








生物技术通报 ›› 2025, Vol. 41 ›› Issue (10): 277-291.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0302
冀梦然( ), 张瑞英, 刘红丹, 冯伟萌, 刘秀玉, 马蕊(
), 张瑞英, 刘红丹, 冯伟萌, 刘秀玉, 马蕊( ), 陈随清(
), 陈随清( )
)
收稿日期:2025-03-20
出版日期:2025-10-26
发布日期:2025-10-28
通讯作者:
马蕊,女,博士,讲师,研究方向 :中药资源与质量评价;E-mail: ruimazy@163.com作者简介:冀梦然,女,硕士研究生,研究方向 :中药资源与质量评价;E-mail: r851368300@163.com
基金资助:
JI Meng-ran( ), ZHANG Rui-ying, LIU Hong-dan, FENG Wei-meng, LIU Xiu-yu, MA Rui(
), ZHANG Rui-ying, LIU Hong-dan, FENG Wei-meng, LIU Xiu-yu, MA Rui( ), CHEN Sui-qing(
), CHEN Sui-qing( )
)
Received:2025-03-20
Published:2025-10-26
Online:2025-10-28
摘要:
目的 探究南阳艾在生长发育过程中萜类成分的变化规律,解析其萜类化合物在积累中的分子催化机制。 方法 采用气相色谱-质谱联用技术(GC-MS)和PacBio平台的单分子实时测序技术(single molecule real-time, SMRT)对南阳艾的嫩叶和老叶进行代谢组学和转录组学联合分析。 结果 嫩叶与老叶代谢组学分析共得到150个差异代谢物,包括萜类(33个)、杂环化合物(26个)和酯类(24个)等,其中老叶相对于嫩叶有149个下调代谢物和1个上调代谢物,共有4个差异代谢物富集到与萜类生物合成有关的代谢通路中。转录组学结果表明,嫩叶和老叶的差异基因有711个,其中406个上调,305个下调,有2个差异基因注释到与萜类合成相关的途径。有12种特定的萜类代谢物参与萜类生物合成,与之相关的关键基因主要有1-脱氧-D-木酮糖-5-磷酸合酶(1-deoxy-D-xylulose-5-phosphate synthase, DXS)、牻牛儿基牻牛儿基焦磷酸合酶(geranylgeranyl pyrophosphate synthase, GGPS)等。 结论 南阳艾嫩叶与老叶的基因表达和代谢水平具有一定差异,但在萜类生物合成途径上基因的表达量差异不大,推测原因为同一时期同一株植物不同发育程度的叶片基因表达差异程度较小。
冀梦然, 张瑞英, 刘红丹, 冯伟萌, 刘秀玉, 马蕊, 陈随清. 转录组与代谢组联合分析南阳艾嫩叶与老叶的萜类成分差异[J]. 生物技术通报, 2025, 41(10): 277-291.
JI Meng-ran, ZHANG Rui-ying, LIU Hong-dan, FENG Wei-meng, LIU Xiu-yu, MA Rui, CHEN Sui-qing. Combined Metabolome and Transcriptome Analysis of the Differences in Terpenoids between New and Old Leaves of Artemisia argyi H. Lév. & Vaniot[J]. Biotechnology Bulletin, 2025, 41(10): 277-291.

图1 南阳艾嫩叶(NS)与老叶(NX)图中从左到右分别为嫩叶(正面)、老叶(正面)、嫩叶(背面)、老叶(背面)
Fig. 1 New leaves and old leaves of A. argyi.New leaves (front), old leaves (front), new leaves (underside), and old leaves (underside) from left to right
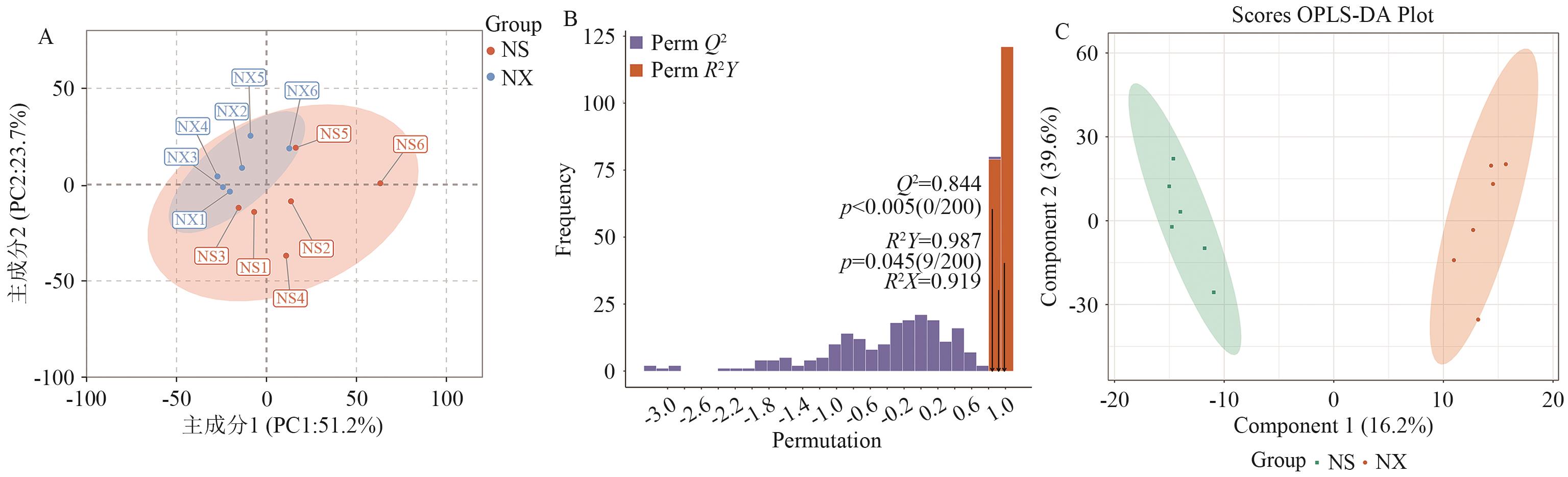
图2 主成分分析与正交-偏最小二乘判别分析A:代谢物主成分分析;B:OPLS-DA模拟验证图;C:OPLS-DA得分图。图A中同一颜色为同一个组别,数字1-6为同组的6个不同重复,如NS1是指嫩叶的第1个重复
Fig. 2 Principal component analysis and orthogonal partial least squares discriminant analysisA: Principal component analysis of metabolites; B: OPLS-DA simulation verification diagram; C: OPLS-DA score graph. The same color in the figure A refers to the same group. Numbers 1 to 6 indicate six different repetitions in the same group, For example, NS1 refers to the first repetition of the new leaf
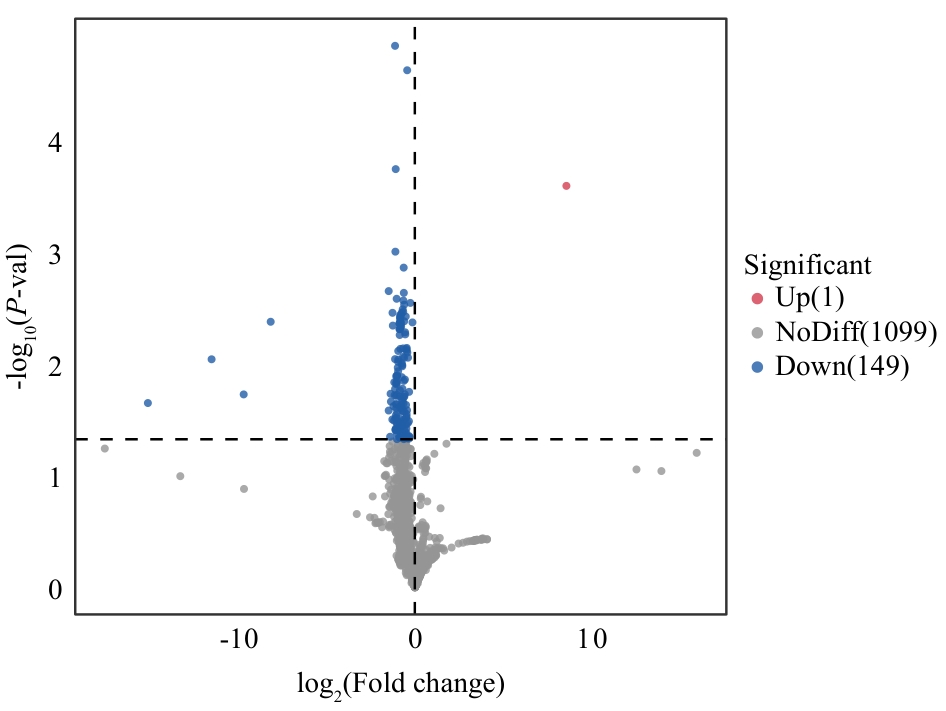
图3 差异代谢物火山图图中红色为上调代谢物(1个),蓝色为下调代谢物(149个),灰色表没有显著差异的代谢物(1 099个)
Fig. 3 Volcano map of differential metabolite componentsIn the figure, red indicates upregulated metabolites (1), blue indicates downregulated metabolites (149), and gray indicates metabolites with no significant differences (1 099)
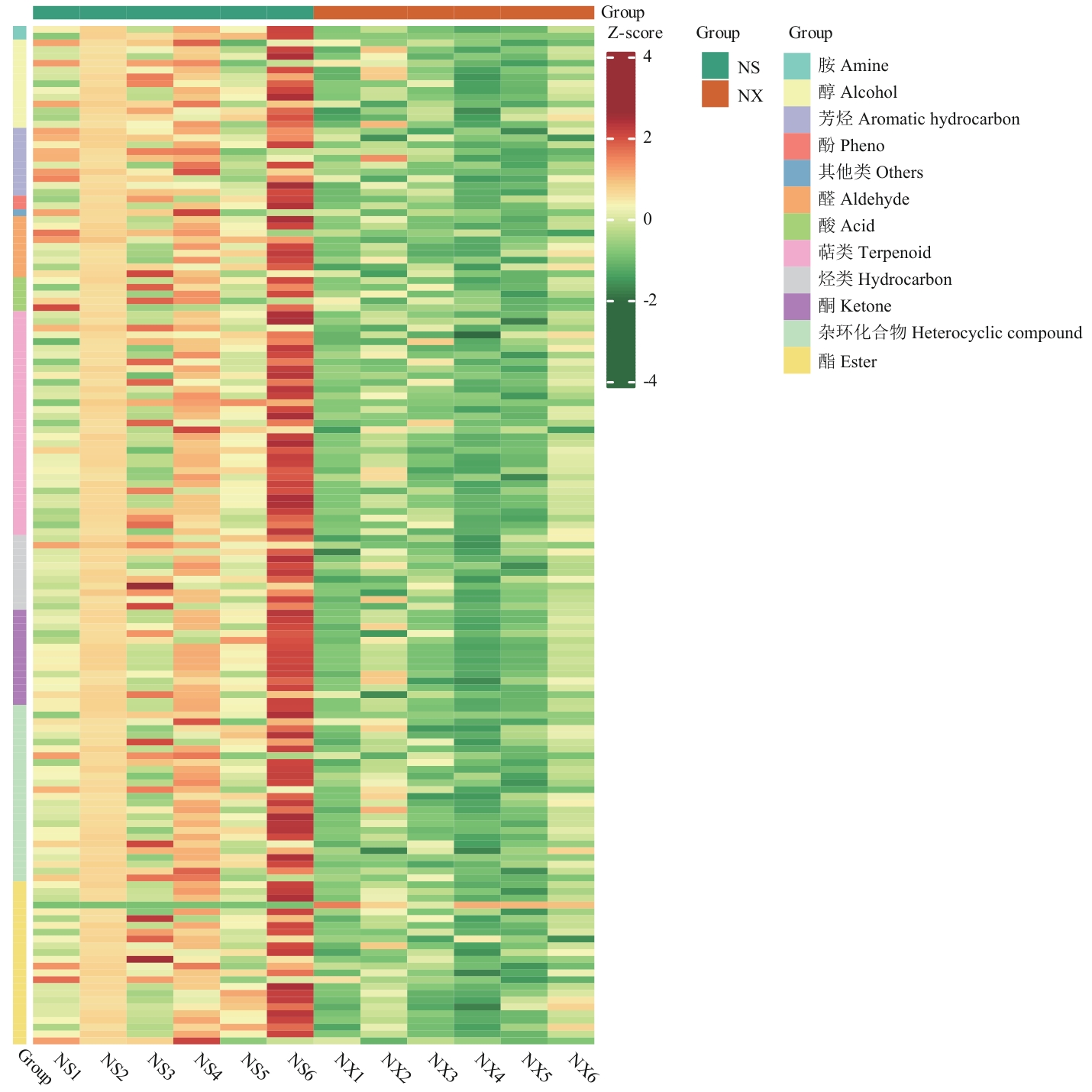
图4 差异代谢物热图NS1-6为嫩叶差异代谢物,NX1-6为老叶差异代谢物;纵坐标为代谢物类别
