus | 固氮 Nitrogen fixation | 铁载体 Siderophore |
|---|---|---|---|---|
| L19 | + | + | - | - |
| G32 | - | + | + | + |
| NZ-4 | + | + | + | + |
| HN-Q-8 | + | + | - | + |
| ZD01 | + | + | + | + |
| 处理 Treatment | 评分 Score |
|---|---|
| CK | 0.052 1 |
| HN-Q-8 | 0.508 1 |
| ZD01 | 0.634 8 |
| L19 | 0.721 7 |
| G32 | 0.798 6 |
| NZ-4 | 0.947 6 |
表4 熵值计算法结果
Table 4 Results via entropy calculation
| 处理 Treatment | 评分 Score |
|---|---|
| CK | 0.052 1 |
| HN-Q-8 | 0.508 1 |
| ZD01 | 0.634 8 |
| L19 | 0.721 7 |
| G32 | 0.798 6 |
| NZ-4 | 0.947 6 |
| 时期 Stage | 处理 Treatment | 细胞分裂素 CTK (mg/g) | 赤霉素 GA3 (mg/g) | 生长素 IAA (mg/g) |
|---|---|---|---|---|
| 15 d | Control | 1.74±0.04a | 2 680.35±334.67a | 23.60±1.75a |
| NZ-4 | 2.07±0.2a | 3 342.54±162.81a | 33.40±3.72a | |
| 25 d | Control | 2.85±0.75a | 3 353.50±597.18a | 30.10±4.84a |
| NZ-4 | 5.96±1.59c | 6 263.64±877.75c | 86.95±11.42c |
表5 贝莱斯芽胞杆菌NZ-4产生的挥发性物质对植物生长激素的影响
Table 5 Effect of VOCs produced by B. velezensis NZ-4 on plant growth hormones
| 时期 Stage | 处理 Treatment | 细胞分裂素 CTK (mg/g) | 赤霉素 GA3 (mg/g) | 生长素 IAA (mg/g) |
|---|---|---|---|---|
| 15 d | Control | 1.74±0.04a | 2 680.35±334.67a | 23.60±1.75a |
| NZ-4 | 2.07±0.2a | 3 342.54±162.81a | 33.40±3.72a | |
| 25 d | Control | 2.85±0.75a | 3 353.50±597.18a | 30.10±4.84a |
| NZ-4 | 5.96±1.59c | 6 263.64±877.75c | 86.95±11.42c |

图2 贝莱斯芽胞杆菌NZ-4产生的挥发性物质对植物生长激素的影响*和**分别表示处理间在0.05和0.01水平差异显著。下同
Fig. 2 Effects of VOCs produced by B. velezensis NZ-4 on plant growth hormones* and ** indicate significant differences between treatments at 0.05 and 0.01 levels, respectively. The same below
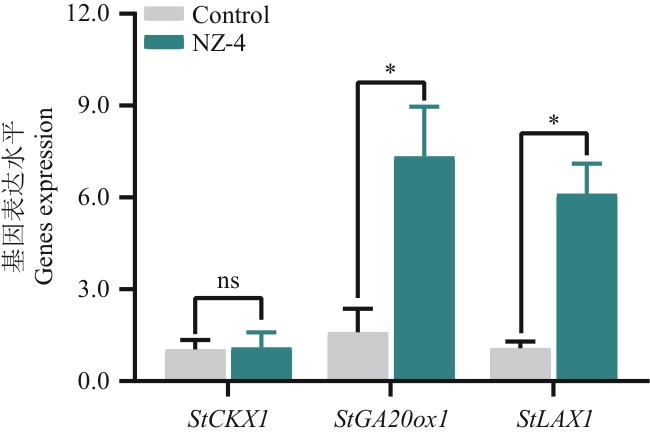
图3 贝莱斯芽胞杆菌NZ-4产生的挥发性物质处理马铃薯后细胞分裂素、赤霉素和生长素相关基因相对表达水平变化
Fig. 3 Changes in the relative expressions of genes related to cytokinin, gibberellin, and auxin in potato treated with VOCs produced by B. velezensis NZ-4
编号 No. | 英文名称 English name | 化合物 Compound | 化合物号 CAS | 分子式 Formula | 保留时间1 Retention time T1(min) | 保留时间2 Retention time T2 (s) | 峰面积值 Peak area ratio (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2-Heptanone | 2-庚酮 | 110-43-0 | C7H14O | 9.756 6 | 1.434 | 58.56 |
| 2 | 3-Ethyltoluene | 3-乙基甲苯 | 620-14-4 | C9H12 | 10.956 9 | 1.795 | 11.39 |
| 3 | 2-Nonanone | 2-壬酮 | 821-55-6 | C9H18O | 17.056 | 1.603 | 9.57 |
| 4 | 2-Decanone | 2-癸酮 | 693-54-9 | C10H20 | 19.155 9 | 1.691 | 3.25 |
| 5 | 3-Undecanone | 3-十一酮 | 2216-87-7 | C11H22 | 22.556 2 | 1.875 | 3.21 |
| 6 | 1-Octanol | 辛醇 | 111-87-5 | C8H18O | 22.156 5 | 0.791 | 2.77 |
| 7 | 3-Undecanol | 3-十一醇 | 6929-8-4 | C11H2O | 26.356 3 | 1.145 | 1.12 |
| 8 | 3-Hydroxy-3-methyl-2-butanone | 3-羟基-3-甲基-2-丁酮 | 115-22-0 | C5H10O2 | 11.756 3 | 0.645 | 1.09 |
