








生物技术通报 ›› 2026, Vol. 42 ›› Issue (7): 1-13.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0964
• 研究报告 •
收稿日期:2025-09-09
出版日期:2026-02-09
发布日期:2026-02-09
通讯作者:
陈洪艳03692@qqhru.edu.cn基金资助:
GAO Fei, ZHANG Yu-xi, MU Di, CHEN Zheng, CHEN Hong-yan( )
)
Received:2025-09-09
Published:2026-02-09
Online:2026-02-09
摘要:
目的 罗伊氏粘液乳杆菌(Limosilactobacillus reuteri)是一种兼性厌氧的乳酸菌,常见于脊椎动物及哺乳动物的肠道内。该菌具有降胆固醇、抑制病原菌生长和增强机体免疫力等多种益生功能,因此分离可广泛应用于食品发酵工业中的菌种至关重要。 方法 利用MRS-CaCO3培养基从小鼠粪便中分离并培养L. reuteri,通过形态学观察、革兰染色、16S rRNA序列分析进行初步鉴定,对符合菌株进行生长性能、抗逆性能、抑菌功效测定,并对菌株进行全基因组测序注释,从基因层面探究其益生机制和安全性。 结果 分离得到的L. reuteri GF304无溶血性,具有良好的生长性能和产酸能力;对酸和胆盐具有较强的耐受性,在人工肠胃液中存活率较高;能有效抑制大肠杆菌、金黄色葡萄球菌的生长,表明其益生特性。全基因组测序分析结果显示,L. reuteri GF304基因组中不存在毒力和耐药基因,且含有耐热胁迫、耐冷胁迫、耐酸、耐胆盐、黏附、抗氧化和有机酸合成相关的抗应激和益生基因。菌株GF304最普遍的CAZy分类为GH、GT。次级代谢产物分析发现菌株GF304具有Type Ⅲ Polyketide Synthase合成基因簇与RiPP合成基因簇。 结论 基于安全的基因组背景(无毒力/耐药基因)和丰富的益生基因簇,结合其优异的生长、抗逆与抑菌性能,L. reuteri GF304 展现出作为益生菌制剂的巨大开发潜力。
高飞, 张宇曦, 穆頔, 陈峥, 陈洪艳. 鼠源罗伊氏黏液乳杆菌的分离鉴定及全基因组测序分析[J]. 生物技术通报, 2026, 42(7): 1-13.
GAO Fei, ZHANG Yu-xi, MU Di, CHEN Zheng, CHEN Hong-yan. Isolation, Identification, and Whole-genome Sequencing Analysis of Murine-Derived Limosilactobacillus reuteri[J]. Biotechnology Bulletin, 2026, 42(7): 1-13.
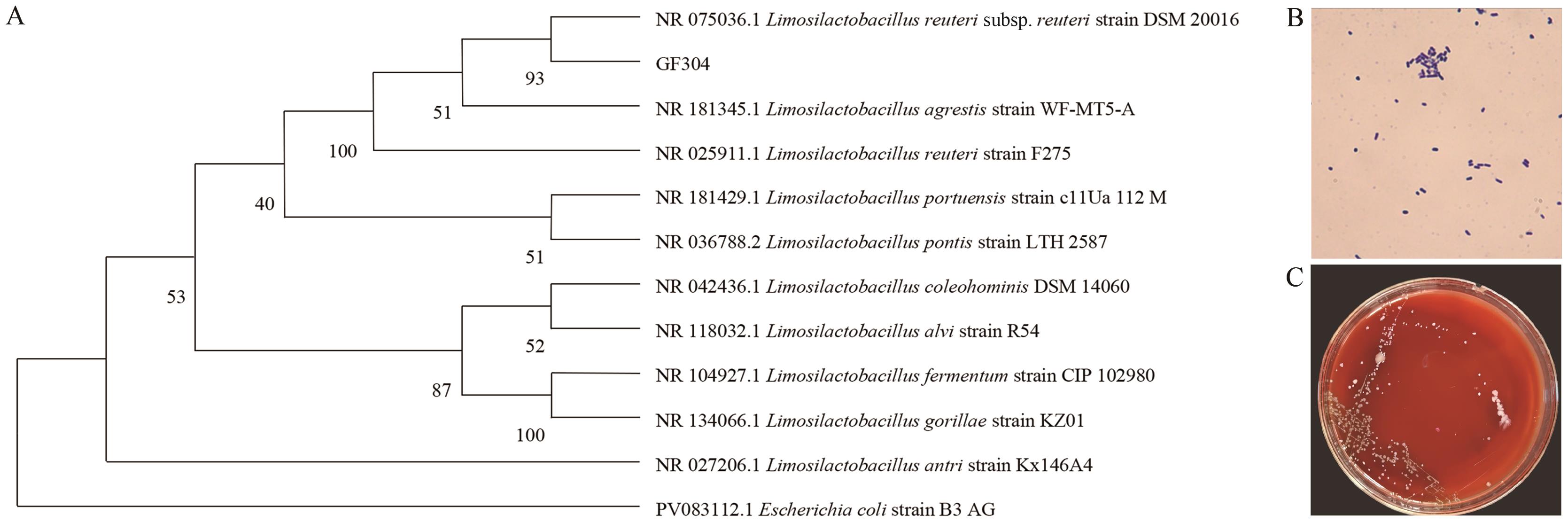
图1 分离菌株的形态学观察、溶血试验与系统发育树A:革兰氏染色镜检(×1 000);B:菌株GF304溶血试验结果;C:菌株GF304系统发育树
Fig. 1 Morphological observation, hemolytic assay, and phylogenetic tree of the isolated strainsA: Gram staining microscopy (×1 000). B: Hemolysis test results of strain GF304. C: Phylogenetic tree of strain GF304
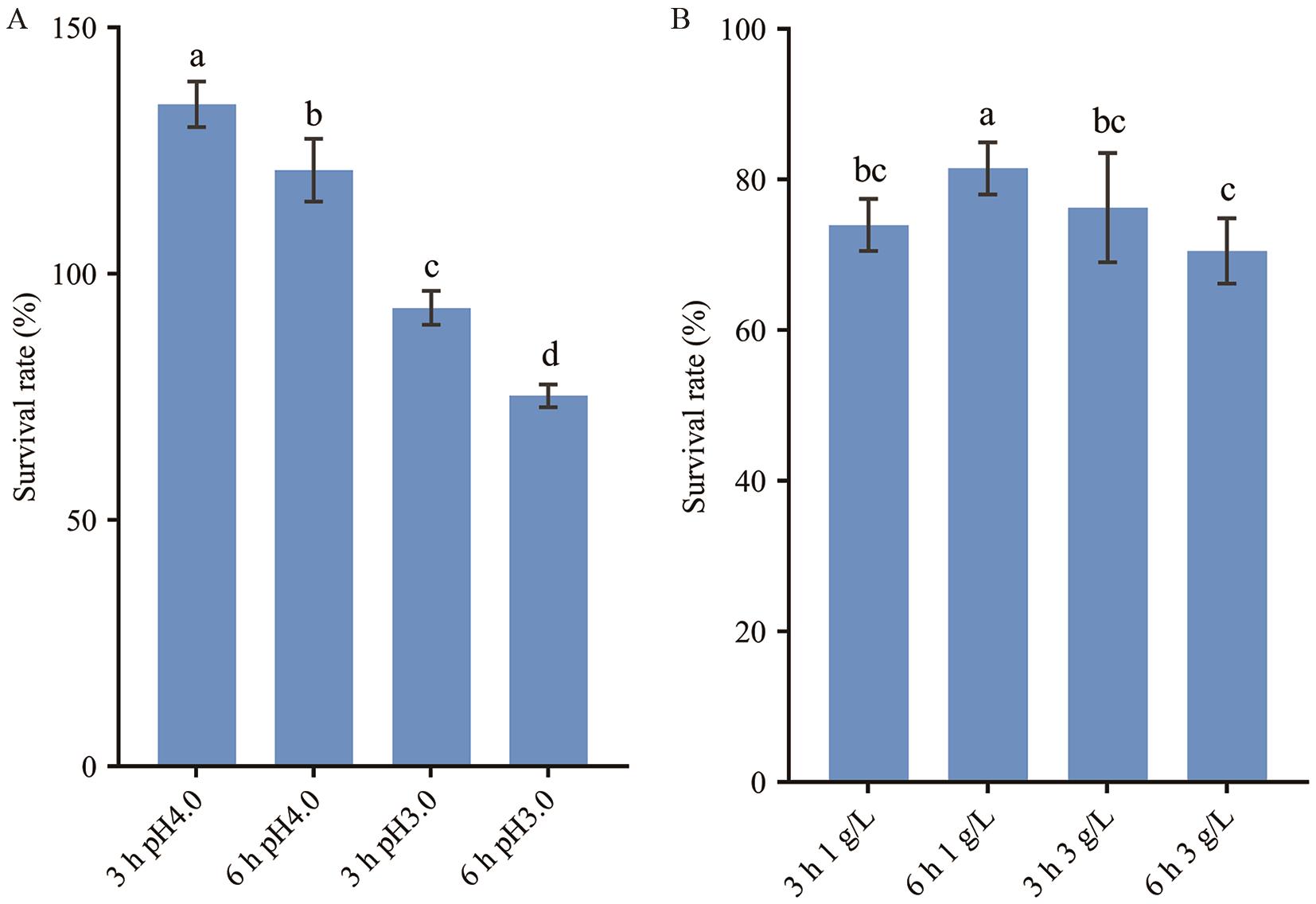
图3 罗伊氏黏液乳杆菌GF304的耐酸和耐胆碱能力A:耐酸能力;B:耐胆碱能力。不同小写字母表示组间差异显著(P<0.05)
Fig. 3 Tolerances to acid and choline of L. reuteri GF304A: Tolerance to acid. B: Tolerance to choline. Different lowercase letters indicate significant differences between groups (P<0.05)
| 菌株 | 人工胃液 | 人工肠液 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 活菌数(×106 CFU·mL-1) | 存活率(%) | 活菌数(×105 CFU·mL-1) | 存活率/% | ||||||
| 0 h | 2 h | 2 h/0 h | 0 h | 2 h | 4 h | 2 h/0 h | 4 h/0 h | ||
| GF304 | 3.26±0.16 | 2.73±0.43 | 83.38±0.09 | 4.75±0.67 | 4.00±0.85 | 2.72±0.70 | 83.66±0.07 | 57.80±0.14 | |
表1 罗伊氏黏液乳杆菌GF304在人工胃液和人工肠液中的存活率
Table 1 Survival rate of L. reuteri GF304 in simulated gastric juice and simulated intestinal juice
| 菌株 | 人工胃液 | 人工肠液 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 活菌数(×106 CFU·mL-1) | 存活率(%) | 活菌数(×105 CFU·mL-1) | 存活率/% | ||||||