Fig. 4 Heatmap of differential metaboliteNS1-6 are differential metabolites of new leaves, and NX1-6 are differential metabolites of old leaves. The ordinate is the metabolite category
序号 No. | 分子式 Molecular formula | 代谢物中文名 Metabolite Chinese name | 代谢物英文名 Metabolite English name | CAS | VIP | P_value |
|---|---|---|---|---|---|---|
| 1 | C15H24 | (4R,4aR,8aR)-十氢-1,6-双(亚甲基)-4-(1-甲基乙基)-萘 | Naphthalene,decahydro-1,6-bis(methylene)-4-(1-methylethyl)-,(4R,4aR,8aR)-rel- | 30021-46-6 | 1.91 | 0.01 |
| 2 | C15H26O | 阔叶缬草醚 | Kessane | 3321-66-2 | 1.36 | 0.05 |
| 3 | C10H16 | α-水芹烯 | α-phellandrene | 99-83-2 | 2.10 | 0.00 |
| 4 | C10H18O | (2R,5R)-2-甲基-5-丙烷-2-基双环(3.1.0)己烷-2-醇 | (2S,5R)-2-methyl-5-propan-2-ylbicyclo(3.1.0)hexan-2-ol | 17699-16-0 | 1.67 | 0.02 |
| 5 | C10H18O | 反式-5-甲基-2-(1-甲基乙基)-环己酮 | Cyclohexanone, 5-methyl-2-(1-methylethyl)-, trans- | 89-80-5 | 1.37 | 0.04 |
| 6 | C15H24 | α-古巴烯 | Copaene | 3856-25-5 | 1.50 | 0.05 |
| 7 | C15H24 | β-瑟林烯 | Naphthalene, decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-, (4aR, 7R, 8aS)- | 17066-67-0 | 1.56 | 0.03 |
| 8 | C15H24 | γ-芹子烯 | (4aR-trans)-decahydro-4a-methyl-1-methylene-7-(1-methylethylidene)-Naphthalene | 515-17-3 | 1.50 | 0.05 |
| 9 | C15H22 | α-姜黄烯 | Benzene, 1-(1, 5-dimethyl-4-hexenyl)-4-methyl- | 644-30-4 | 1.90 | 0.01 |
| 10 | C15H22 | 菖蒲烯 | Naphthalene, 1, 2, 3, 4-tetrahydro-1, 6-dimethyl-4-(1-methylethyl)-, (1S-cis)- | 483-77-2 | 1.58 | 0.03 |
| 11 | C13H20O2 | 4-(2,2,6-三甲基-7-氧杂二环[4.1.0]庚-1-基)-3-丁烯-2-酮 | 3-Buten-2-one, 4-(2, 2, 6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)- | 23267-57-4 | 1.54 | 0.04 |
| 12 | C15H24 | 7,11-二甲基-3-亚甲基十二烷-1,6,10-三烯 | 7, 11-dimethyl-3-methylenedodeca-1, 6, 10-triene | 77129-48-7 | 1.94 | 0.01 |
| 13 | C10H14O2 | 紫苏酸 | 1-Cyclohexene-1-carboxylic acid, 4-(1-methylethenyl)- | 7694-45-3 | 1.54 | 0.03 |
| 14 | C14H16 | 母菊薁 | Chamazulene | 529-05-5 | 2.04 | 0.00 |
| 15 | C15H24 | (+)-β-柏木烯 | 1H-3a, 7-Methanoazulene, octahydro-3, 8, 8-trimethyl-6-methylene-, (3R, 3aS, 7S, 8aS)- | 546-28-1 | 1.85 | 0.01 |
| 16 | C15H24 | (+)-表-双环倍半水芹烯 | (+)-Epi-bicyclosesquiphellandrene | 54274-73-6 | 1.75 | 0.02 |
| 17 | C10H16O | 松樟酮 | Pinocamphone | 547-60-4 | 1.58 | 0.04 |
| 18 | C20H32 | (4aS,8S,8aS)-4,4,8a-三甲基-7-亚甲基-8-(3-甲基戊-2,4-二烯基)-2,3,4a,5,6,8-六氢-1H-萘 | (4aS, 8S, 8aS)-4, 4, 8a-trimethyl-7-methylidene-8-(3-methylpenta-2, 4-dienyl)-2, 3, 4a, 5, 6, 8-hexahydro-1H-naphthalene | 5957-33-5 | 1.23 | 0.03 |
| 19 | C15H24 | 雪松烯 | Cedrene | 11028-42-5 | 2.03 | 0.00 |
| 20 | C10H16O2 | 伊蚁内酯 | Iridomyrmecin | 485-43-8 | 1.90 | 0.02 |
| 21 | C15H20 | α-脱氢芳烃雪松烯 | α-Dehydro-ar-himachalene | 78204-62-3 | 1.70 | 0.01 |
| 22 | C15H24 | α-香柠檬烯 | α-Bergamotene | 17699-05-7 | 1.84 | 0.01 |