| 9 | 1-Decanol | 1-癸醇 | 112-30-1 | C10H2O | 28.156 7 | 0.932 | 0.98 |
| 10 | Chlorobenzene | 氯苯 | 108-90-7 | C6H5Cl | 10.756 4 | 1.124 | 0.94 |
| 11 | 1,2,4,5-Tetramethylbenzene | 1,2,4,5-四甲苯 | 95-93-2 | C10H14 | 18.256 3 | 1.758 | 0.87 |
| 12 | 2-Heptanone, 5-methyl- | 5-甲基-2-庚酮 | 18217-12-4 | C8H16O | 12.256 2 | 1.575 | 0.70 |
| 13 | Dodecanal | 十二醛 | 112-54-9 | C12H24O | 28.256 9 | 1.501 | 0.62 |
| 14 | (Z)-4-decena | 顺-4- 癸烯醛 | 21662-09-9 | C10H18O | 22.256 8 | 1.428 | 0.58 |
| 15 | Pyrazine, 2,5-dimethyl-3-(3-methylbutyl) | 2,5-二甲基-3-(3-甲基丁基)吡嗪 | 18433-98-2 | C11H18N2 | 25.156 | 1.864 | 0.57 |
| 16 | Cyclohexanol | 环己醇 | 108-93-0 | C6H12O | 17.156 2 | 0.786 | 0.56 |
| 17 | 2-Hydroxy-3-pentanone | 2-羟基-3-戊酮 | 5704-20-1 | C5H10O2 | 15.656 5 | 0.65 | 0.52 |
| 18 | 2-Undecanone | 2-十一酮 | 112-12-9 | C11H22O | 24.456 9 | 1.505 | 0.50 |
| 19 | Octane,2,4,6-trimethyl- | 2,4,6-三甲基辛烷 | 62016-37-9 | C11H24 | 10.656 2 | 4.057 | 0.45 |
| 20 | 2-Cyclohexen-1-one | 2-环己烯-1-酮 | 930-68-7 | C6H8O | 18.055 8 | 1.145 | 0.35 |
| 21 | 4-Octanol | 4-辛醇 | 589-62-8 | C8H18O | 16.656 3 | 0.933 | 0.35 |
| 22 | 5-Decanone | 5-癸酮 | 820-29-1 | C10H20O | 18.156 1 | 1.937 | 0.33 |
| 23 | 2-Methyl-3,5-diethylpyrazine | 3,5-二乙基-2-甲基-吡嗪 | 18138-05-1 | C9H14N2 | 20.156 9 | 1.733 | 0.27 |
| 24 | 4-Methyl-5-thiazoleethanol | 4-甲基-5-羟乙基噻唑 | 137-00-8 | C6H9NOS | 41.156 7 | 0.574 | 0.21 |
| 25 | D-(-)-Pantolactone | D-(-)-泛酰内酯 | 599-04-2 | C6H10O3 | 34.856 | 0.527 | 0.18 |
| 26 | Phenethyl isobutyrate | 异丁酸苯乙酯 | 103-48-0 | C12H16O2 | 33.456 4 | 1.354 | 0.04 |
| 27 | Methylhydrazine | 甲基肼 | 60-34-4 | CH6N2 | 20.956 3 | 0.286 | 0.01 |
表6 贝莱斯芽胞杆菌NZ-4挥发性有机物的GC×GC-MS组分鉴定
Table 6 Identification of GC×GC-MS fractions of VOCs from B. velezensis NZ-4
编号 No. | 英文名称 English name | 化合物 Compound | 化合物号 CAS | 分子式 Formula | 保留时间1 Retention time T1(min) | 保留时间2 Retention time T2 (s) | 峰面积值 Peak area ratio (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2-Heptanone | 2-庚酮 | 110-43-0 | C7H14O | 9.756 6 | 1.434 | 58.56 |
| 2 | 3-Ethyltoluene | 3-乙基甲苯 | 620-14-4 | C9H12 | 10.956 9 | 1.795 | 11.39 |
| 3 | 2-Nonanone | 2-壬酮 | 821-55-6 | C9H18O | 17.056 | 1.603 | 9.57 |
| 4 | 2-Decanone | 2-癸酮 | 693-54-9 | C10H20 | 19.155 9 | 1.691 | 3.25 |
| 5 | 3-Undecanone | 3-十一酮 | 2216-87-7 | C11H22 | 22.556 2 | 1.875 | 3.21 |
| 6 | 1-Octanol | 辛醇 | 111-87-5 | C8H18O | 22.156 5 | 0.791 | 2.77 |
| 7 | 3-Undecanol | 3-十一醇 | 6929-8-4 | C11H2O | 26.356 3 | 1.145 | 1.12 |
| 8 | 3-Hydroxy-3-methyl-2-butanone | 3-羟基-3-甲基-2-丁酮 | 115-22-0 | C5H10O2 | 11.756 3 | 0.645 | 1.09 |
