








生物技术通报 ›› 2020, Vol. 36 ›› Issue (12): 216-228.doi: 10.13560/j.cnki.biotech.bull.1985.2020-0365
收稿日期:2020-04-01
出版日期:2020-12-26
发布日期:2020-12-22
作者简介:梁昕鑫,女,硕士研究生,研究方向:酶工程;E-mail:基金资助:
LIANG Xin-xin1( ), TANG Dan1, HUO Yi-xin1,2(
), TANG Dan1, HUO Yi-xin1,2( )
)
Received:2020-04-01
Published:2020-12-26
Online:2020-12-22
摘要:
生物能源的大规模应用有助于环境保护和能源的可持续供给。目前生物燃料工艺开发实现了碳的循环利用,但尚未对氮的循环给予充分的重视。鉴于当前富含蛋白质的废弃物种类繁多且数量巨大,将蛋白质中氨基酸的碳骨架转化为生物燃料、大宗化学品及药物中间体等高附加值化合物正成为一个渐受关注的领域。从氨的生物循环利用角度入手,介绍了利用经光合作用生产的蛋白质等废弃物转化为高附加值化合物的高效、低成本、可持续的生产体系,并综述了进一步优化绿色转化技术所面临的挑战及可能的应对策略,旨为下一步高附加值化合物的低污染和高效生产提供参考。
梁昕鑫, 唐丹, 霍毅欣. 蛋白源生物质的绿色生物转化[J]. 生物技术通报, 2020, 36(12): 216-228.
LIANG Xin-xin, TANG Dan, HUO Yi-xin. Green Biotransformation of Protein-derived Biomass[J]. Biotechnology Bulletin, 2020, 36(12): 216-228.
| 富含蛋白质的生物质残留物/生物质种类 | 蛋白质含量(%,以干重为基准) | 市场价格(¥t-1生物质) | 潜在价值(¥ t-1 生物质) |
|---|---|---|---|
| 含可溶物的干酒糟(DDGS) | 20-40 | 770-1160 | 3100 |
| 甜菜酒渣 | 15-30 | 1160-1390 | 5420 |
| 蓖麻粕 | 30-60 | 770-1010 | 2320 |
| 豆粕 | 45-55 | 2320 | 4640 |
| 家禽羽毛 | 80-90 | 2320 | 2710 |
表1 各种富含蛋白质的生物质残留物中蛋白质的含量、价格和潜在价值
| 富含蛋白质的生物质残留物/生物质种类 | 蛋白质含量(%,以干重为基准) | 市场价格(¥t-1生物质) | 潜在价值(¥ t-1 生物质) |
|---|---|---|---|
| 含可溶物的干酒糟(DDGS) | 20-40 | 770-1160 | 3100 |
| 甜菜酒渣 | 15-30 | 1160-1390 | 5420 |
| 蓖麻粕 | 30-60 | 770-1010 | 2320 |
| 豆粕 | 45-55 | 2320 | 4640 |
| 家禽羽毛 | 80-90 | 2320 | 2710 |
| 蛋白源生物质种类 | Gly | Ala | Val | Leu | Ile | Ser | Thr | Cys | Met | Pro | Phe | Tyr | Trp | Cys | Lys | Arg | Asp/ Asn | Glu/ Gln |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 小麦酒糟(DDGS) | 4.5 | 4.3 | 4.8 | 6.6 | 3.4 | 4.5 | 3.4 | 1.1 | 1.6 | 10.3 | 4.7 | 3.3 | 0.0 | 2.2 | 2.7 | 3.2 | 5.5 | 33.8 |
| 木薯叶 | 7.0 | 7.7 | 0.0 | 11.6 | 6.8 | 6.2 | 0.0 | 1.3 | 2.9 | 0.0 | 7.2 | 5.7 | 2.7 | 3.2 | 8.2 | 7.2 | 8.5 | 13.9 |
| 甜菜酒渣 | 3.6 | 4.5 | 2.9 | 3.2 | 2.7 | 3.2 | 1.9 | 0.0 | 4.2 | 3.2 | 1.5 | 1.9 | 0.8 | 1.2 | 1.7 | 0.8 | 8.0 | 54.8 |
