








生物技术通报 ›› 2021, Vol. 37 ›› Issue (2): 1-14.doi: 10.13560/j.cnki.biotech.bull.1985.2020-0575
• 研究报告 • 下一篇
收稿日期:2020-05-12
出版日期:2021-02-26
发布日期:2021-02-26
作者简介:马旭辉,女,硕士研究生,研究方向:植物分子生物学和基因工程;E-mail: 基金资助:
MA Xu-hui( ), CHEN Ru-mei, LIU Xiao-qing, ZHAO Jun(
), CHEN Ru-mei, LIU Xiao-qing, ZHAO Jun( ), ZHANG Xia(
), ZHANG Xia( )
)
Received:2020-05-12
Published:2021-02-26
Online:2021-02-26
摘要:
褪黑素是一种在生物体内广泛存在的吲哚胺类化合物,参与植物的多种生理和生化过程。近年来研究认为褪黑素可以不同程度地增强植物的抗逆性,但对其作用机理仍知之甚少。通过两种褪黑素的施用方法,详细研究了褪黑素对于玉米根系发育和抗旱性的影响。首先,采用水培根灌褪黑素的方法对玉米幼苗的根系和生长状况进行分析,结果表明施加褪黑素显著提高多种玉米幼苗根系参数,包括根长、根表面积、根体积和侧根数目等。其次,采用盆栽浸种褪黑素的方法,对叶片相对含水量、光合作用、抗氧化酶活性、地上部分生物量等进行测定,结果表明在干旱胁迫条件下,褪黑素浸泡种子的处理方式能够提高植株的光合速率、气孔导度和蒸腾速率,增强抗氧化酶活性以及降低活性氧和丙二醛含量,证明褪黑素促进植物根系发育,减轻氧化损伤,缓解光合抑制,改善植物水分状况,从而提高植物抗旱性。
马旭辉, 陈茹梅, 柳小庆, 赵军, 张霞. 褪黑素对玉米幼苗根系发育和抗旱性的影响[J]. 生物技术通报, 2021, 37(2): 1-14.
MA Xu-hui, CHEN Ru-mei, LIU Xiao-qing, ZHAO Jun, ZHANG Xia. Effects of Melatonin on Root Growth and Drought Tolerance of Maize Seedlings[J]. Biotechnology Bulletin, 2021, 37(2): 1-14.
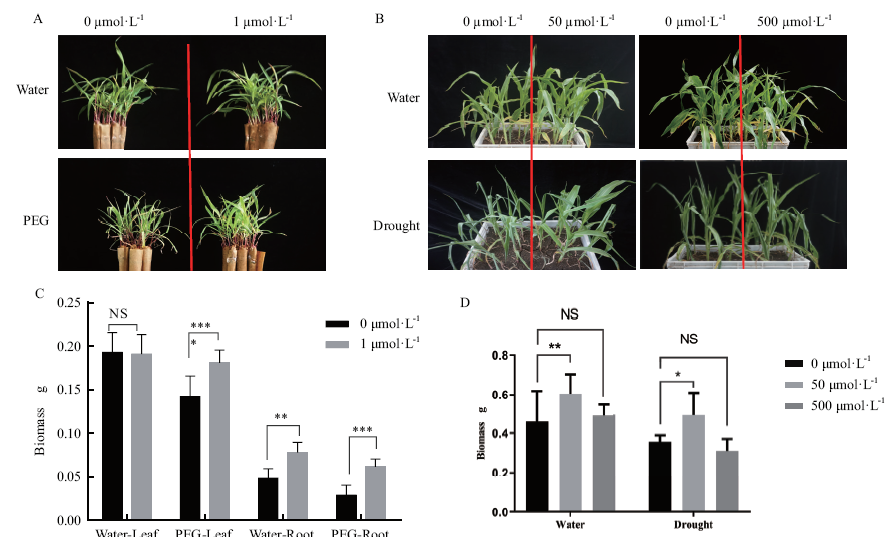
图1 水培根灌和浸种盆栽两种外源褪黑素施加方式对植物生长发育的影响 A:水培法植株的地上部分表型;B:盆栽法植株的地上部分表型;C:水培法植株在正常条件和干旱条件下根系和地上部分的生物量;D:盆栽法植株在正常条件和干旱条件下地上部分的生物量。*表示显著的统计差异,****P<0.0001,***P<0.001,**P<0.01,*P<0.05
| 分类 | 特征 | 缩写 | 单位 | 描述 |
|---|---|---|---|---|
| 主根 | 主根长度 | PAL | cm | 主根(包括主根上的侧根)长度 |
| 主根表面积 | PSA | cm2 | 主根(包括主根上的侧根)表面积 | |
| 主根体积 | PRV | cm3 | 主根(包括主根上的侧根)体积 | |