| 9 | 1-Decanol | 1-癸醇 | 112-30-1 | C10H2O | 28.156 7 | 0.932 | 0.98 |
| 10 | Chlorobenzene | 氯苯 | 108-90-7 | C6H5Cl | 10.756 4 | 1.124 | 0.94 |
| 11 | 1,2,4,5-Tetramethylbenzene | 1,2,4,5-四甲苯 | 95-93-2 | C10H14 | 18.256 3 | 1.758 | 0.87 |
| 12 | 2-Heptanone, 5-methyl- | 5-甲基-2-庚酮 | 18217-12-4 | C8H16O | 12.256 2 | 1.575 | 0.70 |
| 13 | Dodecanal | 十二醛 | 112-54-9 | C12H24O | 28.256 9 | 1.501 | 0.62 |
| 14 | (Z)-4-decena | 顺-4- 癸烯醛 | 21662-09-9 | C10H18O | 22.256 8 | 1.428 | 0.58 |
| 15 | Pyrazine, 2,5-dimethyl-3-(3-methylbutyl) | 2,5-二甲基-3-(3-甲基丁基)吡嗪 | 18433-98-2 | C11H18N2 | 25.156 | 1.864 | 0.57 |
| 16 | Cyclohexanol | 环己醇 | 108-93-0 | C6H12O | 17.156 2 | 0.786 | 0.56 |
| 17 | 2-Hydroxy-3-pentanone | 2-羟基-3-戊酮 | 5704-20-1 | C5H10O2 | 15.656 5 | 0.65 | 0.52 |
| 18 | 2-Undecanone | 2-十一酮 | 112-12-9 | C11H22O | 24.456 9 | 1.505 | 0.50 |
| 19 | Octane,2,4,6-trimethyl- | 2,4,6-三甲基辛烷 | 62016-37-9 | C11H24 | 10.656 2 | 4.057 | 0.45 |
| 20 | 2-Cyclohexen-1-one | 2-环己烯-1-酮 | 930-68-7 | C6H8O | 18.055 8 | 1.145 | 0.35 |
| 21 | 4-Octanol | 4-辛醇 | 589-62-8 | C8H18O | 16.656 3 | 0.933 | 0.35 |
| 22 | 5-Decanone | 5-癸酮 | 820-29-1 | C10H20O | 18.156 1 | 1.937 | 0.33 |
| 23 | 2-Methyl-3,5-diethylpyrazine | 3,5-二乙基-2-甲基-吡嗪 | 18138-05-1 | C9H14N2 | 20.156 9 | 1.733 | 0.27 |
| 24 | 4-Methyl-5-thiazoleethanol | 4-甲基-5-羟乙基噻唑 | 137-00-8 | C6H9NOS | 41.156 7 | 0.574 | 0.21 |
| 25 | D-(-)-Pantolactone | D-(-)-泛酰内酯 | 599-04-2 | C6H10O3 | 34.856 | 0.527 | 0.18 |
| 26 | Phenethyl isobutyrate | 异丁酸苯乙酯 | 103-48-0 | C12H16O2 | 33.456 4 | 1.354 | 0.04 |
| 27 | Methylhydrazine | 甲基肼 | 60-34-4 | CH6N2 | 20.956 3 | 0.286 | 0.01 |

图5 2-庚酮、3-乙基甲苯和2-壬酮对小麦的促生效果A:不同纯品对小麦的促生效果;B:茎长;C:根长
Fig. 5 Growth-promoting effects of 2-heptanone, 3-ethyltoluene, and 2-nonanone on wheatA: Effects of different pure compounds on wheat growth promotion. B: Stem length. C: Root length
| [1] | Thomloudi EE, Tsalgatidou PC, Baira E, et al. Genomic and metabolomic insights into secondary metabolites of the novel Bacillus halotolerans Hil4, an endophyte with promising antagonistic activity against gray mold and plant growth promoting potential [J]. Microorganisms, 2021, 9(12): 2508. |
| [2] | Li H, Guan Y, Dong YL, et al. Isolation and evaluation of endophytic Bacillus tequilensis GYLH001 with potential application for biological control of Magnaporthe oryzae [J]. PLoS One, 2018, 13(10): e0203505. |
| [3] | Zhang D, Yu SQ, Zhao DM, et al. Inhibitory effects of non-volatiles lipopeptides and volatiles ketones metabolites secreted by Bacillus velezensis C16 against Alternaria solani [J]. Biol Control, 2021, 152: 104421. |