| 蓖麻粕 | 5.0 | 4.8 | 5.9 | 7.0 | 4.7 | 6.0 | 3.9 | 2.6 | 1.9 | 4.2 | 4.5 | 0.0 | / | 2.3 | 3.6 | 12.0 | 10.0 | 21.0 |
| 豆粕 | 4.0 | 4.3 | 5.1 | 7.5 | 5.2 | 4.9 | 3.8 | 1.6 | 1.4 | 4.5 | 4.9 | 3.3 | 1.7 | 2.3 | 6.4 | 7.8 | 11.9 | 19.7 |
| 家禽羽毛 | 7.3 | 5.9 | 6.6 | 8.2 | 4.9 | 10.7 | 4.2 | 4.2 | 5.6 | 8.5 | 4.8 | 2.4 | / | 1.3 | 2.3 | 6.7 | 6.6 | 9.9 |
表2 富含蛋白质的生物质废弃物经酸性条件水解后测定的氨基酸组成(湿重%)
| 蛋白源生物质种类 | Gly | Ala | Val | Leu | Ile | Ser | Thr | Cys | Met | Pro | Phe | Tyr | Trp | Cys | Lys | Arg | Asp/ Asn | Glu/ Gln |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 小麦酒糟(DDGS) | 4.5 | 4.3 | 4.8 | 6.6 | 3.4 | 4.5 | 3.4 | 1.1 | 1.6 | 10.3 | 4.7 | 3.3 | 0.0 | 2.2 | 2.7 | 3.2 | 5.5 | 33.8 |
| 木薯叶 | 7.0 | 7.7 | 0.0 | 11.6 | 6.8 | 6.2 | 0.0 | 1.3 | 2.9 | 0.0 | 7.2 | 5.7 | 2.7 | 3.2 | 8.2 | 7.2 | 8.5 | 13.9 |
| 甜菜酒渣 | 3.6 | 4.5 | 2.9 | 3.2 | 2.7 | 3.2 | 1.9 | 0.0 | 4.2 | 3.2 | 1.5 | 1.9 | 0.8 | 1.2 | 1.7 | 0.8 | 8.0 | 54.8 |
| 蓖麻粕 | 5.0 | 4.8 | 5.9 | 7.0 | 4.7 | 6.0 | 3.9 | 2.6 | 1.9 | 4.2 | 4.5 | 0.0 | / | 2.3 | 3.6 | 12.0 | 10.0 | 21.0 |
| 豆粕 | 4.0 | 4.3 | 5.1 | 7.5 | 5.2 | 4.9 | 3.8 | 1.6 | 1.4 | 4.5 | 4.9 | 3.3 | 1.7 | 2.3 | 6.4 | 7.8 | 11.9 | 19.7 |
| 家禽羽毛 | 7.3 | 5.9 | 6.6 | 8.2 | 4.9 | 10.7 | 4.2 | 4.2 | 5.6 | 8.5 | 4.8 | 2.4 | / | 1.3 | 2.3 | 6.7 | 6.6 | 9.9 |
| 氨基酸产品种类 | 主要生产方法 | 市场交易量(Mt) | 价格(¥ t-1) | 主要应用 |
|---|---|---|---|---|
| L-谷氨酸钠 | 谷氨酸棒杆菌发酵 | 3 | 约7750 | 食品增味剂 |
| L-赖氨酸·盐酸盐 | 谷氨酸棒杆菌发酵 | 2.4 | 约9300 | 饲料营养 |
| D,L-蛋氨酸 | 化学合成 | 1 | 约19350 | 饲料营养 |
| L-苏氨酸 | 大肠杆菌发酵 | 0.6 | 约10450 | 食物和饲料营养 |
表3 重要氨基酸的生产及其应用
| 氨基酸产品种类 | 主要生产方法 | 市场交易量(Mt) | 价格(¥ t-1) | 主要应用 |
|---|---|---|---|---|
| L-谷氨酸钠 | 谷氨酸棒杆菌发酵 | 3 | 约7750 | 食品增味剂 |
| L-赖氨酸·盐酸盐 | 谷氨酸棒杆菌发酵 | 2.4 | 约9300 | 饲料营养 |
| D,L-蛋氨酸 | 化学合成 | 1 | 约19350 | 饲料营养 |
| L-苏氨酸 | 大肠杆菌发酵 | 0.6 | 约10450 | 食物和饲料营养 |

图3 大肠杆菌中以氮为中心的代谢工程策略 重编程的转氨和脱氨基循环(A-D)的脱氨基过程,(E)工程酮酸途径。过量表达的酶以红色显示。主要策略为:(1)建立突变库,确定氨基酸降解的相关调控蛋白并使之失活;(2)对分解反应关键酶进行定向进化以获得具有更高反应速度及底物亲和性的突变体,并将其整合入工程菌;(3)通过构建氨泵使蛋白质降解所脱掉的氨排出胞外;(4)构建高效的代谢通路将蛋白质降解所产生的碳骨架转化为高附加值产品;(5)构建人工氨基转移循环并将氨基酸的脱氨反应与不可逆反应偶联,从而实现对所有氨基酸的不可逆脱氨(修改自文献[14])