| 主根长度 | PRL | cm | 主根(不包括主根上的侧根)长度 | |
| 侧根数目 | LRNPR | branch | 主根上侧根的数目 | |
| 种子根 | 种子根的长度 | SAL | cm | 种子根(包括种子根上的侧根)的长度 |
| 种子根的表面积 | SSA | cm2 | 种子根(包括种子根上的侧根)的表面积 | |
| 种子根的体积 | SRV | cm3 | 种子根(包括种子根上的侧根)的体积 | |
| 总根 | 总根长 | TRL | cm | 整个根系的长度 |
| 总根表面积 | TSA | cm2 | 整个根系的表面积 | |
| 总根体积 | TRV | cm3 | 整个根系的体积 |
表1 玉米根系构型构相关特征概述
| 分类 | 特征 | 缩写 | 单位 | 描述 |
|---|---|---|---|---|
| 主根 | 主根长度 | PAL | cm | 主根(包括主根上的侧根)长度 |
| 主根表面积 | PSA | cm2 | 主根(包括主根上的侧根)表面积 | |
| 主根体积 | PRV | cm3 | 主根(包括主根上的侧根)体积 | |
| 主根长度 | PRL | cm | 主根(不包括主根上的侧根)长度 | |
| 侧根数目 | LRNPR | branch | 主根上侧根的数目 | |
| 种子根 | 种子根的长度 | SAL | cm | 种子根(包括种子根上的侧根)的长度 |
| 种子根的表面积 | SSA | cm2 | 种子根(包括种子根上的侧根)的表面积 | |
| 种子根的体积 | SRV | cm3 | 种子根(包括种子根上的侧根)的体积 | |
| 总根 | 总根长 | TRL | cm | 整个根系的长度 |
| 总根表面积 | TSA | cm2 | 整个根系的表面积 | |
| 总根体积 | TRV | cm3 | 整个根系的体积 |
| 分类 | 特征 | 0 μmol/L | 1 μmol/L | Significance | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| 总根 | TRL | 150.375 | 32.656 | 178.512 | 35.414 | ** | |
| TSA | 27.414 | 4.482 | 37.812 | 11.272 | ** | ||
| TRV | 0.564 | 0.096 | 0.593 | 0.100 | *** | ||
| 主根 | PAL | 64.432 | 11.777 | 88.796 | 16.124 | ** | |
| PRL | 23.265 | 0.959 | 28.709 | 0.962 | **** | ||
| PSA | 8.557 | 1.333 | 9.053 | 1.353 | *** | ||
| PRV | 0.348 | 0.487 | 0.370 | 0.463 | **** | ||
| LRNPR | 207 | 21.818 | 239 | 16.244 | **** | ||