| 0 h | 2 h | 2 h/0 h | 0 h | 2 h | 4 h | 2 h/0 h | 4 h/0 h | ||
| GF304 | 3.26±0.16 | 2.73±0.43 | 83.38±0.09 | 4.75±0.67 | 4.00±0.85 | 2.72±0.70 | 83.66±0.07 | 57.80±0.14 | |
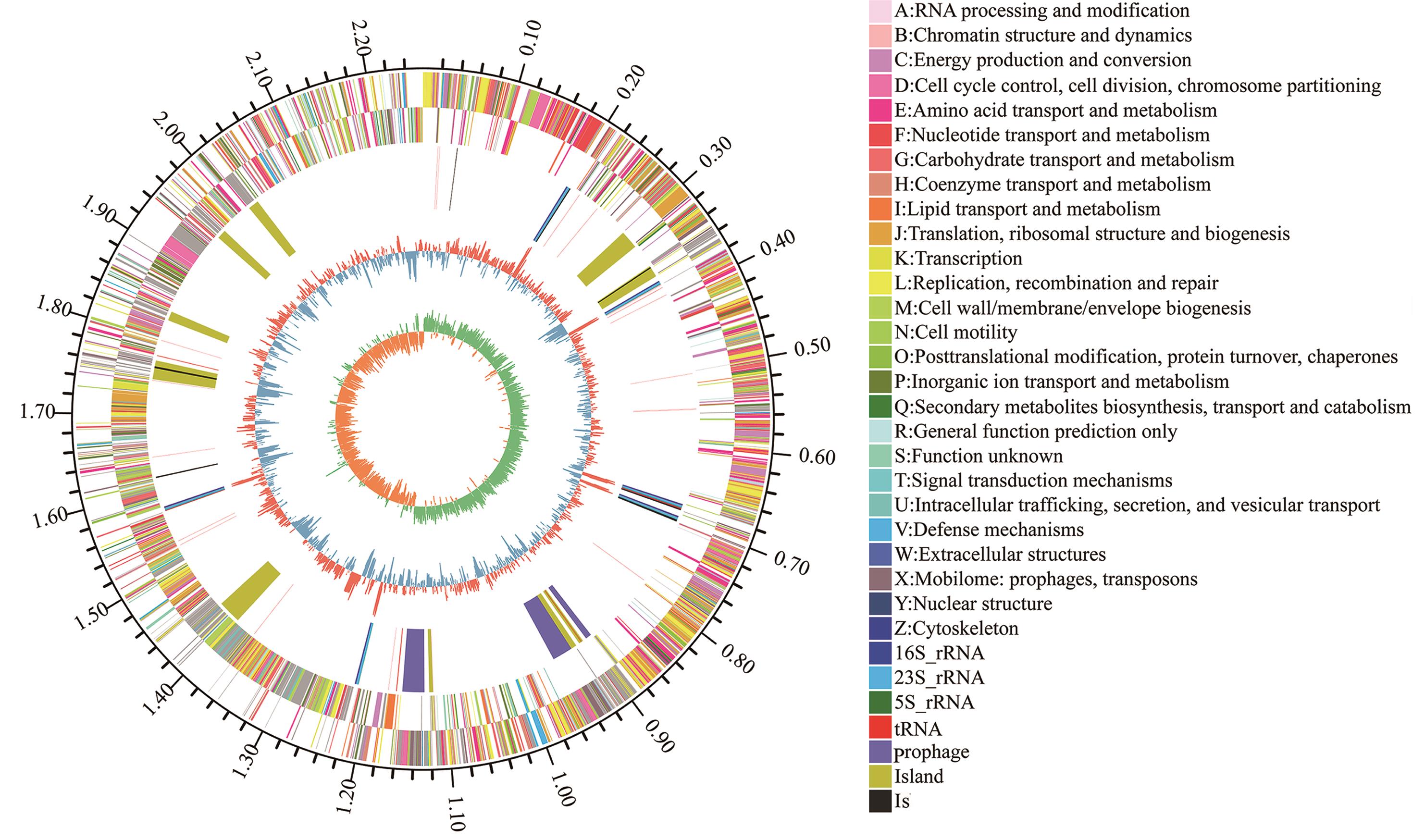
图5 罗伊氏黏液乳杆菌菌株GF304的基因组圈图由外至内各圈层代表:基因组大小标识;按COG功能分类注释的正、负链CDS;rRNA和tRNA的分布;GC含量(红色外突与蓝色内陷分别表示高于和低于全基因组平均GC含量);以及最内层的GC偏好(绿色:G>C;黄色:C>G)
Fig. 5 The circular genome map of L. reuteri strain GF304From the outermost to the innermost layer, the concentric rings represent: the genome scale marker; coding sequences (CDSs) on the positive and negative strands annotated according to COG functional classifications; the distribution of rRNAs and tRNAs; GC content (red outward peaks indicate higher than the average genomic GC content, while blue inward peaks indicate lower than the average); and the innermost layer indicates GC skew (green indicates G > C, yellow indicates C > G)
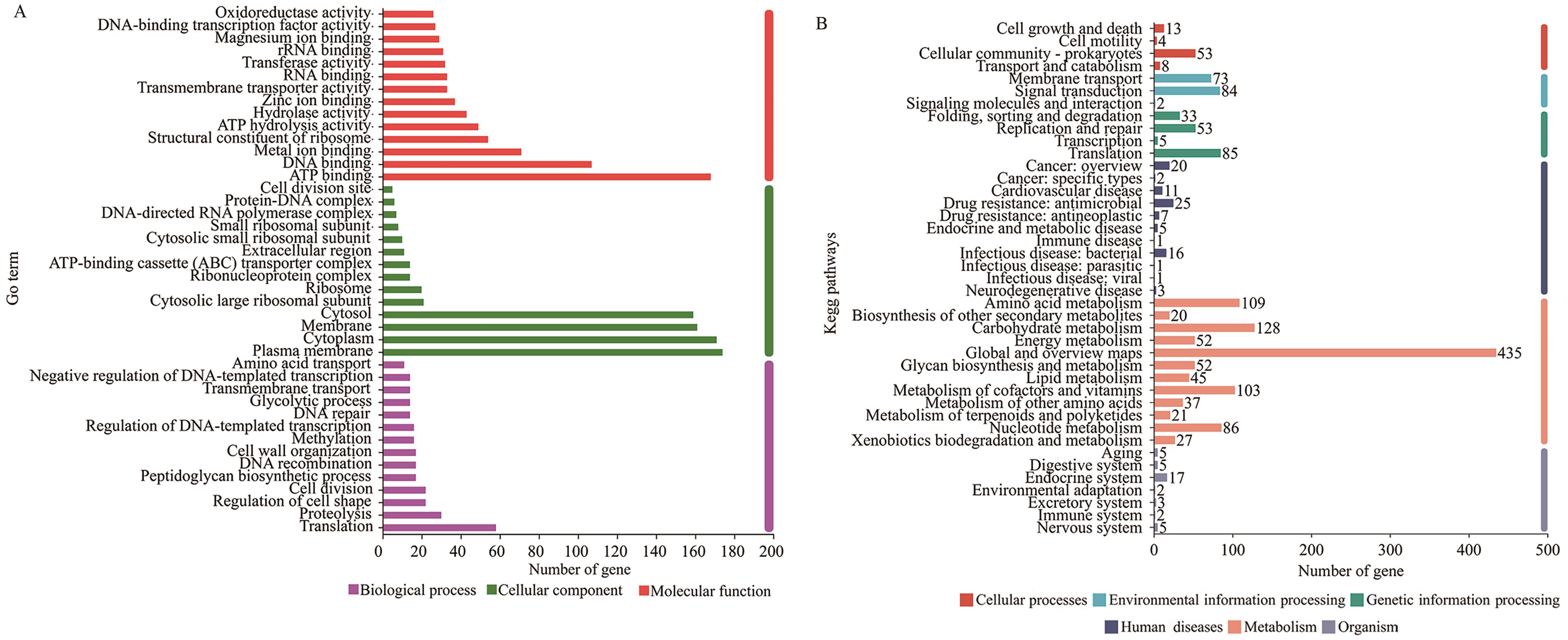
图6 罗伊氏黏液乳杆菌菌株GF304基因组的GO功能分类和KEGG代谢通路分类A:GO分析;B:KEGG分析
Fig. 6 GO functional classification and KEGG metabolic pathway classification of the genome of L. reuteri strain GF304A: GO Analysis. B: KEGG Analysis
| Gene name | Gene product | Gene ID |
|---|---|---|
| Temperature | ||
| groES | Co-chaperone GroES | gene0399 |
| groEL | Chaperonin GroEL | gene0400 |
| smpB | SsrA-binding protein SmpB | gene0450 |
| hrcA | Heat-inducible transcriptional repressor HrcA | gene0768 |
| dnaK | Molecular chaperone DnaK | gene0770 |
| dnaJ | Molecular chaperone DnaJ | gene0771 |
| cspA | Cold shock domain-containing protein,cold-shock protein | gene0676/gene1783 |
| pH | ||
| atpB | F0F1 ATP synthase subunit A | gene0512 |
| atpE | F0F1 ATP synthase subunit C | gene0513 |
| atpF | F0F1 ATP synthase subunit B | gene0514 |
| atpH | ATP synthase F1 subunit delta | gene0515 |
| atpA | F0F1 ATP synthase subunit alpha | gene0516 |
| atpG | F0F1 ATP synthase subunit gamma | gene0517 |
| atpD | F0F1 ATP synthase subunit beta | gene0518 |
| atpC | F0F1 ATP synthase subunit epsilon | gene0519 |
| - | Alkaline shock response membrane anchor protein AmaP | gene0966 |
| arcA | Arginine deiminase | gene0496 |
| arcC | Carbamate kinase | gene0480 |
| argR | Arginine repressor/ArgR family transcriptional regulator | gene0497/gene1329 |
| argG | Argininosuccinate synthase | gene0802 |
| argH | Argininosuccinate lyase | gene0803 |
| gadB | Glutamate decarboxylase | gene0539 |