| 微藻种类 | 蛋白含 量/% | 碳水化合 物含量/% | 脂质含 量/% |
|---|---|---|---|
| Arthrospira maxiuma | 60-71 | 13-16 | 6-7 |
| Synechococcus sp. | 63 | 15 | 11 |
| Spirulina platensis | 46-63 | 8-14 | 4-9 |
| Aphanizomenon flos aquae | 62 | 23 | 3 |
| Euglena gracilis | 39-61 | 14-18 | 14-20 |
| Chlorella vulgaris | 51-58 | 12-17 | 14-22 |
| Chlorella pyrenoidosa | 57 | 26 | 2 |
| Dunaliella salina | 57 | 32 | 6 |
| Scenedesmus obliquus | 50-56 | 10-17 | 12-14 |
| Anabaena cylindrica | 43-56 | 25-30 | 4-7 |
| Chlamydomonas rheinhardii | 48 | 17 | 21 |
| Porphyridium cruentum | 28-39 | 40-57 | 9-14 |
| Spirogyra sp. | 6-20 | 33-4 | 11-21 |
表4 藻类各组分及含量比例(干重百分比)
| 微藻种类 | 蛋白含 量/% | 碳水化合 物含量/% | 脂质含 量/% |
|---|---|---|---|
| Arthrospira maxiuma | 60-71 | 13-16 | 6-7 |
| Synechococcus sp. | 63 | 15 | 11 |
| Spirulina platensis | 46-63 | 8-14 | 4-9 |
| Aphanizomenon flos aquae | 62 | 23 | 3 |
| Euglena gracilis | 39-61 | 14-18 | 14-20 |
| Chlorella vulgaris | 51-58 | 12-17 | 14-22 |
| Chlorella pyrenoidosa | 57 | 26 | 2 |
| Dunaliella salina | 57 | 32 | 6 |
| Scenedesmus obliquus | 50-56 | 10-17 | 12-14 |
| Anabaena cylindrica | 43-56 | 25-30 | 4-7 |
| Chlamydomonas rheinhardii | 48 | 17 | 21 |
| Porphyridium cruentum | 28-39 | 40-57 | 9-14 |
| Spirogyra sp. | 6-20 | 33-4 | 11-21 |
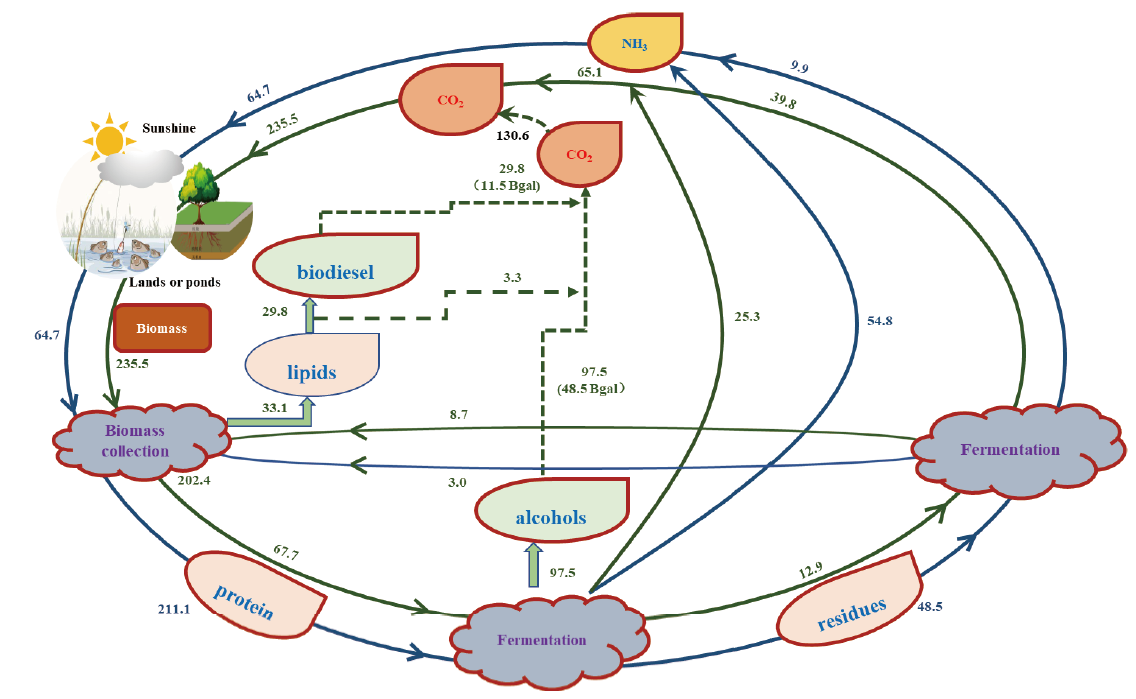
图7 重新施用氨的工艺流程图 碳和氮通量是根据生物量和生物燃料的化学成分计算的。碳(绿色)作为二氧化碳离开系统,后被重新回收成生物质。氮(蓝色)作为氨肥再循环。所有单位每年以百万吨计算,1.360亿t高级醇相当于600亿gal的生物燃料。所有数字都是根据案例研究计算所得,并不是当前生物燃料进程的真实数据。以每年生产600亿gal生物燃料为例(修改自文献[27])
| 微藻生物能源工业化生产中的瓶颈问题 | 氮循环驱动的生产方案以提升商业竞争力 | |
|---|---|---|
| 采收成本 | (1)藻生长速度慢,生物量低 | (1)微藻生物量大,易于采集 |
| (2)同液分离技术直接适用性差 | (2)同液分离技术环境友好 | |
| 培养成本 | (1)开放式培养,易被污染 | (1)开放式培养过程中具有生长优势,不易被污染 |
| (2)封闭式培养藻株生长周期长 | (2)无需营养缺乏环境,藻株生物量积累迅速,蛋白产量高 | |
| (3)生成副产物,降低转化率 | (3)脱氨后碳骨架用于生产高级醇,无其他副产物 | |
| (4)蛋白质和生物量损失显著 | (4)微藻光合固碳合成的蛋白质同时用于藻生长和转化燃料 | |