| 种子根 | SAL | 85.942 | 20.635 | 89.716 | 25.746 | ** | |
| SSA | 18.857 | 3.694 | 28.759 | 2.878 | *** | ||
| SRV | 0.216 | 0.255 | 0.223 | 0.192 | * | ||
表2 正常条件下水培根灌褪黑素对根系构型相关性状影响的统计总结
| 分类 | 特征 | 0 μmol/L | 1 μmol/L | Significance | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| 总根 | TRL | 150.375 | 32.656 | 178.512 | 35.414 | ** | |
| TSA | 27.414 | 4.482 | 37.812 | 11.272 | ** | ||
| TRV | 0.564 | 0.096 | 0.593 | 0.100 | *** | ||
| 主根 | PAL | 64.432 | 11.777 | 88.796 | 16.124 | ** | |
| PRL | 23.265 | 0.959 | 28.709 | 0.962 | **** | ||
| PSA | 8.557 | 1.333 | 9.053 | 1.353 | *** | ||
| PRV | 0.348 | 0.487 | 0.370 | 0.463 | **** | ||
| LRNPR | 207 | 21.818 | 239 | 16.244 | **** | ||
| 种子根 | SAL | 85.942 | 20.635 | 89.716 | 25.746 | ** | |
| SSA | 18.857 | 3.694 | 28.759 | 2.878 | *** | ||
| SRV | 0.216 | 0.255 | 0.223 | 0.192 | * | ||
| 分类 | 特征 | 0 μmol/L | 1 μmol/L | Significance | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| 总根 | TRL | 105.7 | 20.737 | 132.2 | 9.196 | **** | |
| TSA | 15.81 | 4.531 | 25.82 | 3.389 | *** | ||
| TRV | 0.332 | 0.058 | 0.469 | 0.06 | *** | ||
| 主根 | PAL | 53.954 | 7.444 | 61.309 | 11.265 | **** | |
| PRL | 18.267 | 1.989 | 21.062 | 2.677 | **** | ||
| PSA | 7.155 | 1.142 | 8.162 | 0.933 | * | ||
| PRV | 0.159 | 0.013 | 0.312 | 0.014 | ** | ||
| LRNPR | 119 | 18.788 | 132 | 24.615 | ** | ||
| 种子根 | SAL | 51.746 | 21.368 | 70.891 | 15.744 | *** | |