| gadC | Gamma-aminobutyrate antiporte/glutamate/gamma-aminobutyrate family transporter YjeM | gene0542/gene0540/gene1458/gene1618 |
| nhaC | Na+/H+ antiporter NhaC family protein/Na+/H+ antiporter NhaC | gene0171/gene2094 |
| Bile salt resistance | ||
| cbh | Choloylglycine hydrolase | gene0801 |
| ppaC | Manganese-dependent inorganic pyrophosphatase | gene0959 |
| Production of adhesion molecules | ||
| ltaS | LTA synthase family protein | gene1935/gene2098 |
| Oxidative resistance | ||
| trxA | Thioredoxin | gene0582/gene2088 |
| trxB | Thioredoxin-disulfide reductase | gene0423 |
| gshA | Glutamate--cysteine ligase | gene0072/gene1632 |
| pepN | M1 family metallopeptidase | gene2145 |
| nfrA1 | NADPH-dependent oxidoreductase | gene1863 |
| Riboflavin biosynthesis | ||
| ribD | Bifunctional diaminohydroxyphosphoribosylaminopyrimidine Deaminase/5-amino-6-(5-phosphoribosylamino)uracil reductase RibD | gene0993 |
| ribE | riboflavin synthase | gene0994 |
| ribBA | Bifunctional 3,4-dihydroxy-2-butanone-4-phosphate synthase/GTP cyclohydrolase Ⅱ | gene0995 |
| ribH | 6,7-dimethyl-8-ribityllumazine synthase | gene0996 |
| ribF | Riboflavin biosynthesis protein RibF | gene0766 |
| ribT | Reductase | gene0825 |
| Organic acid biosynthesis | ||
| ackA | Acetate kinase | gene0604 |
表2 菌株GF304具有的益生特性相关基因
Table 2 Genes related to probiotic properties in Limosilactobacillus reuteri strain GF304
| Gene name | Gene product | Gene ID |
|---|---|---|
| Temperature | ||
| groES | Co-chaperone GroES | gene0399 |
| groEL | Chaperonin GroEL | gene0400 |
| smpB | SsrA-binding protein SmpB | gene0450 |
| hrcA | Heat-inducible transcriptional repressor HrcA | gene0768 |
| dnaK | Molecular chaperone DnaK | gene0770 |
| dnaJ | Molecular chaperone DnaJ | gene0771 |
| cspA | Cold shock domain-containing protein,cold-shock protein | gene0676/gene1783 |
| pH | ||
| atpB | F0F1 ATP synthase subunit A | gene0512 |
| atpE | F0F1 ATP synthase subunit C | gene0513 |
| atpF | F0F1 ATP synthase subunit B | gene0514 |
| atpH | ATP synthase F1 subunit delta | gene0515 |
| atpA | F0F1 ATP synthase subunit alpha | gene0516 |
| atpG | F0F1 ATP synthase subunit gamma | gene0517 |
| atpD | F0F1 ATP synthase subunit beta | gene0518 |
| atpC | F0F1 ATP synthase subunit epsilon | gene0519 |
| - | Alkaline shock response membrane anchor protein AmaP | gene0966 |
| arcA | Arginine deiminase | gene0496 |
| arcC | Carbamate kinase | gene0480 |