| [4] | Morath SU, Hung R, Bennett JW. Fungal volatile organic compounds: a review with emphasis on their biotechnological potential [J]. Fungal Biol Rev, 2012, 26(2/3): 73-83. |
| [5] | Arrebola E, Sivakumar D, Korsten L. Effect of volatile compounds produced by Bacillus strains on postharvest decay in Citrus [J]. Biol Control, 2010, 53(1): 122-128. |
| [6] | Lemfack MC, Nickel J, Dunkel M, et al. mVOC: a database of microbial volatiles [J]. Nucl Acids Res, 2014, 42(D1): D744-D748. |
| [7] | Asari S, Matzén S, Petersen MA, et al. Multiple effects of Bacillus amyloliquefaciens volatile compounds: plant growth promotion and growth inhibition of phytopathogens [J]. FEMS Microbiol Ecol, 2016, 92(6): fiw070. |
| [8] | Wang Y, Li YX, Yang JL, et al. Microbial volatile organic compounds and their application in microorganism identification in foodstuff [J]. Trac Trends Anal Chem, 2016, 78: 1-16. |
| [9] | Stahl PD, Parkin TB. Microbial production of volatile organic compounds in soil microcosms [J]. Soil Sci Soc Am J, 1996, 60(3): 821-828. |
| [10] | Zhang HM, Kim MS, Krishnamachari V, et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis [J]. Planta, 2007, 226(4): 839-851. |
| [11] | Meldau DG, Long HH, Baldwin IT. A native plant growth promoting bacterium, Bacillus sp. B55, rescues growth performance of an ethylene-insensitive plant genotype in nature [J]. Front Plant Sci, 2012, 3: 112. |
| [12] | Xie XT, Zhang HM, Paré PW. Sustained growth promotion in Arabidopsis with long-term exposure to the beneficial soil bacterium Bacillus subtilis (GB03) [J]. Plant Signal Behav, 2009, 4(10): 948-953. |
| [13] | Liu HW, Brettell LE. Plant defense by VOC-induced microbial priming [J]. Trends Plant Sci, 2019, 24(3): 187-189. |
| [14] | van Agtmaal M, Straathof AL, Termorshuizen A, et al. Volatile-mediated suppression of plant pathogens is related to soil properties and microbial community composition [J]. Soil Biol Biochem, 2018, 117: 164-174. |
| [15] | Zou CS, Li ZF, Yu DQ. Bacillus megaterium strain XTBG34 promotes plant growth by producing 2-pentylfuran [J]. J Microbiol, 2010, 48(4): 460-466. |
| [16] | Meldau DG, Meldau S, Hoang LH, et al. Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition [J]. Plant Cell, 2013, 25(7): 2731-2747. |
| [17] | Zhao DY, Jiao JH, Du BH, et al. Volatile organic compounds from Lysinibacillus macroides regulating the seedling growth of Arabidopsis thaliana [J]. Physiol Mol Biol Plants, 2022, 28(11-12): 1997-2009. |
| [18] | Hao HT, Zhao X, Shang QH, et al. Comparative digital gene expression analysis of the Arabidopsis response to volatiles emitted by Bacillus amyloliquefaciens [J]. PLoS One, 2016, 11(8): e0158621. |