| SSA | 8.655 | 4.477 | 17.658 | 5.169 | *** | ||
| SRV | 0.123 | 0.266 | 0.158 | 0.187 | ** | ||
表3 PEG6000 诱导的干旱胁迫条件下水培添加褪黑素对根系构型相关性状影响的统计总结
| 分类 | 特征 | 0 μmol/L | 1 μmol/L | Significance | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| 总根 | TRL | 105.7 | 20.737 | 132.2 | 9.196 | **** | |
| TSA | 15.81 | 4.531 | 25.82 | 3.389 | *** | ||
| TRV | 0.332 | 0.058 | 0.469 | 0.06 | *** | ||
| 主根 | PAL | 53.954 | 7.444 | 61.309 | 11.265 | **** | |
| PRL | 18.267 | 1.989 | 21.062 | 2.677 | **** | ||
| PSA | 7.155 | 1.142 | 8.162 | 0.933 | * | ||
| PRV | 0.159 | 0.013 | 0.312 | 0.014 | ** | ||
| LRNPR | 119 | 18.788 | 132 | 24.615 | ** | ||
| 种子根 | SAL | 51.746 | 21.368 | 70.891 | 15.744 | *** | |
| SSA | 8.655 | 4.477 | 17.658 | 5.169 | *** | ||
| SRV | 0.123 | 0.266 | 0.158 | 0.187 | ** | ||
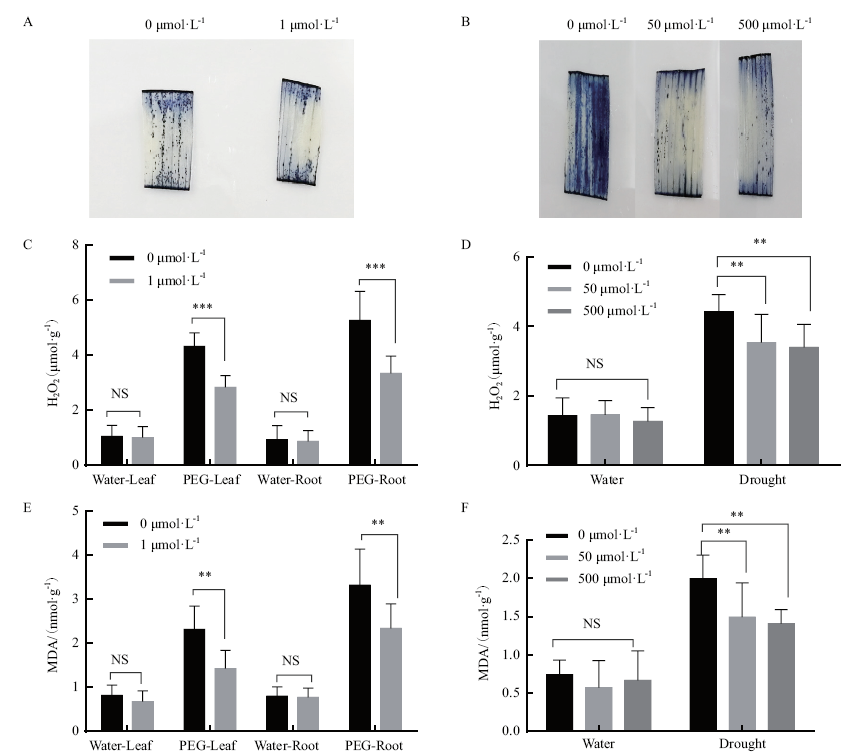
图3 水培根灌和浸种盆栽两种褪黑素处理方式对ROS和MDA含量的影响 A,B:组织化学染色测定超氧阴离子O2-;C,D:过氧化氢(H2O2)的含量;E,F:丙二醛(MDA)的含量。A、C、E测定的是水培法的植株,B、D、F测定的是盆栽法的植株。*P≤ 0.05;**P ≤ 0.01;*** P≤ 0.001;**** P≤ 0.0001
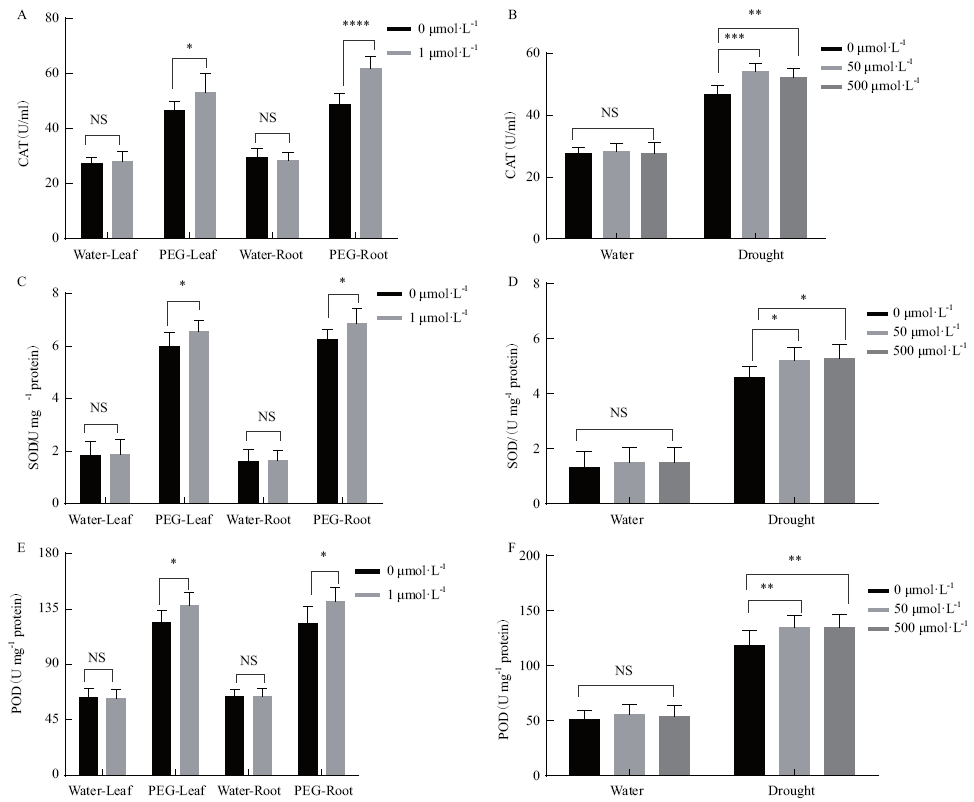
图5 水培根灌和浸种盆栽两种褪黑素处理方式对玉米植株抗氧化酶活性的影响 A,B:过氧化氢酶(CAT)活性;C,D:超氧化物歧化酶(SOD)活性;E,F:过氧化物酶(POD)活性;A、C、E测定的是水培法的植株,B、D、F测定的是是盆栽法的植株。*P≤ 0.05;**P ≤ 0.01;*** P≤ 0.001;**** P≤ 0.0001
| [1] |
Daryanto S, Wang L, Jacinthe P-A. Global synjournal of drought effects on maize and wheat production[J]. PLoS One, 2016,11(5):e0156362.