| argR | Arginine repressor/ArgR family transcriptional regulator | gene0497/gene1329 |
| argG | Argininosuccinate synthase | gene0802 |
| argH | Argininosuccinate lyase | gene0803 |
| gadB | Glutamate decarboxylase | gene0539 |
| gadC | Gamma-aminobutyrate antiporte/glutamate/gamma-aminobutyrate family transporter YjeM | gene0542/gene0540/gene1458/gene1618 |
| nhaC | Na+/H+ antiporter NhaC family protein/Na+/H+ antiporter NhaC | gene0171/gene2094 |
| Bile salt resistance | ||
| cbh | Choloylglycine hydrolase | gene0801 |
| ppaC | Manganese-dependent inorganic pyrophosphatase | gene0959 |
| Production of adhesion molecules | ||
| ltaS | LTA synthase family protein | gene1935/gene2098 |
| Oxidative resistance | ||
| trxA | Thioredoxin | gene0582/gene2088 |
| trxB | Thioredoxin-disulfide reductase | gene0423 |
| gshA | Glutamate--cysteine ligase | gene0072/gene1632 |
| pepN | M1 family metallopeptidase | gene2145 |
| nfrA1 | NADPH-dependent oxidoreductase | gene1863 |
| Riboflavin biosynthesis | ||
| ribD | Bifunctional diaminohydroxyphosphoribosylaminopyrimidine Deaminase/5-amino-6-(5-phosphoribosylamino)uracil reductase RibD | gene0993 |
| ribE | riboflavin synthase | gene0994 |
| ribBA | Bifunctional 3,4-dihydroxy-2-butanone-4-phosphate synthase/GTP cyclohydrolase Ⅱ | gene0995 |
| ribH | 6,7-dimethyl-8-ribityllumazine synthase | gene0996 |
| ribF | Riboflavin biosynthesis protein RibF | gene0766 |
| ribT | Reductase | gene0825 |
| Organic acid biosynthesis | ||
| ackA | Acetate kinase | gene0604 |
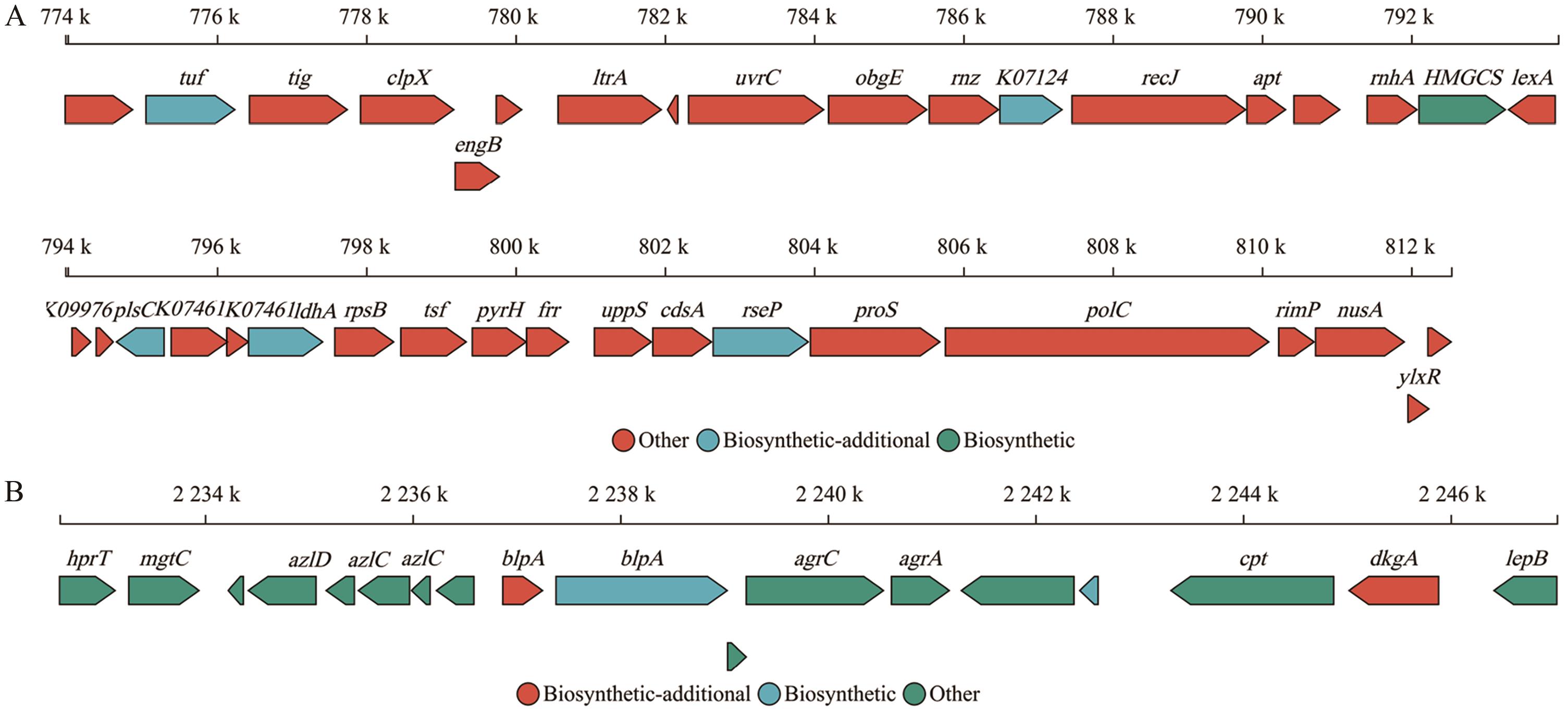
图7 罗伊氏黏液乳杆菌菌株GF304的次级代谢产物合成基因簇分析A:T3PKS合成基因簇;B:RiPP-like合成基因簇
Fig. 7 Analysis of secondary metabolite biosynthetic gene clusters in L. reuteri strain GF304A: T3PKS biosynthetic gene cluster. B: RiPP-like biosynthetic gene cluster
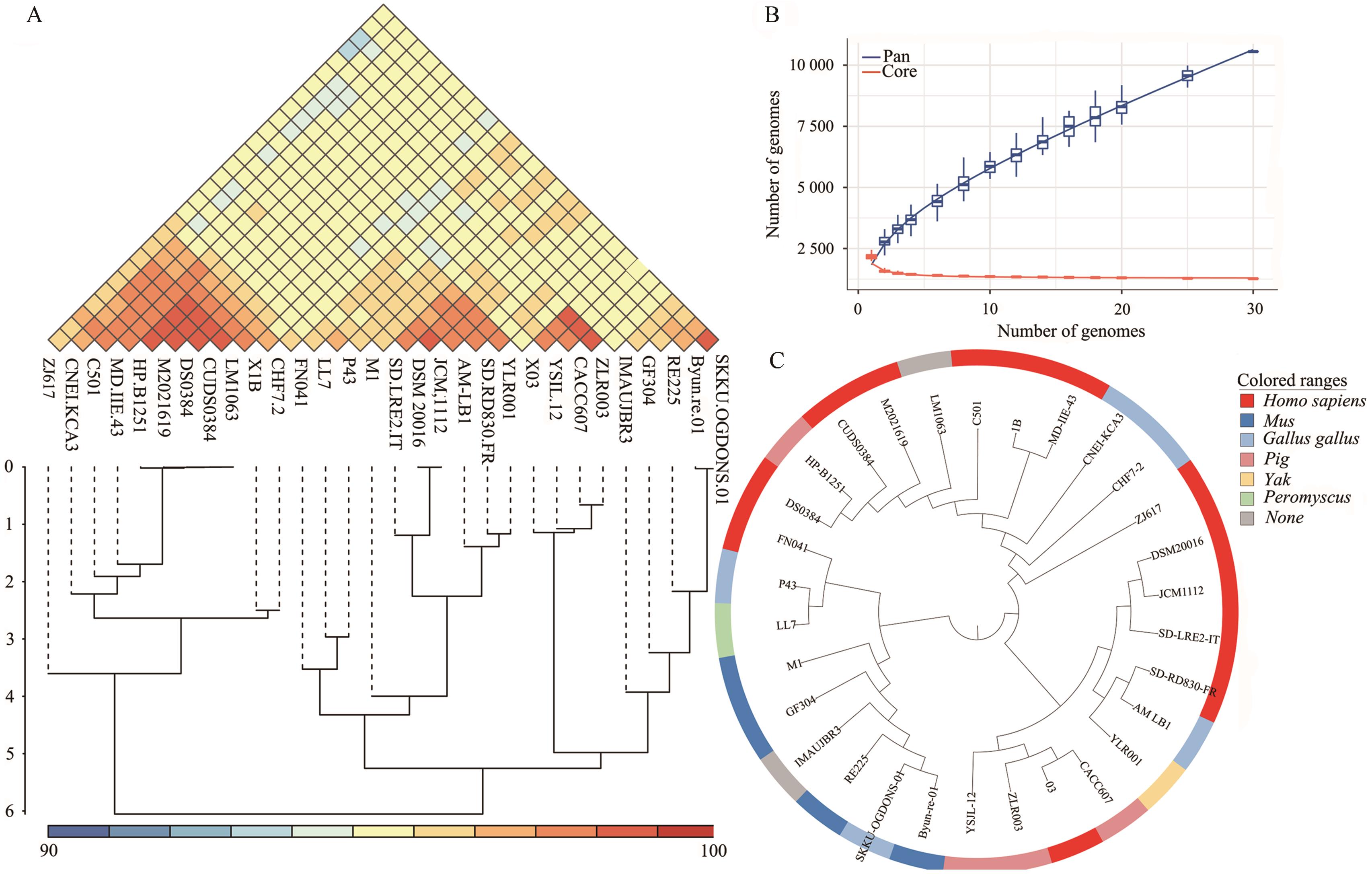
图8 罗伊氏黏液乳杆菌菌株GF304的比较基因组分析A:ANI结果;B:泛基因集和核心基因集曲线;C:基于核心基因构建的系统发育树
Fig. 8 Comparative genomic analysis of L. reuteri strain GF304A: ANI results. B: Pan-gene and core gene curves. C: Core gene-based phylogenetic tree
| [1] | Afrc RF. Probiotics in man and animals [J]. J Appl Bacteriol, 1989, 66(5): 365-378. |
| [2] | Lata P, Savitri. Probiotics and human health [J]. Res J Biotech, 2023, 18(7): 173-180. |
| [3] | Cristofori F, Dargenio VN, Dargenio C, et al. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body [J]. Front Immunol, 2021, 12: 578386. |
| [4] | 程雨, 谢坤, 姜艳平, 等. 兔源罗伊乳杆菌的分离鉴定及益生效果评价 [J]. 中国兽医学报, 2024, 44(10): 2136-2144, 2293. |