| 23 | C15H24 | (1R,3aS,8aS)-7-异丙基-1,4-二甲基-1,2,3,3a,6,8a-六氢茚 | (1R, 3aS, 8aS)-7-Isopropyl-1, 4-dimethyl-1, 2, 3, 3a, 6, 8a-hexahydroazulene | 36577-33-0 | 1.86 | 0.01 |
| 24 | C10H16O | 2-(4-甲基-2,4-环己二烯)-2-丙醇 | 2-(4-Methyl-2, 4-cyclohexadienyl)-2-propanol | 1686-20-0 | 1.59 | 0.03 |
| 25 | C15H24 | [1S-(1R*,9S*)]10,10-二甲基-2,6-双(亚甲基)-双环[7.2.0]十一烷 | Bicyclo[7.2.0]undecane, 10, 10-dimethyl-2, 6-bis(methylene)-, [1S-(1R*, 9S*)]- | 136296-38-3 | 1.38 | 0.05 |
| 26 | C15H24 | 10,10-二甲基-2,6-二亚甲基双环[7.2.0]十一烷 | 10, 10-Dimethyl-2, 6-dimethylenebicyclo[7.2.0]undecane | 357414-37-0 | 1.86 | 0.01 |
| 27 | C10H16O | 5-甲基-2-(1-甲基亚乙基)-环己酮 | Cyclohexanone, 5-methyl-2-(1-methylethylidene)- | 15932-80-6 | 1.80 | 0.02 |
| 28 | C15H24 | (1S,4S,4aR)-1-异丙基-4-甲基-7-亚甲基-1,2,3,4,4a,5,6,7-八氢萘 | (1S, 4S, 4aR)-1-Isopropyl-4-methyl-7-methylene-1, 2, 3, 4, 4a, 5, 6, 7-octahydronaphthalene | 157477-72-0 | 1.85 | 0.02 |
| 29 | C15H24 | 缬草-4,7(11)-二烯 | Valerena-4, 7(11)-diene | 351222-66-7 | 1.88 | 0.02 |
| 30 | C15H24 | (4R,4aS,6S)-4,4a-二甲基-6-(丙-1-烯-2-基)-1,2,3,4,4a,5,6,7-八氢萘 | (4R, 4aS, 6S)-4, 4a-Dimethyl-6-(prop-1-en-2-yl)- 1, 2, 3, 4, 4a, 5, 6, 7-octahydronaphthalene | 823810-22-6 | 1.83 | 0.01 |
| 31 | C15H24 | (1R,3aR,4aR,8aR)-1,4,4,6-四甲基-1,2,3,3a,4,4a,7,8-八氢环戊烯[ | (1R, 3aR, 4aR, 8aR)-1, 4, 4, 6-Tetramethyl-1, 2, 3, 3a, 4, 4a, 7, 8-octahydrocyclopenta[ | 94482-89-0 | 1.70 | 0.02 |
| 32 | C15H22 | (2R,4aS)-4a,8-二甲基-2-(丙-1-烯-2-基)-1,2,3,4,4a,5-六氢萘 | (2R, 4aS)-4a, 8-Dimethyl-2-(prop-1-en-2-yl)-1, 2, 3, 4, 4a, 5-hexahydronaphthalene | 82462-31-5 | 1.64 | 0.03 |
| 33 | C15H24 | 2,6,6,8-四甲基三环[5.3.1.01,5]十一碳-9-烯 | 2, 6, 6, 8-tetramethyltricyclo[5.3.1.01,5]undec-9-ene | 21996-77-0 | 1.54 | 0.05 |
表1 萜类差异代谢物
Table 1 Terpenoid differential metabolites
序号 No. | 分子式 Molecular formula | 代谢物中文名 Metabolite Chinese name | 代谢物英文名 Metabolite English name | CAS | VIP | P_value |
|---|---|---|---|---|---|---|
| 1 | C15H24 | (4R,4aR,8aR)-十氢-1,6-双(亚甲基)-4-(1-甲基乙基)-萘 | Naphthalene,decahydro-1,6-bis(methylene)-4-(1-methylethyl)-,(4R,4aR,8aR)-rel- | 30021-46-6 | 1.91 | 0.01 |
| 2 | C15H26O | 阔叶缬草醚 | Kessane | 3321-66-2 | 1.36 | 0.05 |
| 3 | C10H16 | α-水芹烯 | α-phellandrene | 99-83-2 | 2.10 | 0.00 |
| 4 | C10H18O | (2R,5R)-2-甲基-5-丙烷-2-基双环(3.1.0)己烷-2-醇 | (2S,5R)-2-methyl-5-propan-2-ylbicyclo(3.1.0)hexan-2-ol | 17699-16-0 | 1.67 | 0.02 |
| 5 | C10H18O | 反式-5-甲基-2-(1-甲基乙基)-环己酮 | Cyclohexanone, 5-methyl-2-(1-methylethyl)-, trans- | 89-80-5 | 1.37 | 0.04 |
| 6 | C15H24 | α-古巴烯 | Copaene | 3856-25-5 | 1.50 | 0.05 |
| 7 | C15H24 | β-瑟林烯 | Naphthalene, decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-, (4aR, 7R, 8aS)- | 17066-67-0 | 1.56 | 0.03 |
| 8 | C15H24 | γ-芹子烯 | (4aR-trans)-decahydro-4a-methyl-1-methylene-7-(1-methylethylidene)-Naphthalene | 515-17-3 | 1.50 | 0.05 |
| 9 | C15H22 | α-姜黄烯 | Benzene, 1-(1, 5-dimethyl-4-hexenyl)-4-methyl- | 644-30-4 | 1.90 | 0.01 |