doi: 10.1371/journal.pone.0156362 URL pmid: 27223810 |
| [2] |
Singh B UK. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress[J]. Plant Growth Regulation, 2003,39:137-141.
doi: 10.1023/A:1022556103536 URL |
| [3] | Lerner AB, Case JD, takahashi Y, et al. Isolation of melatonin, the pineal gland factor that lightens melanocytes[J]. Journal of the American Chemical Society, 1958,80(10):2587. |
| [4] |
Tan DX, Hardeland R, Manchester LC, et al. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science[J]. Journal of Experimental Botany, 2012,63(2):577-597.
doi: 10.1093/jxb/err256 URL |
| [5] |
Arnao MB, Hernandez-Ruiz J. Melatonin:plant growth regulator and/or biostimulator during stress?[J]. Trends in Plant Science, 2014,19(12):789-797.
doi: 10.1016/j.tplants.2014.07.006 URL |
| [6] |
Hardeland R. Melatonin in plants and other phototrophs:advances and gaps concerning the diversity of functions[J]. Journal of Experimental Botany, 2015,66(3):627-646.
doi: 10.1093/jxb/eru386 URL pmid: 25240067 |
| [7] |
Kanwar MK, Yu J, Zhou J. Phytomelatonin:Recent advances and future prospects[J]. Journal of Pineal Research, 2018,65(4):e12526.
doi: 10.1111/jpi.12526 URL pmid: 30256447 |
| [8] |
Vadez V. Root hydraulics:The forgotten side of roots in drought adaptation[J]. Field Crops Research, 2014,165:15-24.
doi: 10.1016/j.fcr.2014.03.017 URL |
| [9] |
Lynch J. Root architecture and plant productivity[J]. Plant Physiology, 1995,109(1):7-13.
doi: 10.1104/pp.109.1.7 URL pmid: 12228579 |
| [10] |
Hochholdinger F, Woll K, Sauer M, et al. Genetic dissection of root formation in maize(Zea mays L.)reveals root-type specific developmental programmes[J]. Annals of Botany, 2004,93(4):359-368.
doi: 10.1093/aob/mch056 URL pmid: 14980975 |
| [11] |
Zhang N, Zhao B, Zhang HJ, et al. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber(Cucumis sativus L.)[J]. Journal of Pineal Research, 2013,54(1):15-23.
doi: 10.1111/j.1600-079X.2012.01015.x URL pmid: 22747917 |
| [12] |
Zhang N, Zhang HJ, Zhao B, et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation[J]. Journal of Pineal Research, 2014,56(1):39-50.
doi: 10.1111/jpi.12095 URL |
| [13] |
Wang P, Sun X, Li C, et al. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple[J]. Journal of Pineal Research, 2013,54(3):292-302.
doi: 10.1111/jpi.12017 URL pmid: 23106234 |
| [14] | Xu XD, Sun Y, Guo XQ, et al. Effects of exogenous melatonin on ascorbate metabolism system in cucumber seedlings under high temperature stress[J]. Chinese Journal of Applied Ecology, 2010,21(10):2580-2586. |
| [15] | Gao QH, Jia SS, Miao YM, et al. Effects of exogenous melatonin on nitrogen metabolism and osmotic adjustment substances of melon seedlings under sub-low temperature[J]. Chinese Journal of Applied Ecology, 2016,27(2):519-524. |
| [16] |
Kostopoulou Z, Therios I, Roumeliotis E, et al. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings[J]. Plant Physiology and Biochemistry, 2015,86:155-165.