| Cheng Y, Xie K, Jiang YP, et al. Isolation and identification of rabbit-derived Lactobacillus reuteri and evaluation of its probiotic function [J]. Chin J Vet Sci, 2024, 44(10): 2136-2144, 2293. | |
| [5] | Falcinelli S, Rodiles A, Hatef A, et al. Influence of probiotics administration on gut microbiota core: a review on the effects on appetite control, glucose, and lipid metabolism [J]. J Clin Gastroenterol, 2018, 52(): S50-S56. |
| [6] | 徐乐, 陈诗宇, 王上, 等. 茶花鸡源罗伊特氏黏液乳杆菌CHF7-2的益生特性及全基因组测序分析 [J]. 微生物学报, 2025, 65(3): 1197-1218. |
| Xu L, Chen SY, Wang S, et al. Probiotic characterization and whole genome sequencing of Limosilactobacillus reuteri CHF7-2 from Chahua chicken [J]. Acta Microbiol Sin, 2025, 65(3): 1197-1218. | |
| [7] | 国家卫生健康委员会. 解读关于《可用于食品的菌种名单》和《可用于婴幼儿食品的菌种名单》更新的公告(2022年第4号) [J]. 饮料工业, 2022, 25(5): 3-4. |
| National Health Commission of the People’s Republic of China. Interpretation of the announcement on updating the list of bacteria used in food and the list of bacteria used in infant food (No.4, 2022) [J]. Beverage Ind, 2022, 25(5): 3-4. | |
| [8] | Abuqwider J, Altamimi M, Mauriello G. Limosilactobacillus reuteri in health and disease [J]. Microorganisms, 2022, 10(3): 522. |
| [9] | 张晓莉, 杨勇, 王光强, 等. 基于全基因组和比较基因组分析罗伊氏粘液乳杆菌的益生特性 [J]. 中国食品学报, 2025, 25(8): 8-20. |
| Zhang XL, Yang Y, Wang GQ, et al. Probiotic properties of Limosilactobacillus reuteri based on whole-genome and comparative genome analysis [J]. J Chin Inst Food Sci Technol, 2025, 25(8): 8-20. | |
| [10] | 张媛媛, 赵梦迪, 李悦垚, 等. 犬源罗伊氏乳杆菌LRA7对比格犬短链脂肪酸含量、肠道菌群及代谢组的影响 [J]. 动物营养学报, 2025, 37(4): 2648-2660. |
| Zhang YY, Zhao MD, Li YY, et al. Effects of canine-derived Lactobacillus reuteri LRA7 on short-chain fatty acid contents, intestinal flora and metabolome in beagle dogs [J]. Chin J Anim Nutr, 2025, 37(4): 2648-2660. | |
| [11] | 刘春艳. 猪源罗伊氏乳杆菌对断奶仔猪肠道屏障功能和细胞外基质的影响 [D]. 南宁: 广西大学, 2023. |
| Liu CY. Effects of Lactobacillus reuteri from pigs on intestinal barrier function and extracellular matrix of weaned piglets [D]. Nanning: Guangxi University, 2023. | |
| [12] | 杨巍巍, 彭子维, 谢昊炅, 等. 罗伊氏黏液乳杆菌的分离鉴定及对瘤胃体外发酵参数的影响 [J]. 饲料工业, 2025, 46(15): 143-150. |
| Yang WW, Peng ZW, Xie HJ, et al. Isolation and identification of Limosilactobacillus reuteri and its effects on rumen fermentation parameters in vitro [J]. Feed Ind, 2025, 46(15): 143-150. | |
| [13] | 王志刚, 徐伟慧, 张迎, 等. 一种谷氨酸浓缩母液的常温脱盐以及资源化利用方法及其装置: CN115872482B [P]. 2023-08-11. |