| 10 | C15H22 | 菖蒲烯 | Naphthalene, 1, 2, 3, 4-tetrahydro-1, 6-dimethyl-4-(1-methylethyl)-, (1S-cis)- | 483-77-2 | 1.58 | 0.03 |
| 11 | C13H20O2 | 4-(2,2,6-三甲基-7-氧杂二环[4.1.0]庚-1-基)-3-丁烯-2-酮 | 3-Buten-2-one, 4-(2, 2, 6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)- | 23267-57-4 | 1.54 | 0.04 |
| 12 | C15H24 | 7,11-二甲基-3-亚甲基十二烷-1,6,10-三烯 | 7, 11-dimethyl-3-methylenedodeca-1, 6, 10-triene | 77129-48-7 | 1.94 | 0.01 |
| 13 | C10H14O2 | 紫苏酸 | 1-Cyclohexene-1-carboxylic acid, 4-(1-methylethenyl)- | 7694-45-3 | 1.54 | 0.03 |
| 14 | C14H16 | 母菊薁 | Chamazulene | 529-05-5 | 2.04 | 0.00 |
| 15 | C15H24 | (+)-β-柏木烯 | 1H-3a, 7-Methanoazulene, octahydro-3, 8, 8-trimethyl-6-methylene-, (3R, 3aS, 7S, 8aS)- | 546-28-1 | 1.85 | 0.01 |
| 16 | C15H24 | (+)-表-双环倍半水芹烯 | (+)-Epi-bicyclosesquiphellandrene | 54274-73-6 | 1.75 | 0.02 |
| 17 | C10H16O | 松樟酮 | Pinocamphone | 547-60-4 | 1.58 | 0.04 |
| 18 | C20H32 | (4aS,8S,8aS)-4,4,8a-三甲基-7-亚甲基-8-(3-甲基戊-2,4-二烯基)-2,3,4a,5,6,8-六氢-1H-萘 | (4aS, 8S, 8aS)-4, 4, 8a-trimethyl-7-methylidene-8-(3-methylpenta-2, 4-dienyl)-2, 3, 4a, 5, 6, 8-hexahydro-1H-naphthalene | 5957-33-5 | 1.23 | 0.03 |
| 19 | C15H24 | 雪松烯 | Cedrene | 11028-42-5 | 2.03 | 0.00 |
| 20 | C10H16O2 | 伊蚁内酯 | Iridomyrmecin | 485-43-8 | 1.90 | 0.02 |
| 21 | C15H20 | α-脱氢芳烃雪松烯 | α-Dehydro-ar-himachalene | 78204-62-3 | 1.70 | 0.01 |
| 22 | C15H24 | α-香柠檬烯 | α-Bergamotene | 17699-05-7 | 1.84 | 0.01 |
| 23 | C15H24 | (1R,3aS,8aS)-7-异丙基-1,4-二甲基-1,2,3,3a,6,8a-六氢茚 | (1R, 3aS, 8aS)-7-Isopropyl-1, 4-dimethyl-1, 2, 3, 3a, 6, 8a-hexahydroazulene | 36577-33-0 | 1.86 | 0.01 |
| 24 | C10H16O | 2-(4-甲基-2,4-环己二烯)-2-丙醇 | 2-(4-Methyl-2, 4-cyclohexadienyl)-2-propanol | 1686-20-0 | 1.59 | 0.03 |
| 25 | C15H24 | [1S-(1R*,9S*)]10,10-二甲基-2,6-双(亚甲基)-双环[7.2.0]十一烷 | Bicyclo[7.2.0]undecane, 10, 10-dimethyl-2, 6-bis(methylene)-, [1S-(1R*, 9S*)]- | 136296-38-3 | 1.38 | 0.05 |
| 26 | C15H24 | 10,10-二甲基-2,6-二亚甲基双环[7.2.0]十一烷 | 10, 10-Dimethyl-2, 6-dimethylenebicyclo[7.2.0]undecane | 357414-37-0 | 1.86 | 0.01 |
| 27 | C10H16O | 5-甲基-2-(1-甲基亚乙基)-环己酮 | Cyclohexanone, 5-methyl-2-(1-methylethylidene)- | 15932-80-6 | 1.80 | 0.02 |
| 28 | C15H24 | (1S,4S,4aR)-1-异丙基-4-甲基-7-亚甲基-1,2,3,4,4a,5,6,7-八氢萘 | (1S, 4S, 4aR)-1-Isopropyl-4-methyl-7-methylene-1, 2, 3, 4, 4a, 5, 6, 7-octahydronaphthalene | 157477-72-0 | 1.85 | 0.02 |
| 29 | C15H24 | 缬草-4,7(11)-二烯 | Valerena-4, 7(11)-diene | 351222-66-7 | 1.88 | 0.02 |
| 30 | C15H24 | (4R,4aS,6S)-4,4a-二甲基-6-(丙-1-烯-2-基)-1,2,3,4,4a,5,6,7-八氢萘 | (4R, 4aS, 6S)-4, 4a-Dimethyl-6-(prop-1-en-2-yl)- 1, 2, 3, 4, 4a, 5, 6, 7-octahydronaphthalene | 823810-22-6 | 1.83 | 0.01 |
| 31 | C15H24 | (1R,3aR,4aR,8aR)-1,4,4,6-四甲基-1,2,3,3a,4,4a,7,8-八氢环戊烯[ | (1R, 3aR, 4aR, 8aR)-1, 4, 4, 6-Tetramethyl-1, 2, 3, 3a, 4, 4a, 7, 8-octahydrocyclopenta[ | 94482-89-0 | 1.70 | 0.02 |