URL pmid: 25500452 |
| [17] |
Zhang N, Sun Q, Zhang H, et al. Roles of melatonin in abiotic stress resistance in plants[J]. Journal of Experimental Botany, 2015,66(3):647-656.
doi: 10.1093/jxb/eru336 URL pmid: 25124318 |
| [18] | Xu L, Zhang F, Tang M, et al. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants[J]. Journal of Pineal Research, 2020: e12659. |
| [19] |
Yang H, Dai LJ, Wei YX, et al. Melatonin enhances salt stress tolerance in rubber tree(Hevea brasiliensis)seedlings[J]. Industrial Crops and Products, 2020,145:111990.
doi: 10.1016/j.indcrop.2019.111990 URL |
| [20] |
Meng JF, Xu TF, Wang ZZ, et al. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress:antioxidant metabolites, leaf anatomy, and chloroplast morphology[J]. Journal of Pineal Research, 2014,57(2):200-212.
doi: 10.1111/jpi.12159 URL |
| [21] |
Li D, Wei J, Peng Z, et al. Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in Arabidopsis[J]. Journal of Pineal Research, 2020,68(3):e12640.
doi: 10.1111/jpi.12640 URL pmid: 32064655 |
| [22] | Turner NC. Techniques and experimental approaches for the measurement of plant water status[J]. Plant Soil, 1981,58(1):339-366. |
| [23] |
Wei J, Li DX, Zhang JR, et al. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana[J]. Journal of Pineal Research, 2018,65(2):e12500.
URL pmid: 29702752 |
| [24] |
Tan DX, Manchester LC, Terron MP, et al. One molecule, many derivatives:a never-ending interaction of melatonin with reactive oxygen and nitrogen species?[J]. Journal of Pineal Research, 2007,42(1):28-42.
doi: 10.1111/j.1600-079X.2006.00407.x URL pmid: 17198536 |
| [25] | Turner NC, Wright GC, Siddique KHH. Adaptation of grain legumes(pulses)to water-limited environments[J]. Advances in Agronomy, 2001,71:193-231. |
| [26] | Subbarao GV, Johansen C, Slinkard AE, et al. Strategies for improving drought resistance in grain legumes[J]. Critical Reviews in Plant Sciences, 1995,14(6):469-523. |
| [27] | Kavar T, Maras M, Kidric M, et al. Identification of genes involved in the response of leaves of Phaseolus vulgaris to drought stress[J]. Molecular Breeding, 2008,21(2):159-172. |
| [28] |
Chen Q, Qi WB, Reiter RJ, et al. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea[J]. Journal of Plant Physiology, 2009,166(3):324-328.
doi: 10.1016/j.jplph.2008.06.002 URL pmid: 18706737 |
| [29] |
Sarropoulou VN, Therios IN, Dimassi-Theriou KN. Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P(Prunus cerasus L.), Gisela 6(P. cerasus x P. canescens), and MxM 60(P. avium x P. mahaleb)[J]. Journal of Pineal Research, 2012,52(1):38-46.
URL pmid: 21749439 |
| [30] |
Zhou Y, Chen M, Guo J, et al. Overexpression of soybean DREB1 enhances drought stress tolerance of transgenic wheat in the field[J]. Journal of Experimental Botany, 2020,71(6):1842-1857.
doi: 10.1093/jxb/erz569 URL pmid: 31875914 |
| [31] | de Souza TC, Magalhães PC, de Castro EM, et al. ABA application to maize hybrids contrasting for drought tolerance:changes in water parameters and in antioxidant enzyme activity[J]. Plant Growth Regulation, 2014,73(3):205-217. |
| [32] |
Chen YE, Cui JM, Su YQ, et al. Comparison of phosphorylation and assembly of photosystem complexes and redox homeostasis in two wheat cultivars with different drought resistance[J]. Scientific Reports, 2017,7(1):12718.
URL pmid: 28983110 |
| [33] |
Tan DX, Manchester LC, Reiter RJ, et al. Significance of melatonin in antioxidative defense system:reactions and products[J]. Biological Signals and Receptors, 2000,9(3-4):137-159.