| Wang ZG, Xu WH, Zhang Y, et al. A method and apparatus for room temperature desalination and resource utilization of glutamic acid concentrate mother liquor: CN115872482B [P]. 2023-08-11. | |
| [14] | 农业农村部办公厅关于印发《直接饲喂微生物和发酵制品生产菌株鉴定及其安全性评价指南》的通知(农办牧[2021]43号) [J]. 中华人民共和国农业农村部公报, 2021(11): 97-111. |
| Circular of the general office of the ministry of agriculture and rural affairs on printing and distributing the guidelines on identification and safety evaluation of direct-fed microbials and fermented-food-derived bacterial strains [J]. Gaz Minist Agric Rural Aff People’s Repub China, 2021(11): 97-111. | |
| [15] | Zhou WQ, Gao S, Zheng J, et al. Identification of an Aerococcus urinaeequi isolate by whole genome sequencing and average nucleotide identity analysis [J]. J Glob Antimicrob Resist, 2022, 29: 353-359. |
| [16] | 李兰溪. 植物乳杆菌LPJZ-658对成犬血液指标、消化吸收及肠道菌群的影响 [D]. 长春: 吉林农业大学, 2024. |
| Li LX. Effects of Lactiplantibacillus plantarum LPJZ-658 on blood indexes, digestion and metabolism, and intestinal microbiota in dogs [D].Changchun: Jilin Agricultural University, 2024. | |
| [17] | 仇聪蕊, 舒祥力, 张云飞, 等. 一株猪源罗伊氏黏液乳杆菌的分离鉴定及其益生特性研究 [J]. 中国兽医科学, 2025, 55(9): 1216-1226. |
| Qiu CR, Shu XL, Zhang YF, et al. Isolation, identification and probiotic characteristics analysis of Limosilactobacillus reuteri from porcine [J]. Chin Vet Sci, 2025, 55(9): 1216-1226. | |
| [18] | 林龙镇, 邹卫玲, 李安章, 等. 产酸、耐酸乳酸菌的分离鉴定及益生特性 [J]. 华南农业大学学报, 2018, 39(2): 95-102. |
| Lin LZ, Zou WL, Li AZ, et al. Isolation, identification and probiotic characteristics of acidproducing and acid-resistant Lactobacillus strains [J]. J South China Agric Univ, 2018, 39(2): 95-102. | |
| [19] | Wu JJ, Zhou QY, Liu DM, et al. Evaluation of the safety and probiotic properties of Lactobacillus gasseri LGZ1029 based on whole genome analysis [J]. LWT, 2023, 184: 114759. |
| [20] | Yogeswara IBA, Maneerat S, Haltrich D. Glutamate decarboxylase from lactic acid bacteria-a key enzyme in GABA synthesis [J]. Microorganisms, 2020, 8(12): 1923. |
| [21] | Yang H, He MW, Wu CD. Cross protection of lactic acid bacteria during environmental stresses: Stress responses and underlying mechanisms [J]. LWT, 2021, 144: 111203. |
| [22] | Chintakovid N, Singkhamanan K, Yaikhan T, et al. Probiogenomic analysis of Lactiplantibacillus plantarum SPS109: a potential GABA-producing and cholesterol-lowering probiotic strain [J]. Heliyon, 2024, 10(13): e33823. |