| 32 | C15H22 | (2R,4aS)-4a,8-二甲基-2-(丙-1-烯-2-基)-1,2,3,4,4a,5-六氢萘 | (2R, 4aS)-4a, 8-Dimethyl-2-(prop-1-en-2-yl)-1, 2, 3, 4, 4a, 5-hexahydronaphthalene | 82462-31-5 | 1.64 | 0.03 |
| 33 | C15H24 | 2,6,6,8-四甲基三环[5.3.1.01,5]十一碳-9-烯 | 2, 6, 6, 8-tetramethyltricyclo[5.3.1.01,5]undec-9-ene | 21996-77-0 | 1.54 | 0.05 |
| Sample | Length (bp) |
|---|---|
| Min-length | 59 |
| Max-length | 5 317 |
| Mean-length | 1 666 |
| N50 | 1 792 |
表2 Polished consensus统计结果
Table 2 Statistical results of polished consensus
| Sample | Length (bp) |
|---|---|
| Min-length | 59 |
| Max-length | 5 317 |
| Mean-length | 1 666 |
| N50 | 1 792 |
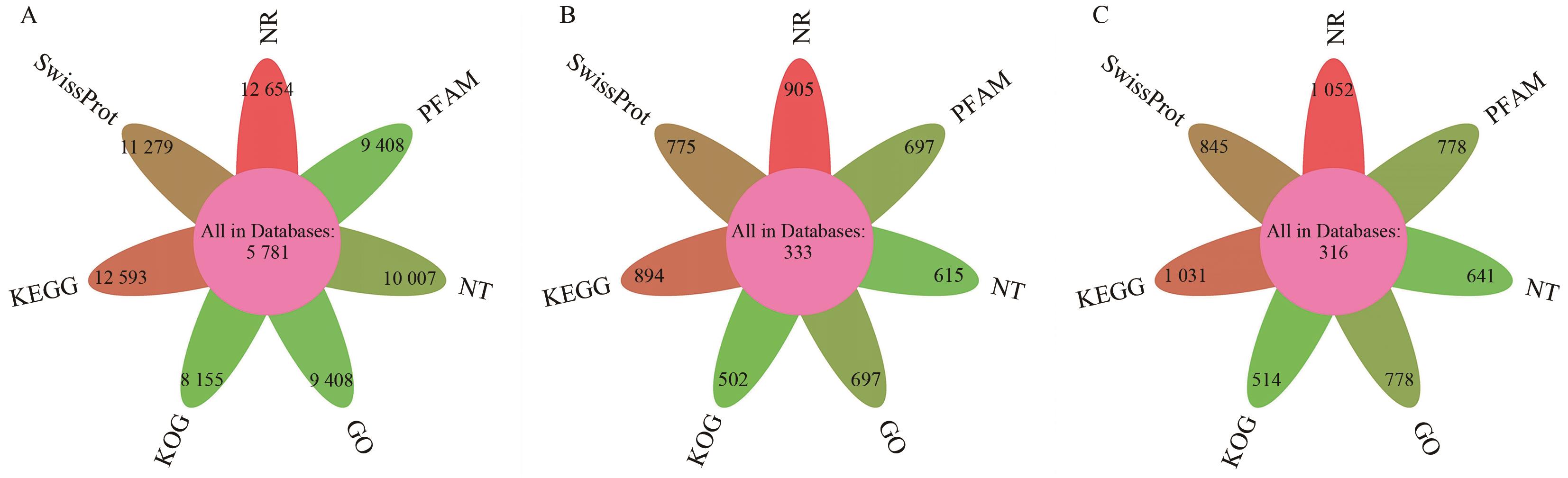
图6 功能注释统计图A:iso; B:unmap; C:novel; iso:结构注释后的转录本;unmap:未比对到参考基因组上的转录本;novel:新基因。下同
Fig. 6 Statistical diagram of functional annotationA: iso; B: unmap; C: novel. iso: The transcripts after structural annotation. unmap: The transcripts on the reference genome were not matched. novel: New gene. The same below
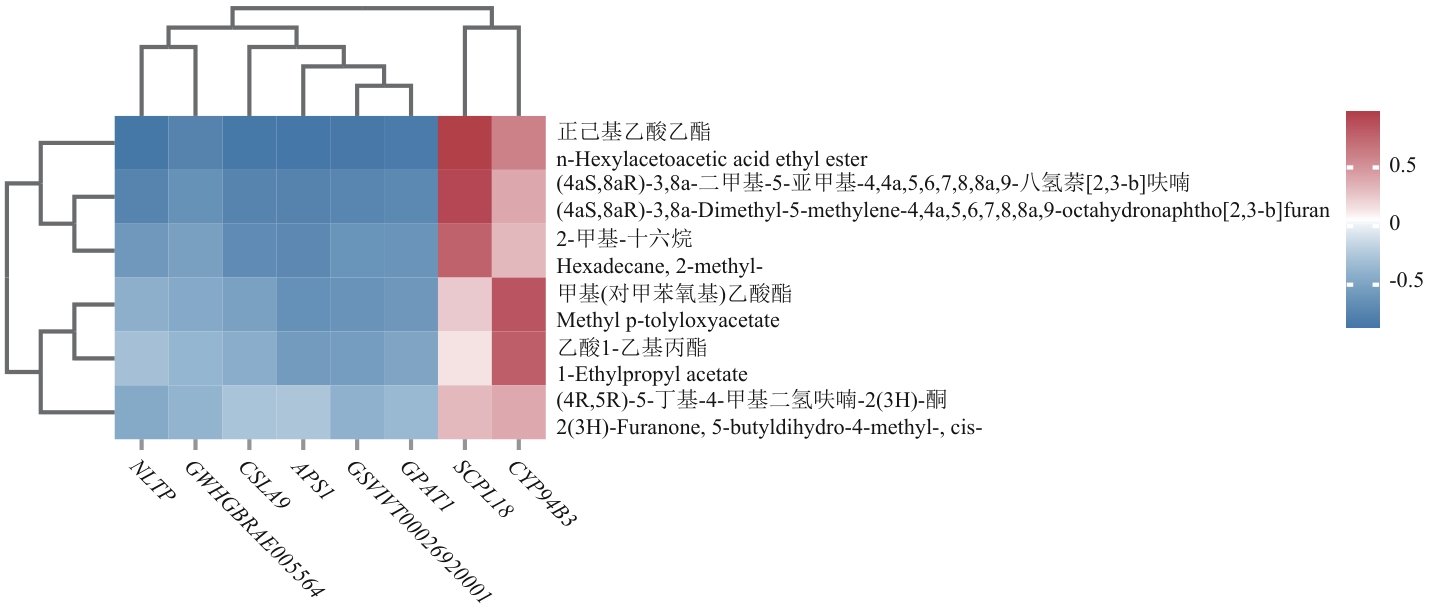
图11 差异基因和差异代谢物关联热图图中红色表示正相关,蓝色表示负相关,横坐标为基因名称,其中GWHGBRAE005564为基因测序代码,未查找到所属基因
Fig. 11 Heat map of differential genes and differential metabolites associationRed indicates positive correlation and blue indicates negative correlation. The horizontal coordinate indicates the name of the gene. Among them, GWHGBRAE005564 is the gene sequencing code, and the corresponding gene was not found
| [1] | 国家药典委员会.中华人民共和国药典 [M]. 一部. 北京: 中国医药科技出版社, 2020. |
| Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China [M]. Volume I.Beijing:China Medical Science Press, 2020. | |
| [2] | 计辰洋, 任燕, 翟煦, 等. 基于功效古今演变的艾叶研究进展与应用思考 [J]. 世界中医药, 2024, 19(2): 279-284. |
| Ji CY, Ren Y, Zhai X, et al. Research progress and application of Artemisia argyi leaf based on the evolution of efficacy from ancient to modern times [J]. World Chin Med, 2024, 19(2): 279-284. | |
| [3] | 王倩倩, 郭蕊, 张丹, 等. 基于UPLC和HS-GC-MS的不同道地产区艾叶药材化学成分比较分析 [J]. 中国中药杂志, 2023, 48(20): 5509-5518. |
| Wang QQ, Guo R, Zhang D, et al. Comparison of chemical constituents in Artemisiae Argyi Folium from different Dao-di producing areas based on UPLC and HS-GC-MS [J]. China Journal of Chinese Materia Medica, 2023, 48(20): 5509-5518. | |
| [4] | 兰晓燕, 朱龙波, 黄显章, 等. 艾叶中主要化学成分的鉴定及其含量测定研究 [J]. 中草药, 2021, 52(24): 7630-7637. |
| Lan XY, Zhu LB, Huang XZ, et al. Study on identification and quantitation of main compounds in Artemisiae Argyi Folium [J]. Chinese Traditional and Herbal Drugs, 2021, 52(24): 7630-7637. | |
| [5] | 孙宗淼, 许秀萍. 艾不同部位有效成分含量的比较 [J]. 山西医科大学学报, 2023, 54(1): 114-118. |
| Sun ZM, Xu XP. Comparative study on contents of active components in different parts of Artemisia argyi Levl. [J]. Journal of Shanxi Medical University, 2023, 54(1): 114-118. | |