URL pmid: 10899700 |
| [34] |
Shi HT, Jiang C, Ye TT, et al. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass[Cynodon dactylon(L). Pers. ]by exogenous melatonin[J]. Journal of Experimental Botany, 2015,66(3):681-694.
doi: 10.1093/jxb/eru373 URL pmid: 25225478 |
| [35] |
Zhao G, Zhao Y, Yu X, et al. Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed(Brassica napus L.)seedlings[J]. International Journal of Molecular Sciences, 2018,19(7).
doi: 10.3390/ijms19072128 URL pmid: 30037122 |
| [36] | Gu Q, Chen Z, Yu X, et al. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis[J]. Plant Science, 2017,261:28-37. |
| [37] |
Sun C, Lv T, Huang L, et al. Melatonin ameliorates aluminum toxicity through enhancing aluminum exclusion and reestablishing redox homeostasis in roots of wheat[J]. Journal of Pineal Research, 2020,68(4):e12642.
doi: 10.1111/jpi.12642 URL pmid: 32092171 |
| [38] |
Zuo B, Zheng X, He P, et al. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants[J]. Journal of Pineal Research, 2014,57(4):408-417.
doi: 10.1111/jpi.12180 URL pmid: 25250844 |
| [39] |
Shi H, Qian Y, Tan DX, et al. Melatonin induces the transcripts of CBF/DREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis[J]. Journal of Pineal Research, 2015,59(3):334-342.
doi: 10.1111/jpi.12262 URL pmid: 26182834 |
| [40] | Zuo Z, Sun L, Wang T, et al. Melatonin improves the photosynthetic carbon assimilation and antioxidant capacity in wheat exposed to nano-zno stress[J]. Molecules, 2017,22(10):1727. |
| [41] |
Arnao MB, Hernandez-Ruiz J. Melatonin:a new plant hormone and/or a plant master regulator?[J]. Trends in Plant Science, 2019,24(1):38-48.
doi: 10.1016/j.tplants.2018.10.010 URL pmid: 30446305 |
| [42] |
Wei W, Li QT, Chu YN, et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants[J]. Journal of Experimental Botany, 2015,66(3):695-707.
URL pmid: 25297548 |
| [43] |
Maxwell K, Johnson GN. Chlorophyll fluorescence-a practical guide[J]. Journal of Experimental Botany, 2000,51(345):659-668.
URL pmid: 10938857 |
| [44] | Xin CP, Yang J, Zhu XG. A model of chlorophyll a fluorescence induction kinetics with explicit description of structural constraints of individual photosystem II units[J]. Photosynjournal Research, 2013,117(1-3):339-354. |
| [45] |
Nikolaou A, Bernardi A, Meneghesso A, et al. A model of chlorophyll fluorescence in microalgae integrating photoproduction, photoinhibition and photoregulation[J]. Journal of Biotechnology, 2015,194:91-99.
doi: 10.1016/j.jbiotec.2014.12.001 URL pmid: 25527384 |
| [46] | Ye J, Wang S, Deng X, et al. Melatonin increased maize(Zea mays L.)seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage[J]. Acta Physiologiae Plantarum, 2016,38(2):48. |
| [47] |
Sokolovic D, Djordjevic B, Kocic G, et al. The effects of melatonin on oxidative stress parameters and dna fragmentation in testicular tissue of rats exposed to microwave radiation[J]. Advances in Clinical and Experimental Medicine, 2015,24(3):429-436.
doi: 10.17219/acem/43888 URL pmid: 26467130 |
| [48] | Sun Q, Zhang N, Wang J, et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life[J]. Journal of Experimental Botany, 2015,66(3):657-668. |
| [49] |
Sun Q, Zhang N, Wang J, et al. A label-free differential proteomics analysis reveals the effect of melatonin on promoting fruit ripening and anthocyanin accumulation upon postharvest in tomato[J]. Journal of Pineal Research, 2016,61(2):138-153.