| [23] | Kompramool S, Singkhamanan K, Pomwised R, et al. Genomic insights into Pediococcus pentosaceus ENM104: a probiotic with potential antimicrobial and cholesterol-reducing properties [J]. Antibiotics, 2024, 13(9): 813. |
| [24] | Bäumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut [J]. Nature, 2016, 535(7610): 85-93. |
| [25] | 李彩玉, 刘东慧, 权衡, 等. 益生菌细菌素防御病毒和细菌感染的机制研究 [J]. 中国兽医科学, 2024, 54(6): 808-815. |
| Li CY, Liu DH, Quan H, et al. Mechanism study of probiotics bacteriocins defense against viral and bacterial infections [J]. Chin Vet Sci, 2024, 54(6): 808-815. | |
| [26] | Ginsburg I. Role of lipoteichoic acid in infection and inflammation [J]. Lancet Infect Dis, 2002, 2(3): 171-179. |
| [27] | Averill-bates DA. The antioxidant glutathione [M]//Antioxidants. Amsterdam: Elsevier, 2023: 109-141. |
| [28] | Li XM, Zhang BX, Yan CX, et al. A fast and specific fluorescent probe for thioredoxin reductase that works via disulphide bond cleavage [J]. Nat Commun, 2019, 10: 2745. |
| [29] | Suwannasom N, Kao I, Pruß A, et al. Riboflavin: the health benefits of a forgotten natural vitamin [J]. Int J Mol Sci, 2020, 21(3): 950. |
| [30] | Martin-Gallausiaux C, Marinelli L, Blottière HM, et al. SCFA: mechanisms and functional importance in the gut [J]. Proc Nutr Soc, 2021, 80(1): 37-49. |
| [31] | Smythe P, Efthimiou G. In silico genomic and metabolic atlas of Limosilactobacillus reuteri DSM 20016: an insight into human health [J]. Microorganisms, 2022, 10(7): 1341. |
| [32] | Douillard FP, Ribbera A, Kant R, et al. Comparative genomic and functional analysis of 100 Lactobacillus rhamnosus strains and their comparison with strain GG [J]. PLoS Genet, 2013, 9(8): e1003683. |
| [33] | 苏世友, 滕超, 张维, 等. 细菌Ⅲ型聚酮合酶研究进展 [J]. 中国农业科技导报, 2013, 15(6): 119-129. |
| Su SY, Teng C, Zhang W, et al. Progress on type Ⅲ polyketide synthase from bacteria [J]. J Agric Sci Technol, 2013, 15(6): 119-129. | |
| [34] | Benjdia A, Balty C, Berteau O. Radical SAM enzymes in the biosynthesis of ribosomally synthesized and post-translationally modified peptides (RiPPs) [J]. Front Chem, 2017, 5: 87. |
| [35] | 汪金秀, 张琪, 丁伟, 等. 核糖体肽生物合成中的典型翻译后修饰研究 [J]. 生物技术通报, 2020, 36(10): 215-225. |
| Wang JX, Zhang Q, Ding W, et al. Classic post-translational modification in ribosomally synthesized and post-translationally modified peptides biosynthesis [J]. Biotechnol Bull, 2020, 36(10): 215-225. | |