| (5)培养组分无法循环使用,高污染 | (5)氨基酸脱氨产生的NH3可用于微藻蛋白的合成与生长 | |
表5 微藻生物能源工业化生产概况一览表
| 微藻生物能源工业化生产中的瓶颈问题 | 氮循环驱动的生产方案以提升商业竞争力 | |
|---|---|---|
| 采收成本 | (1)藻生长速度慢,生物量低 | (1)微藻生物量大,易于采集 |
| (2)同液分离技术直接适用性差 | (2)同液分离技术环境友好 | |
| 培养成本 | (1)开放式培养,易被污染 | (1)开放式培养过程中具有生长优势,不易被污染 |
| (2)封闭式培养藻株生长周期长 | (2)无需营养缺乏环境,藻株生物量积累迅速,蛋白产量高 | |
| (3)生成副产物,降低转化率 | (3)脱氨后碳骨架用于生产高级醇,无其他副产物 | |
| (4)蛋白质和生物量损失显著 | (4)微藻光合固碳合成的蛋白质同时用于藻生长和转化燃料 | |
| (5)培养组分无法循环使用,高污染 | (5)氨基酸脱氨产生的NH3可用于微藻蛋白的合成与生长 | |
| [1] |
Scott E, Peter F, Sanders J. Biomass in the manufacture of industrial products—the use of proteins and amino acids[J]. Applied Microbiology and Biotechnology, 2007,75(4):751-762.
URL pmid: 17387469 |
| [2] |
Corma A, Iborra S, Velty A. Chemical routes for the transformation of biomass into chemicals[J]. Chemical Reviews, 2007,107(6):2411-2502.
doi: 10.1021/cr050989d URL pmid: 17535020 |
| [3] |
Dusselier M, Mascal M, Sels BF. Top chemical opportunities from carbohydrate biomass:A chemist’s view of the biorefinery[J]. Topics in Current Chemistry, 2014,353:1-40.
doi: 10.1007/128_2014_544 URL pmid: 24842622 |
| [4] |
Clarens AF, Resurreccion EP, White MA, et al. Environmental life cycle comparison of algae to other bioenergy feedstocks[J]. Environmental Science and Technology, 2010,44(5):1813-1819.
URL pmid: 20085253 |
| [5] |
Brentner LB, Eckelman MJ, Zimmerman JB. Combinatorial life cycle assessment to inform process design of industrial production of algal biodiesel[J]. Environmental Science and Technology, 2011,45(16):7060-7067.
URL pmid: 21662987 |
| [6] | Erisman JW, Van Grinsven H, Leip A, et al. Nitrogen and biofuels;an overview of the current state of knowledge[J]. Nutrient Cycling in Agroecosystems, 2010,86(2):211-223. |
| [7] |
Ha GS, El-Dalatony MM, Kim DH, et al. Biocomponent-based microalgal transformations into biofuels during the pretreatment and fermentation process[J]. Bioresource Technology, 2020,302:122809.