URL pmid: 26820691 |
| [50] |
Ahmad S, Kamran M, Ding R, et al. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings[J]. PeerJ, 2019,7:e7793.
doi: 10.7717/peerj.7793 URL pmid: 31616591 |
| [51] | Huang B, Chen YE, Zhao YQ, et al. Exogenous melatonin alleviates oxidative damages and protects photosystemii in maize seedlings under drought stress[J]. Front Plant Science, 2019,10:677. |
| [1] | 杨志晓, 侯骞, 刘国权, 卢志刚, 曹毅, 芶剑渝, 王轶, 林英超. 不同抗性烟草品系Rubisco及其活化酶对赤星病胁迫的响应[J]. 生物技术通报, 2023, 39(9): 202-212. |
| [2] | 康凌云, 韩露露, 韩德平, 陈建胜, 甘瀚凌, 邢凯, 马友记, 崔凯. 褪黑素缓解空肠黏膜上皮细胞氧化损伤的效果研究[J]. 生物技术通报, 2023, 39(9): 291-299. |
| [3] | 张岳一, 兰社益, 裴海闰, 封棣. 多菌种联用发酵燕麦麸皮工艺优化及发用功效评价[J]. 生物技术通报, 2023, 39(9): 58-70. |
| [4] | 魏茜雅, 秦中维, 梁腊梅, 林欣琪, 李映志. 褪黑素种子引发处理提高朝天椒耐盐性的作用机制[J]. 生物技术通报, 2023, 39(7): 160-172. |
| [5] | 游子娟, 陈汉林, 邓辅财. 鱼皮生物活性肽的提取及功能活性研究进展[J]. 生物技术通报, 2023, 39(7): 91-104. |
| [6] | 李帜奇, 袁月, 苗荣庆, 庞秋颖, 张爱琴. 盐胁迫盐芥和拟南芥褪黑素含量及合成相关基因表达模式分析[J]. 生物技术通报, 2023, 39(5): 142-151. |
| [7] | 王春语, 李政君, 王平, 张丽霞. 高粱表皮蜡质缺失突变体sb1抗旱生理生化分析[J]. 生物技术通报, 2023, 39(5): 160-167. |
| [8] | 翟莹, 李铭杨, 张军, 赵旭, 于海伟, 李珊珊, 赵艳, 张梅娟, 孙天国. 异源表达大豆转录因子GmNF-YA19提高转基因烟草抗旱性[J]. 生物技术通报, 2023, 39(5): 224-232. |
| [9] | 王琪, 胡哲, 富薇, 李光哲, 郝林. 伯克霍尔德氏菌GD17对黄瓜幼苗耐干旱的调节[J]. 生物技术通报, 2023, 39(3): 163-175. |
| [10] | 杨茂, 林宇丰, 戴阳朔, 潘素君, 彭伟业, 严明雄, 李魏, 王冰, 戴良英. OsDIS1通过抗氧化途径负调控水稻耐旱性[J]. 生物技术通报, 2023, 39(2): 88-95. |
| [11] | 赵佳, 赵飞燕, 沈馨, 高广琦, 孙志宏. 乳酸菌抗氧化活性及其应用研究进展[J]. 生物技术通报, 2023, 39(11): 182-190. |
| [12] | 阮航, 多浩源, 范文艳, 吕清晗, 姜述君, 朱生伟. AtERF49在拟南芥应答盐碱胁迫中的作用[J]. 生物技术通报, 2023, 39(1): 150-156. |
| [13] | 朱金成, 杨洋, 娄慧, 张薇. 外源褪黑素调控棉花枯萎病抗性研究[J]. 生物技术通报, 2023, 39(1): 243-252. |
| [14] | 陈光, 李佳, 杜瑞英, 王旭. pOsHAK1:OsFLN2提高水稻的糖代谢水平和抗旱性[J]. 生物技术通报, 2022, 38(8): 92-100. |
| [15] | 袁存霞, 李艳楠, 张肖冲, 杨瑞, 刘建利, 李靖宇. As3+胁迫下Bacillus sp. ZJS3菌株的生理生化响应特性[J]. 生物技术通报, 2022, 38(7): 236-246. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||