| [19] | Jiang CH, Xie YS, Zhu K, et al. Volatile organic compounds emitted by Bacillus sp. JC03 promote plant growth through the action of auxin and strigolactone [J]. Plant Growth Regul, 2019, 87(2): 317-328. |
| [20] | Ryu CM, Farag MA, Hu CH, et al. Bacterial volatiles promote growth in Arabidopsis [J]. Proc Natl Acad Sci USA, 2003, 100(8): 4927-4932. |
| [21] | Ramírez V, Munive JA, Cortes L, et al. Long-chain hydrocarbons (C21, C24, and C31) released by Bacillus sp. MH778713 break dormancy of mesquite seeds subjected to chromium stress [J]. Front Microbiol, 2020, 11: 741. |
| [22] | Cordovez V, Carrion VJ, Etalo DW, et al. Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil [J]. Front Microbiol, 2015, 6: 1081. |
| [23] | Effah E, Holopainen JK, McCormick AC. Potential roles of volatile organic compounds in plant competition [J]. Perspect Plant Ecol Evol Syst, 2019, 38: 58-63. |
| [24] | 强然, 张岱, 杨喆, 等. 解淀粉芽胞杆菌L19对马铃薯枯萎病菌的抑制及植株的促生作用 [J]. 园艺学报, 2024, 51(4): 875-892. |
| Qiang R, Zhang D, Yang Z, et al. Inhibition of potato Fusarium wilt and growth promotion by Bacillus amyloliquefaciens L19 [J]. Acta Horticulturae Sinica, 2024, 51(4): 875-892. | |
| [25] | 李铮, 王金辉, 丁丽丽, 等. 贝莱斯芽孢杆菌菌株NZ-4生防潜能及基因组学分析 [J]. 江苏农业科学, 2023,51(2): 117-125. |
| Li Z, Wang JH, Ding LL, et al. Biocontrol potential and genomic analysis of Bacillus velezensis strain NZ-4 [J]. Jiangsu Agricultural Sciences, 2023, 51(2): 117-125. | |
| [26] | 张岱. 枯草芽胞杆菌ZD01次级代谢产物对马铃薯早疫病菌的抑制机理 [D]. 保定: 河北农业大学, 2023. |
| Zhang D. The antifungal mechanism of secondary metabolites produced by Bacillus subtilis ZD01 against Alternaria solani [D]. Baoding: Hebei Agricultural University, 2023. | |
| [27] | 高学策, 张岱, 魏笑薇, 等. 马铃薯黑痣病生防芽胞杆菌的筛选及其次级代谢产物的抑菌特性 [J]. 西南农业学报, 2023, 36(9): 1942-1949. |
| Gao XC, Zhang D, Wei XW, et al. Screening of biocontrol Bacillus against potato black scurf and antimicrobial characteristics of its secondary metabolites [J]. Southwest China Journal of Agricultural Sciences, 2023, 36(9): 1942-1949. | |
| [28] | 朱明明, 张岱, 赵冬梅, 等. 马铃薯黑痣病生防芽孢杆菌的筛选与鉴定 [J]. 江苏农业科学, 2018, 46(14): 97-101. |
| Zhu MM, Zhang D, Zhao DM, et al. Screening and identification of antagonistic Bacillus against potato black scurf [J]. Jiangsu Agric Sci, 2018, 46(14): 97-101. | |
| [29] | Zhao J, Zhou ZJ, Bai XF, et al. A novel of new class II bacteriocin from Bacillus velezensis HN-Q-8 and its antibacterial activity on Streptomyces scabies [J]. Front Microbiol, 2022, 13: 943232. |
| [30] | 宁楚涵, 李文彬, 张晨, 等. 定殖植物根内和根围放线菌的分离鉴定及其体外抑菌促生效应 [J]. 微生物学报, 2019, 59(10): 2024-2037. |