URL pmid: 31981806 |
| [8] | Deoliveira V, Brooks K, Nogueira L. A short introduction to the Distillers’ dried grains export market[R]. Lincoln:University of Nebraska-Lincoln, Department of Agricultural Economics, 2017. |
| [9] |
Sanders J, Scott E, Weusthuis R, et al. Bio-refinery as the bio-inspired process to bulk chemicals[J]. Macromolecular Bioscience, 2007,7(2):105-117.
URL pmid: 17295397 |
| [10] |
Wijffels RH, Barbosa MJ. An outlook on microalgal biofuels[J]. Science, 2010,329(5993):796-799.
URL pmid: 20705853 |
| [11] | Erisman JW, Sutton MA, Galloway J, et al. How a century of ammonia synjournal changed the world[J]. Nature Geoscience, 2008,1(10):636-639. |
| [12] |
Chojnacka K, Moustakas K, Witek-Krowiak A. Bio-based fertilizers:A practical approach towards circular economy[J]. Bioresource Technology, 2020,295:122223.
doi: 10.1016/j.biortech.2019.122223 URL pmid: 31623921 |
| [13] |
Vaneeckhaute C, Styles D, Prade T, et al. Closing nutrient loops through decentralized anaerobic digestion of organic residues in agricultural regions:A multi-dimensional sustainability assessment[J]. Resources Conservation and Recycling, 2018,136:110-117.
doi: 10.1016/j.resconrec.2018.03.027 URL |
| [14] | Huo YX, Cho KM, Rivera JGL, et al. Conversion of proteins into biofuels by engineering nitrogen flux[J]. Nature Biotechnology, 2011,29(4):346. |
| [15] |
Branduardi P, Longo V, Berterame NM, et al. A novel pathway to produce butanol and isobutanol in Saccharomyces cerevisiae[J]. Biotechnology for Biofuels, 2013,6(1):68.
URL pmid: 23642236 |
| [16] |
Choi KY, Wernick DG, Tat CA, et al. Consolidated conversion of protein waste into biofuels and ammonia using Bacillus subtilis[J]. Metabolic Engineering, 2014,23:53-61.
doi: 10.1016/j.ymben.2014.02.007 URL pmid: 24566040 |
| [17] |
Mikami Y, Yoneda H, Tatsukami Y, et al. Ammonia production from amino acid-based biomass-like sources by engineered Escherichia coli[J]. AMB Express, 2017,7(1):83.
URL pmid: 28429328 |
| [18] | Ma L, Guo L, Yang Y, et al. Protein-based biorefining driven by nitrogen-responsive transcriptional machinery[J]. Biotechnology for Biofuels, 2020,13(1):1-14. |
| [19] |
Jach ME, Baj T, Juda M, et al. Statistical evaluation of growth parameters in biofuel waste as a culture medium for improved production of single cell protein and amino acids by Yarrowia lipolytica[J]. AMB Express, 2020,10(1):35.
doi: 10.1186/s13568-020-00968-x URL pmid: 32072349 |
| [20] |
Tuck CO, Pérez E, Horváth IT, et al. Valorization of biomass:deriving more value from waste[J]. Science, 2012,337(6095):695-699.
URL pmid: 22879509 |
| [21] |
De-Schouwer F, Claes L, Vandekerkhove A, et al. Protein-rich biomass waste as a resource for future biorefineries:State of the art, challenges, and opportunities[J]. ChemSusChem, 2019,12(7):1272-1303.
doi: 10.1002/cssc.201802418 URL pmid: 30667150 |
| [22] |
El-Dalatony MM, Saha S, Govindwar SP, et al. Biological conversion of amino acids to higher alcohols[J]. Trends in Biotechnology, 2019,37(8):855-869.
URL pmid: 30871798 |
| [23] | Bozell JJ, Petersen GR. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited[J]. Green Chemistry, 2010,12(4):539-554. |
| [24] |
Huber GW and Corma A. Synergies between bio-and oil refineries for the production of fuels from biomass[J]. Angewandte Chemie International Edition, 2007,46(38):7184-7201.
URL pmid: 17610226 |
| [25] |
Besson ML, Gallezot P, Pinel C. Conversion of biomass into chemicals over metal catalysts[J]. Chemical Reviews, 2014,114(3):1827-1870.