| [36] | Bonelli RR, Schneider T, Sahl HG, et al. Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies [J]. Antimicrob Agents Chemother, 2006, 50(4): 1449-1457. |
| [37] | 柳少燕, 陈捷胤, 李蕾, 等. 拮抗菌与病原菌碳水化合物酶类比较分析 [J]. 基因组学与应用生物学, 2013, 32(1): 97-104. |
| Liu SY, Chen JY, Li L, et al. Comparative analysis of the carbohydrate-active enzymes between antagonistic microorganism and plant pathogen [J]. Genom Appl Biol, 2013, 32(1): 97-104. | |
| [38] | Oehme DP, Shafee T, Downton MT, et al. Differences in protein structural regions that impact functional specificity in GT2 family β-glucan synthases [J]. PLoS One, 2019, 14(10): e0224442. |
| [39] | Inglin RC, Meile L, Stevens MJA. Clustering of pan- and core-genome of Lactobacillus provides novel evolutionary insights for differentiation [J]. BMC Genom, 2018, 19(1): 284. |
| [1] | 吕镇, 甘恬, 霍思羽, 赵晨笛, 赵梦瑶, 李亚涛, 马玉超, 耿玉清. 产Surfactin贝莱斯芽胞杆菌C5A-1的鉴定和所产Surfactin对植物的促生效果[J]. 生物技术通报, 2025, 41(9): 265-276. |
| [2] | 苏秀敏, 韩文清, 王佼, 李鹏, 王秋兰, 李万星, 曹晋军. 哈茨木霉M408的分离鉴定、生物学特性及对番茄早疫病的生防效果[J]. 生物技术通报, 2025, 41(9): 277-288. |
| [3] | 廉少杰, 唐胜硕, 康传利, 刘磊, 郑德强, 杜帅, 汤丽伟, 张美霞, 刘蔷. 高产银耳多糖酶菌株的分离、鉴定、发酵条件优化及其酶的特性分析[J]. 生物技术通报, 2025, 41(9): 302-313. |
| [4] | 闫梦阳, 梁晓阳, 戴君昂, 张妍, 关团, 张辉, 刘良波, 孙志华. 阿莫西林降解菌的筛选及降解机制研究[J]. 生物技术通报, 2025, 41(9): 314-325. |
| [5] | 李亚涛, 张志鹏, 赵梦瑶, 吕镇, 甘恬, 魏浩, 吴书凤, 马玉超. 根瘤菌Bd1的全基因组分析及TetR3对细胞生长和结瘤的负调控功能[J]. 生物技术通报, 2025, 41(9): 289-301. |
| [6] | 张茹, 李一鸣, 张桐溪, 孙占斌, 任清, 潘寒姁. 厚朴中1株高产厚朴酚与和厚朴酚菌株的分离鉴定及其“发汗”工艺优化[J]. 生物技术通报, 2025, 41(8): 322-334. |
| [7] | 吴泽银, 黄晨阳, 赵梦然, 张利姣, 姚方杰. 短柄白黄侧耳CCMSSC 04611基因组特异性分析[J]. 生物技术通报, 2025, 41(5): 320-332. |
| [8] | 张慧, 卢文才, 王冬, 刘倩, 马连杰. 一株高产吲哚乙酸的Bacillus cereus YT2-1C的鉴定及促生作用[J]. 生物技术通报, 2025, 41(5): 300-309. |
| [9] | 项波卡, 周钻钻, 冯佳卉, 夏琛, 李奇, 陈春. 一株烟叶霉变真菌的分离鉴定及其致霉因素研究[J]. 生物技术通报, 2025, 41(2): 321-330. |
| [10] | 徐远志, 胡珊, 代思泽, 游帅, 郑明明, 单凯. 高活性脂肪酶的发掘、评价及在甘油二脂合成中应用[J]. 生物技术通报, 2025, 41(11): 177-189. |
| [11] | 苗昊翔, 张颖, 郭世鹏, 张健. 一株高产γ-氨基丁酸短乳杆菌TCCC13007全基因组测序及比较基因组分析[J]. 生物技术通报, 2025, 41(11): 166-176. |
| [12] | 张婷, 万雨欣, 徐伟慧, 王志刚, 陈文晶, 胡云龙. 一株玉米根际促生菌Leclercia adecarboxylata LN01促生效果研究及其基因组分析[J]. 生物技术通报, 2025, 41(1): 263-275. |
| [13] | 张亚亚, 李盼盼, 高惠惠, 贾晨波, 徐春燕. 基于根表真菌群落与病原菌鉴定探究‘宁杞5号’枸杞根腐病的发生机制[J]. 生物技术通报, 2024, 40(9): 238-248. |
| [14] | 王芳, 于璐, 齐泽铮, 周长军, 于吉东. 大豆镰刀菌根腐病拮抗菌的筛选及生防效果[J]. 生物技术通报, 2024, 40(7): 216-225. |
| [15] | 周江鸿, 夏菲, 仲丽, 仇兰芬, 李广, 刘倩, 张国锋, 邵金丽, 李娜, 车少臣. 黄栌枯萎病拮抗细菌CCBC3-3-1的全基因组测序及比较基因组分析[J]. 生物技术通报, 2024, 40(7): 235-246. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||