| Ning CH, Li WB, Zhang C, et al. Isolation and identification of antagonizing and growthpromoting Actinobacteria colonized in plant roots and rhizosphere [J]. Acta Microbiol Sin, 2019, 59(10): 2024-2037. | |
| [31] | 刘泽平, 王志刚, 徐伟慧, 等. 水稻根际促生菌的筛选鉴定及促生能力分析 [J]. 农业资源与环境学报, 2018, 35(2): 119-125. |
| Liu ZP, Wang ZG, Xu WH, et al. Screen, identification and analysis on the growth-promoting ability for the rice growth-promoting rhizobacteria [J]. J Agric Resour Environ, 2018, 35(2): 119-125. | |
| [32] | Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores [J]. Anal Biochem, 1987, 160(1): 47-56. |
| [33] | 朱喜安, 魏国栋. 熵值法中无量纲化方法优良标准的探讨 [J]. 统计与决策, 2015, 31(2): 12-15. |
| Zhu XA, Wei GD. Discussion on the excellent standard of dimensionless method in entropy method [J]. Stat Decis, 2015, 31(2): 12-15. | |
| [34] | 罗梦娜. 生长调节剂对小麦和玉米内源激素的影响 [D]. 杨凌: 西北农林科技大学, 2019. |
| Luo MN. Effect of growth regulator on endogenous hormones in wheat and maize [D]. Yangling: Northwest A & F University, 2019. | |
| [35] | 李葵秀, 罗崇玉, 傅琪景, 等. 马铃薯StDRO2基因的生物信息学分析 [J]. 西南农业学报, 2021, 34(4): 679-688. |
| Li KX, Luo CY, Fu QJ, et al. Bioinformatics analysis of potato StDRO2 gene [J]. Southwest China J Agric Sci, 2021, 34(4): 679-688. | |
| [36] | Syed-Ab-Rahman SF, Carvalhais LC, Chua ET, et al. Soil bacterial diffusible and volatile organic compounds inhibit Phytophthora capsici and promote plant growth [J]. Sci Total Environ, 2019, 692: 267-280. |
| [37] | Ghazala I, Chiab N, Saidi MN, et al. Volatile organic compounds from Bacillus mojavensis I4 promote plant growth and inhibit phytopathogens [J]. Physiol Mol Plant Pathol, 2022, 121: 101887. |
| [38] | He AL, Zhao LY, Ren W, et al. A volatile producing Bacillus subtilis strain from the rhizosphere of Haloxylon ammodendron promotes plant root development [J]. Plant Soil, 2023, 486(1): 661-680. |
| [39] | Rath M, Mitchell TR, Gold SE. Volatiles produced by Bacillus mojavensis RRC101 act as plant growth modulators and are strongly culture-dependent [J]. Microbiol Res, 2018, 208: 76-84. |
| [40] | Fincheira P, Venthur H, Mutis A, et al. Growth promotion of Lactuca sativa in response to volatile organic compounds emitted from diverse bacterial species [J]. Microbiol Res, 2016, 193: 39-47. |
| [41] | Byrne ME. Making leaves [J]. Curr Opin Plant Biol, 2012, 15(1): 24-30. |
| [42] | Dudareva N, Negre F, Nagegowda DA, et al. Plant volatiles: recent advances and future perspectives [J]. Crit Rev Plant Sci, 2006, 25(5): 417-440. |
| [43] | Zou XL, Ning JQ, Zhao XJ, et al. Bacillus velezensis LY7 promotes pepper growth and induces resistance to Colletotrichum scovillei [J]. Biol Control, 2024, 192: 105480. |