URL pmid: 24083630 |
| [26] |
Gallezot P. Conversion of biomass to selected chemical products[J]. Chemical Society Reviews, 2012,41(4):1538-1558.
URL pmid: 21909591 |
| [27] | Huo YX, Wernick DG, Liao JC. Toward nitrogen neutral biofuel production[J]. Curr Opin Biotechnolo, 2012,23(3):406-413. |
| [28] | Werpy T, Petersen G. Top value added chemicals from biomass:volume I--results of screening for potential candidates from sugars and synreport gas[R]. United States:National Renewable Energy Lab., Golden, CO (US), 2004. |
| [29] |
Kumar K, Dasgupta CN, Nayak B, et al. Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria[J]. Bioresource Technology, 2011,102(8):4945-4953.
URL pmid: 21334885 |
| [30] |
Gong Y, Jiang M. Biodiesel production with microalgae as feedstock:from strains to biodiesel[J]. Biotechnology Letters, 2011,33(7):1269-1284.
URL pmid: 21380528 |
| [31] |
Becker E. Micro-algae as a source of protein[J]. Biotechnology Advances, 2007,25(2):207-210.
doi: 10.1016/j.biotechadv.2006.11.002 URL pmid: 17196357 |
| [32] |
Shen CR, Liao JC. Metabolic engineering of Escherichia coli for 1-butanol and 1-propanol production via the keto-acid pathways[J]. Metabolic Engineering, 2008,10(6):312-320.
doi: 10.1016/j.ymben.2008.08.001 URL pmid: 18775501 |
| [33] |
Atsumi S, Hanai T, Liao JC. Non-fermentative pathways for synjournal of branched-chain higher alcohols as biofuels[J]. Nature, 2008,451(7174):86.
URL pmid: 18172501 |
| [34] | Wood W, Gunsalus IC. Serine and threonine deaminases of Escherichia coli:Activators for a cell-free enzyme[J]. Journal of Biological Chemistry, 1949,181(1):171-182. |
| [35] | Arie BB, Sima SF, Huang LL, et al. Methods for the preparation of para-hydroxycinnamic acid and cinnamic acid at alkaline pH:US, 8003356 B2[P]. 2011-08-23. |
| [36] | Brenda Database[DB/OL]. Available from: http://www.brenda. uni-koeln. de. |
| [37] |
Shen CR, Lan EI, Dekishima Y, et al. Driving forces enable high-titer anaerobic 1-butanol synjournal in Escherichia coli[J]. Appl Environ Microbiol, 2011,77(9):2905-2915.
URL pmid: 21398484 |
| [38] |
Choi YJ, Park JH, Kim TY, et al. Metabolic engineering of Escherichia coli for the production of 1-propanol[J]. Metabolic Engineering, 2012,14(5):477-486.
URL pmid: 22871504 |
| [39] |
Wang X, Xu N, Hu S, et al. d-1, 2, 4-Butanetriol production from renewable biomass with optimization of synthetic pathway in engineered Escherichia coli[J]. Bioresource Technology, 2018,250:406-412.
doi: 10.1016/j.biortech.2017.11.062 URL pmid: 29195152 |
| [40] | Li SY, Ng IS, Chen PT, et al. Biorefining of protein waste for production of sustainable fuels and chemicals[J]. Biotechnology for Biofuels, 2018,11(1):256. |
| [41] |
Lee J, Jang YS, Choi SJ, et al. Metabolic engineering of Clostridium acetobutylicum ATCC 824 for isopropanol-butanol-ethanol fermentation[J]. Applied and Environmental Microbiology, 2012,78(5):1416-1423.
URL pmid: 22210214 |
| [42] |
Ohisa N, Yamaguchi M, Kurihara N. Lindane degradation by cell-free extracts of Clostridium rectum[J]. Archives of Microbiology, 1980,125(3):221-225.
URL pmid: 6155109 |
| [43] | Zhao J, Lu C, Chen CC, et al. Biological production of butanol and higher alcohols[M]// Yang ST, El-Enshasy HA, Thongchul N. Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers. Wiley, 2013: 235-262. |
| [44] | Sangavai C, Chellapandi P. Amino acid catabolism-directed biofuel production in Clostridium sticklandii:an insight into model-driven systems engineering[J]. Biotechnol Rep, 2017,16:32-43. |
| [45] |
Milias-Argeitis A, Oliveira AP, Gerosa L, et al. Elucidation of genetic interactions in the yeast GATA-factor network using Bayesian model selection[J]. PLoS Computational Biology, 2016,12(3):e1004784.
doi: 10.1371/journal.pcbi.1004784 URL pmid: 26967983 |
| [46] | Ogata K, Uchiyama K, Yamada H. Microbial formation of cinnamic acid from phenylalanine[J]. Agricultural and Biological Chemistry, 1966,30(3):311-312. |
| [47] | Tang L. Amino acid catabolism and antibiotic synjournal:valine is a source of precursors for macrolide biosynjournal in Streptomyces ambofaciens and Streptomyces fradiae[J]. Journal of Bacteriology, 1994,16:32-43. |
| [48] |
Norton JE, Sokath JR. Oxidation of D- and L-valine by enzymes of Pseudomonas aeruginosa[J]. J Bacteriol, 1966,92(1):116-120.