| [6] | 任凌丽, 李浩, 钱雪, 等. 新鲜艾叶与陈化艾叶挥发油化学成分研究 [J]. 中国野生植物资源, 2022, 41(11): 39-42, 55. |
| Ren LL, Li H, Qian X, et al. Study on chemical composition of volatile oil from fresh and aged Artemisia argyi leaves [J]. Chinese Wild Plant Resources, 2022, 41(11): 39-42, 55. | |
| [7] | 李玉萍, 魏莉霞, 张东佳, 等. 艾草的研究现状、应用与展望 [J]. 中国种业, 2024(8): 21-27. |
| Li YP, Wei LX, Zhang DJ, et al. Research status, applications and prospect of Artemisia argyi [J]. China Seed Ind, 2024(8): 21-27. | |
| [8] | 蒋小洁, 陈贻豪, 宋紫欣, 等. 艾草的药理作用及其机制研究进展 [J]. 华夏医学, 2023, 36(6): 182-188. |
| Jiang XJ, Chen YH, Song ZX, et al. Research progress in the pharmacology and mechanism of Artemisia argyi [J]. Acta Med Sin, 2023, 36(6): 182-188. | |
| [9] | Liu Y, He YN, Wang F, et al. From longevity grass to contemporary soft gold: Explore the chemical constituents, pharmacology, and toxicology of Artemisia argyi H.Lév. & vaniot essential oil [J]. J Ethnopharmacol, 2021, 279: 114404. |
| [10] | 兰晓燕, 张元, 朱龙波, 等. 艾叶化学成分、药理作用及质量研究进展 [J]. 中国中药杂志, 2020, 45(17): 4017-4030. |
| Lan XY, Zhang Y, Zhu LB, et al. Research progress on chemical constituents from Artemisiae argyi Folium and their pharmacological activities and quality control [J]. China J Chin Mater Med, 2020, 45(17): 4017-4030. | |
| [11] | 朱晔, 马蕊, 陈灵丽, 等. 艾的基原考证 [J].中医学报, 2024: 1-10. |
| Zhu Y, Ma R, Chen LL, et al. Textual research on traditional Chinese medicine Artemisia argyi [J]. Acta Chinese Medicine, 2024: 1-10. | |
| [12] | 庄二平, 郑真. 南阳市艾草产业发展分析 [J]. 农村经济与科技, 2022, 33(3): 147-149. |
| Zhuang EP, Zheng Z. Analysis on the development of wormwood industry in Nanyang city [J]. Rural Econ Sci Technol, 2022, 33(3): 147-149. | |
| [13] | 时敏, 王瑶, 周伟, 等. 药用植物萜类化合物的生物合成与代谢调控研究进展 [J]. 中国科学: 生命科学, 2018, 48(4): 352-364. |
| Shi M, Wang Y, Zhou W, et al. Research progress in terms of the biosynthesis and regulation of terpenoids from medicinal plants [J]. Sci Sin Vitae, 2018, 48(4): 352-364. | |
| [14] | 孙丽超, 李淑英, 王凤忠, 等. 萜类化合物的合成生物学研究进展 [J]. 生物技术通报, 2017, 33(1): 64-75. |
| Sun LC, Li SY, Wang FZ, et al. Research progresses in the synthetic biology of terpenoids [J]. Biotechnol Bull, 2017, 33(1): 64-75. | |
| [15] | 零唯, 覃艳红,黄鼎, 等. 绞股蓝萜类合成酶(TPS)基因家族鉴定及其在非生物胁迫下的表达分析 [J]. 中国中药杂志, 2023, 48(4): 930-938. |
| Ling W, Qin YH, Huang D, et al. Identification of terpene synthase gene family in Gynostemma pentaphyllum and expression pattern analysis under abiotic stresses [J]. China Journal of Chinese Materia Medica, 2023, 48(4): 930-938. | |
| [16] | Wolkoff P, Clausen PA, Wilkins CK, et al. Formation of strong airway irritants in terpene/ozone mixtures [J]. Indoor Air, 2000, 10(2): 82-91. |
| [17] | Wang X, Pereira JH, Tsutakawa S, et al. Efficient production of oxidized terpenoids via engineering fusion proteins of terpene synthase and cytochrome P450 [J]. Metab Eng, 2021, 64: 41-51. |