| [44] | Tahir HAS, Gu Q, Wu HJ, et al. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2 [J]. Front Microbiol, 2017, 8: 171. |
| [45] | Kwon YS, Ryu CM, Lee S, et al. Proteome analysis of Arabidopsis seedlings exposed to bacterial volatiles [J]. Planta, 2010, 232(6): 1355-1370. |
| [46] | Ren FF, Liu N, Gao B, et al. Identification of Stutzerimonas stutzeri volatile organic compounds that enhance the colonization and promote tomato seedling growth [J]. J Appl Microbiol, 2024, 135(10): lxae248. |
| [47] | Wu YC, Zhou JY, Li CG, et al. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens [J]. Microbiologyopen, 2019, 8(8): e00813. |
| [48] | Cole LK, Blum MS. Antifungal properties of the insect alarm pheromones, citral, 2-heptanone, and 4-methyl-3-heptanone [J]. Mycologia, 1975, 67(4): 701-708. |
| [49] | Zhu M, Chen Y, Zhao NH, et al. Multiple olfactory pathways contribute to the lure process of Caenorhabditis elegans by pathogenic bacteria [J]. Sci China Life Sci, 2021, 64(8): 1346-1354. |
| [1] | 白雨果, 李婉迪, 梁建萍, 石志勇, 卢庚龙, 刘红军, 牛景萍. 哈茨木霉T9131对黄芪幼苗的促生机理[J]. 生物技术通报, 2025, 41(8): 175-185. |
| [2] | 孙孟雪, 张艺潆, 徐鹏, 孙卓, 王云贺, 韩忠明. 防风内生细菌的分离鉴定及其促生特性分析[J]. 生物技术通报, 2025, 41(7): 299-311. |
| [3] | 张钧杰, 刘爽, 胡明珠, 石雪瑞, 代金霞. 荒漠植物根际土壤固氮微生物的筛选及其抗逆促生特性[J]. 生物技术通报, 2025, 41(6): 317-326. |
| [4] | 张慧, 卢文才, 王冬, 刘倩, 马连杰. 一株高产吲哚乙酸的Bacillus cereus YT2-1C的鉴定及促生作用[J]. 生物技术通报, 2025, 41(5): 300-309. |
| [5] | 夏馨媛, 薛道晟, 李鑫静, 龙俊杰, 陆开形, 丁沃娜, 李梦莎. 稻油轮作土壤多功能促生菌的鉴定及其对油菜生长和根际细菌群落的影响[J]. 生物技术通报, 2025, 41(4): 289-301. |
| [6] | 马耀武, 张麒宇, 杨淼, 蒋诚, 张振宇, 张伊琳, 李梦莎, 许嘉阳, 张斌, 崔光周, 姜瑛. 烟草根际促生菌的筛选鉴定及促生性能研究[J]. 生物技术通报, 2025, 41(3): 271-281. |
| [7] | 刘克寒, 杨升辉, 黄巧云, 崔文靖. 黑龙江大豆根瘤菌及根际促共生菌株的筛选及应用[J]. 生物技术通报, 2025, 41(1): 252-262. |
| [8] | 张婷, 万雨欣, 徐伟慧, 王志刚, 陈文晶, 胡云龙. 一株玉米根际促生菌Leclercia adecarboxylata LN01促生效果研究及其基因组分析[J]. 生物技术通报, 2025, 41(1): 263-275. |
| [9] | 刘文志, 贺丹, 李鹏, 傅应林, 张译心, 温华杰, 于文清. 多粘类芽胞杆菌新菌株X-11及其对番茄和水稻的促生效应[J]. 生物技术通报, 2024, 40(9): 249-259. |
| [10] | 杜仲阳, 杨泽, 梁梦静, 刘义珍, 崔红利, 史达明, 薛金爱, 孙岩, 张春辉, 季春丽, 李润植. 纳米硒(SeNPs)缓解烟草幼苗铅胁迫和促生效应[J]. 生物技术通报, 2024, 40(7): 183-196. |
| [11] | 范宗强, 冯靖涵, 郑丽雪, 王硕, 彭向前, 陈芳. 枯草芽孢杆菌B579对黄瓜枯萎病的防治及其诱导抗性研究[J]. 生物技术通报, 2024, 40(7): 226-234. |
| [12] | 孙亚楠, 王春雪, 王欣, 杜秉海, 刘凯, 汪城墙. 萎缩芽孢杆菌CNY01的生防特性及其对玉米的抗盐促生作用[J]. 生物技术通报, 2024, 40(5): 248-260. |
| [13] | 王佳玮, 李晨, 刘建利, 周世杰, 易嘉敏, 杨谨源, 康鹏. 内生真菌接种方式对青贮玉米幼苗生长的影响[J]. 生物技术通报, 2024, 40(4): 189-202. |
| [14] | 高志伟, 魏明, 于祖隆, 伍国强, 魏俊龙. 耐盐植物促生菌W-1鉴定及其对红豆草耐盐性的影响[J]. 生物技术通报, 2024, 40(4): 217-227. |
| [15] | 李雪, 李容欧, 孔美懿, 黄磊. 解淀粉芽孢杆菌SQ-2对水稻的促生作用[J]. 生物技术通报, 2024, 40(2): 109-119. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||