URL pmid: 4957429 |
| [49] | Izaguirre JK, Dietrich T, Villarán MC, et al. Protein hydrolysate from organic fraction of municipal solid waste compost as nitrogen source to produce lactic acid by Lactobacillus fermentum ATCC 9338 and Lactobacillus plantarum NCIMB 8826[J]. Process Biochemistry, 2020,88:15-21. |
| [50] |
Ho SH, Chen CY, Lee DJ, et al. Perspectives on microalgal CO2-emission mitigation systems—a review[J]. Biotechnology Advances, 2011,29(2):189-198.
doi: 10.1016/j.biotechadv.2010.11.001 URL pmid: 21094248 |
| [51] |
Peccia J, Haznedaroglu B, Gutierrez J, et al. Nitrogen supply is an important driver of sustainable microalgae biofuel production[J]. Trends in Biotechnology, 2013,31(3):134-138.
URL pmid: 23414785 |
| [52] |
Mielenz JR. Biofuels from protein[J]. Nature Biotechnology, 2011,29(4):327.
doi: 10.1038/nbt.1838 URL pmid: 21478847 |
| [53] |
Wang G, Huang M, Nielsen J. Exploring the potential of Saccharo-myces cerevisiae for biopharmaceutical protein production[J]. Curr Opin Biotechnol, 2017,48:77-84.
URL pmid: 28410475 |
| [54] |
Li W, Chen SJ, Wang JH, et al. Genetic engineering to alter carbon flux for various higher alcohol productions by Saccharomyces cerevisiae for Chinese Baijiu fermentation[J]. Applied Microbiology and Biotechnology, 2018,102(4):1783-1795.
doi: 10.1007/s00253-017-8715-5 URL pmid: 29305698 |
| [55] |
Atsumi S, Liao JC. Directed evolution of Methanococcus jannaschii citramalate synthase for biosynjournal of 1-propanol and 1-butanol by Escherichia coli[J]. Applied and Environmental Microbiology, 2008,74(24):7802-7808.
doi: 10.1128/AEM.02046-08 URL pmid: 18952866 |
| [56] |
Bond-Watts BB, Bellerose RJ, Chang MC. Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways[J]. Nature Chemical Biology, 2011,7(4):222.
doi: 10.1038/nchembio.537 URL pmid: 21358636 |
| [57] |
Cann AF, Liao JC. Production of 2-methyl-1-butanol in engineered Escherichia coli[J]. Applied Microbiology and Biotechnology, 2008,81(1):89-98.
doi: 10.1007/s00253-008-1631-y URL pmid: 18758769 |
| [58] | Hanai T, Atsumi S, Liao J. Engineered synthetic pathway for isopropanol production in Escherichia coli[J]. Applied and Environmental Microbiology, 2007,73(24):7814-7818. |
| [59] |
Connor MR, Cann AF, Liao JC. 3-Methyl-1-butanol production in Escherichia coli:random mutagenesis and two-phase fermentation[J]. Applied Microbiology and Biotechnology, 2010,86(4):1155-1164.
URL pmid: 20072783 |
| [60] |
Rodolfi L, Chini Zittelli G, Bassi N, et al. Microalgae for oil:Strain selection, induction of lipid synjournal and outdoor mass cultivation in a low-cost photobioreactor[J]. Biotechnology and Bioengineering, 2009,102(1):100-112.