| [18] | 徐俊, 叶雨晴, 牛雅静, 等. 菊花根状茎发育的转录组分析 [J]. 生物技术通报, 2023, 39(10): 231-245. |
| Xu J, Ye YQ, Niu YJ, et al. Transcriptome analysis of rhizome development in Chrysanthemum morifolium [J]. Biotechnol Bull, 2023, 39(10): 231-245. | |
| [19] | Arick M 2nd, Hsu CY. Differential gene expression analysis of plants [J]. Methods Mol Biol, 2018, 1783: 279-298. |
| [20] | 刘慧敏, 张悦, 王佳艺, 等. 代谢组学前沿技术进展及在中药现代研究中的应用 [J]. 中草药, 2024, 55(3): 969-977. |
| Liu HM, Zhang Y, Wang JY, et al. Advances in frontier technologies in metabolomics and their application in modern research of Chinese medicine [J]. Chin Tradit Herb Drugs, 2024, 55(3): 969-977. | |
| [21] | 崔芙岩, 杨佳颖, 王志刚, 等. 代谢组学在中医药领域的应用与展望 [J]. 中草药, 2022, 53(14): 4512-4526. |
| Cui FY, Yang JY, Wang ZG, et al. Application and prospect of metabolomics in traditional Chinese medicine research [J]. Chin Tradit Herb Drugs, 2022, 53(14): 4512-4526. | |
| [22] | Fraga-Corral M, Carpena M, Garcia-Oliveira P, et al. Analytical metabolomics and applications in health, environmental and food science [J]. Crit Rev Anal Chem, 2022, 52(4): 712-734. |
| [23] | 梁婉凤, 曾菁菁, 胡若群, 等. 转录组与代谢组分析不同生长时期金线莲类胡萝卜素的积累 [J]. 生物技术通报, 2024, 40(10): 262-274. |
| Liang WF, Zeng JJ, Hu RQ, et al. Transcriptional and metabolomic analysis of carotenoid accumulation in Anoectochilus roxburghii during different growth periods [J]. Biotechnol Bull, 2024, 40(10): 262-274. | |
| [24] | 薛守宇, 朱涛, 李冰冰, 等. 转录组和代谢组联合分析在植物中的应用研究 [J]. 山西农业大学学报: 自然科学版, 2022, 42(3): 1-13. |
| Xue SY, Zhu T, Li BB, et al. Application research of combined transcriptome with metabolome in plants [J]. J Shanxi Agric Univ Nat Sci Ed, 2022, 42(3): 1-13. | |
| [25] | 张改君, 苗静, 郭丽颖, 等. 多组学联用在中药作用机制研究中的应用 [J]. 中草药, 2021, 52(10): 3112-3120. |
| Zhang GJ, Miao J, Guo LY, et al. Application of multi-omics combination in mechanism studies of traditional Chinese medicine [J]. Chinese Traditional and Herbal Drugs, 2021, 52(10): 3112-3120. | |
| [26] | Wang SS, Liu L, Mi XZ, et al. Multi-omics analysis to visualize the dynamic roles of defense genes in the response of tea plants to gray blight [J]. Plant J, 2021, 106(3): 862-875. |
| [27] | 罗元明, 杨福全. 多组学前沿技术专刊序言 [J]. 生物工程学报, 2022, 38(10): 3571-3580. |
| Luo YM, Yang FQ. Preface for special issue on multi-omics frontier technologies [J]. Chinese Journal of Biotechnology, 2022, 38(10): 3571-3580. | |
| [28] | 张利苹, 王俊玲, 李振华, 等. 花生红色种皮花青素生物合成转录-代谢组学联合分析 [J]. 植物遗传资源学报, 2024, 25(10): 1767-1780. |
| Zhang LP, Wang JL, Li ZH, et al. Transcriptomics-metabolomics combined analysis highlight the anthocyanin biosynthesis mechanism of red testa in peanut(Arachis hypogaea L.) [J]. Journal of Plant Genetic Resources, 2024, 25(10): 1767-1780. | |
| [29] | Chen HY, Guo MX, Dong ST, et al. A chromosome-scale genome assembly of Artemisia argyi reveals unbiased subgenome evolution and key contributions of gene duplication to volatile terpenoid diversity [J]. Plant Commun, 2023, 4(3): 100516. |
| [30] | 奥斯伯FM, 马学军, 舒跃龙. 精编分子生物学实验指南 [M]. 北京: 科学出版社, 2005. |
| Ausubel FM, Ma XJ, Shu YL. Short protocols in molecular biology [M]. Beijing: Science Press, 2005. | |
| [31] | Wu TD, Watanabe CK. GMAP: a genomic mapping and alignment program for mRNA and EST sequences [J]. Bioinformatics, 2005, 21(9): 1859-1875. |
| [32] | 董树廷. 基于代谢组和单细胞转录组的艾叶腺毛发育及倍半萜生物合成研究 [D]. 北京:北京协和医学院, 2024. |
| Dong ST. Analysis of glandular trichome development and sesquiterpene biosynthesis in Artemisia argyi based on metabolome and single-cell transcriptome [D]. Beijing: Peking Union Medical College, 2024. | |
| [33] | Lee S, Won HJ, Ban S, et al. Integrative analysis of metabolite and transcriptome reveals biosynthetic pathway and candidate genes for eupatilin and jaceosidin biosynthesis in Artemisia argyi [J]. Front Plant Sci, 2023, 14: 1186023. |
| [34] | Zhang KP, Wang NH, Gao XQ, et al. Integrated metabolite profiling and transcriptome analysis reveals tissue-specific regulation of terpenoid biosynthesis in Artemisia argyi [J]. Genomics, 2022, 114(4): 110388. |
| [1] | 刘语诗, 李镇, 邹宇琛, 汤维维, 李彬. 药用植物空间代谢组学研究进展[J]. 生物技术通报, 2025, 41(9): 22-31. |
| [2] | 刘建国, 刘格儿, 郭颖欣, 王斌, 王玉昆, 卢金凤, 黄文庭, 朱云娜. 转录组和代谢组联合解析‘桂柚1号’和‘沙田柚’果实品质差异[J]. 生物技术通报, 2025, 41(9): 168-181. |
| [3] | 刘泽洲, 段乃彬, 岳丽昕, 王清华, 姚行浩, 高莉敏, 孔素萍. 大蒜叶片蜡质成分分析及蜡质缺失基因Ggl-1筛选[J]. 生物技术通报, 2025, 41(9): 219-231. |
| [4] | 闫梦阳, 梁晓阳, 戴君昂, 张妍, 关团, 张辉, 刘良波, 孙志华. 阿莫西林降解菌的筛选及降解机制研究[J]. 生物技术通报, 2025, 41(9): 314-325. |
| [5] | 张雅祺, 王芹芹, 沈夏, 李旭苗, 高敏, 李军, 李辰, 王慧. 食管鳞状细胞癌早期进展风险的代谢物预警模型[J]. 生物技术通报, 2025, 41(9): 335-344. |
| [6] | 柴军发, 洪波, 贾彦霞. 转录组和代谢组联合分析三株蜡蚧轮枝菌菌株毒力差异[J]. 生物技术通报, 2025, 41(8): 311-321. |
| [7] | 蔡如凤, 杨宇轩, 于基正, 李佳楠. 人工智能重塑蛋白质工程:从结构解析到合成生物学的算法革命[J]. 生物技术通报, 2025, 41(8): 1-10. |
| [8] | 白雨果, 李婉迪, 梁建萍, 石志勇, 卢庚龙, 刘红军, 牛景萍. 哈茨木霉T9131对黄芪幼苗的促生机理[J]. 生物技术通报, 2025, 41(8): 175-185. |
| [9] | 王月琛, 韩鑫骐, 魏文敏, 崔兆兰, 罗阳美, 陈鹏如, 王海岗, 刘龙龙, 张莉, 王纶. 黍稷落粒的生物学基础研究及落粒调控基因的鉴定[J]. 生物技术通报, 2025, 41(7): 164-171. |
| [10] | 李成花, 豆飞飞, 任毓昭, 刘彩霞, 刘凤楼, 王掌军, 李清峰. 外施水杨酸对白粉菌侵染小麦的影响及白粉菌转录组分析[J]. 生物技术通报, 2025, 41(7): 272-280. |
| [11] | 郭秀娟, 冯宇, 吴瑞香, 王利琴, 杨建春. Ca2+处理对胡麻种子萌发影响的转录组分析[J]. 生物技术通报, 2025, 41(7): 139-149. |
| [12] | 蒋天威, 马培杰, 李亚娇, 陈才俊, 刘晓霞, 王小利. 二穗短柄草对光周期的代谢响应分析[J]. 生物技术通报, 2025, 41(7): 237-247. |
| [13] | 张越, 毕钰, 慕雪男, 郑子薇, 王志刚, 徐伟慧. 小麦赤霉病拮抗菌JB7的生防特性[J]. 生物技术通报, 2025, 41(7): 261-271. |
| [14] | 高婧, 陈益存, 高暝, 赵耘霄, 汪阳东. 植物单宁合成调控及其对环境的响应机制[J]. 生物技术通报, 2025, 41(7): 49-59. |
| [15] | 吴娅, 姚润, 杨含婷, 刘微, 杨帅, 宋驰, 陈士林. 凤梨薄荷SDR基因家族全基因组鉴定及表达分析[J]. 生物技术通报, 2025, 41(5): 175-185. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||