URL pmid: 18683258 |
| [61] | 谢平. 论蓝藻水华的发生机制---从生物进化, 生物地球化学和生态学视点[M]. 北京: 科学出版社, 2007. |
| Xie P. On the mechanism of cyanobacteria blooms:From the perspectives of biological evolution, biogeochemistry and ecology[M]. Beijing: Science Press, 2007. | |
| [62] | Wu W, Davis R, Wu B. Improved algal biofuel yield through bioconversion of proteins to mixed alcohols and sesquiterpenes[M]. United States:National Nuclear Security Administration, 2015: 26-30. |
| [63] | Sheehan J, Dunahay T, Benemann J, et al. Look back at the US department of energy’s aquatic species program:biodiesel from algae;close-out report[R]. United States:National Renewable Energy Lab., Golden, CO. (US), 1998. |
| [64] | Posten C. Design principles of photo-bioreactors for cultivation of microalgae[J]. Engineering in Life Sciences, 2009,9(3):165-177. |
| [65] | Xu L, Weathers PJ, Xiong XR, et al. Microalgal bioreactors:challenges and opportunities[J]. Engineering in Life Sciences, 2009,9(3):178-189. |
| [66] |
Bozarth A, Maier UG, Zauner S. Diatoms in biotechnology:modern tools and applications[J]. Applied microbiology and biotechnology, 2009,82(2):195-201.
URL pmid: 19082585 |
| [67] | Kumar V, Nanda M, Joshi H, et al. Production of biodiesel and bioethanol using algal biomass harvested from fresh water river[J]. Renewable Energy, 2018,116:606-612. |
| [68] | Baral NR, Shah A. Techno-economic analysis of cellulosic butanol production from corn stover through acetone-butanol-ethanol fermentation[J]. Energy and Fuels, 2016,30(7):5779-5790. |
| [1] | 梅欢, 李玥, 刘可蒙, 刘吉华. 小檗碱桥酶高效原核表达及生物合成l-SLR的研究[J]. 生物技术通报, 2023, 39(7): 277-287. |
| [2] | 赵宝顶, 吕佳, 申玉玉, 桂玲, 陈钟秀, 陈杰, 路福平, 黎明. 基于信号肽和分子伴侣策略促进大肠杆菌高效转化尿苷[J]. 生物技术通报, 2022, 38(11): 238-249. |
| [3] | 孙腾, 徐刘佳, 郑明明. 硫辛酸衍生物的制备与活性研究进展[J]. 生物技术通报, 2020, 36(4): 41-46. |
| [4] | 赵祥杰, 杨文君, 杨荣玲, 吴婷婷, 王朝宇, 许宁宁, 何佳美. 花色苷生物转化修饰的研究进展[J]. 生物技术通报, 2019, 35(10): 205-211. |
| [5] | 高庆龙, 陈升宝, 田文佳, 张学铭, 马玉超. 代谢工程法强化恶臭假单胞菌利用木质素积累PHA的能力[J]. 生物技术通报, 2018, 34(10): 92-99. |
| [6] | 白龙,李春美,吕途,杜颖,杨玥,田沈. 生物转化能源草制取纤维素乙醇的研究进展[J]. 生物技术通报, 2017, 33(5): 50-56. |
| [7] | 朱凤芝, 程赪, 刘祥胜, 张昆, 王立爽, 王敏, 骆健美. 利用响应面法优化巴卡亭III生成10-DAB的工艺条件[J]. 生物技术通报, 2017, 33(4): 238-246. |
| [8] | 何庆林, 白艳芬, 周威, 殷华, 陈宁, 庄以彬, 刘涛. 利用糖基转移酶UGT73B6在大肠杆菌中生物催化酚苷类天然产物[J]. 生物技术通报, 2017, 33(11): 136-142. |
| [9] | 张丛丛,陈彩霞,陈笑,温雅,晏礼明,陶勇. 全细胞催化法生产N-乙酰神经氨酸的研究进展[J]. 生物技术通报, 2015, 31(4): 175-183. |
| [10] | 王艳霞,刘祥胜,王敏,骆健美. 利用分子生物学技术提高微生物有机溶剂耐受性的研究进展[J]. 生物技术通报, 2015, 31(10): 77-88. |
| [11] | 赵婧楠, 李志敏, 叶勤. Pseudomonas sp. F-12发酵优化及转化合成半胱氨酸的研究[J]. 生物技术通报, 2014, 0(10): 207-214. |
| [12] | 于雷, 李成龙, 于珊珊. 人参皂苷CK 的研究进展[J]. 生物技术通报, 2013, 0(1): 31-35. |
| [13] | 胡晓清, 郭雯, 韩国强. 微生物转化生产S-腺苷甲硫氨酸[J]. 生物技术通报, 2012, 0(12): 33-39. |
| [14] | 赵东;荆玮;姚盟成;董逸楠;李玉;. 京尼平的微生物转化及其交联特性的测定分析[J]. , 2012, 0(07): 146-151. |
| [15] | 杨光富;魏云林;. 假单胞菌研究现状及应用前景[J]. , 2011, 0(01): 37-39. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||