








生物技术通报 ›› 2022, Vol. 38 ›› Issue (8): 135-141.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1509
收稿日期:2021-12-06
出版日期:2022-08-26
发布日期:2022-09-14
作者简介:索青青,女,硕士研究生,研究方向:分子植物病理学;E-mail: 基金资助:
SUO Qing-qing( ), WU Nan, YANG Hui, LI Li(
), WU Nan, YANG Hui, LI Li( ), WANG Xi-feng
), WANG Xi-feng
Received:2021-12-06
Published:2022-08-26
Online:2022-09-14
摘要:
咖啡酰辅酶A-O-甲基转移酶(caffeoyl coenzyme A-O-methyltransferase,CCoAOMT)在植物木质素生物合成过程中发挥重要作用。目前关于水稻咖啡酰辅酶A-O-甲基转移酶的研究主要集中在基因水平,对其在蛋白水平上的功能研究却相当少。通过PCR扩增技术克隆得到OsCCoAOMT1基因,并成功构建了原核表达载体pET30a(+)-OsCCoAOMT1,转化大肠杆菌,表达出了重组蛋白。蛋白经纯化后,注射新西兰公兔,成功制备了多克隆抗体。Western blot检测表明该抗体能特异性地结合水稻不同组织的该蛋白。利用制备的抗体对水稻茎原生质体进行免疫荧光标记,激光共聚焦显微镜观察发现在Ubi:OsCCoAOMT1-GFP转基因水稻和野生型水稻原生质体里均可标记到该蛋白的信号,且转基因水稻融合蛋白的GFP信号可以与该蛋白抗体的荧光信号共定位,同时结合水稻核质蛋白分离的Western blot检测结果可知OsCCoAOMT1蛋白在主要分布在细胞质中。表明制备的抗体能用于该蛋白的特异性检测,为后续对水稻咖啡酰辅酶A-O-甲基转移酶的功能研究奠定了良好基础。
索青青, 吴楠, 杨慧, 李莉, 王锡锋. 水稻咖啡酰辅酶A-O-甲基转移酶基因的原核表达、抗体制备和应用[J]. 生物技术通报, 2022, 38(8): 135-141.
SUO Qing-qing, WU Nan, YANG Hui, LI Li, WANG Xi-feng. Prokaryotic Expression,Antibody Preparation and Application of Rice Caffeoyl Coenzyme A-O-methyltransferase Gene[J]. Biotechnology Bulletin, 2022, 38(8): 135-141.
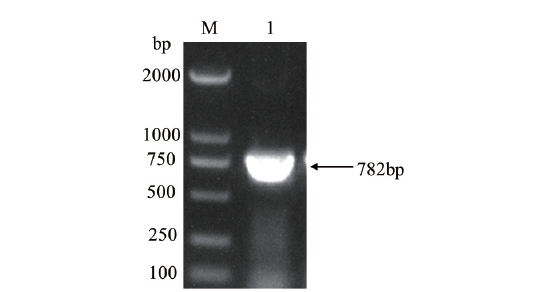
图1 OsCCoAOMT1基因的PCR扩增结果 M:DL 2000 DNA marker;1:OsCCoAOMT1基因扩增产物
Fig.1 PCR amplifying product of OsCCoAOMT1 gene M:DL 2000 DNA marker;1:PCR product of OsCCoAOMT1
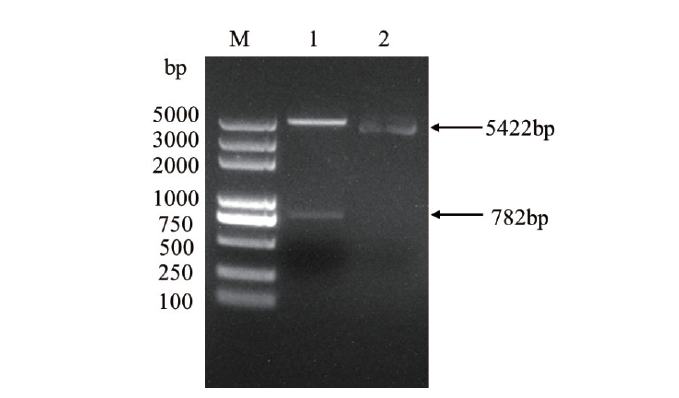
图2 重组质粒pET30a(+)-OsCCoAOMT1的酶切鉴定 M:DL 5000 DNA marker;1:Nde I和Hind III酶切pET30a(+)-OsCCoAOMT1;2:pET30a(+)空载体
Fig.2 Restriction identification of recombinant plasmid pET30a(+)-OsCCoAOMT1 M:DL 5000 DNA marker;1:pET30a(+)-OsCCoAOMT1 digested by Nde I and Hind III;2:pET30a(+)plasmid
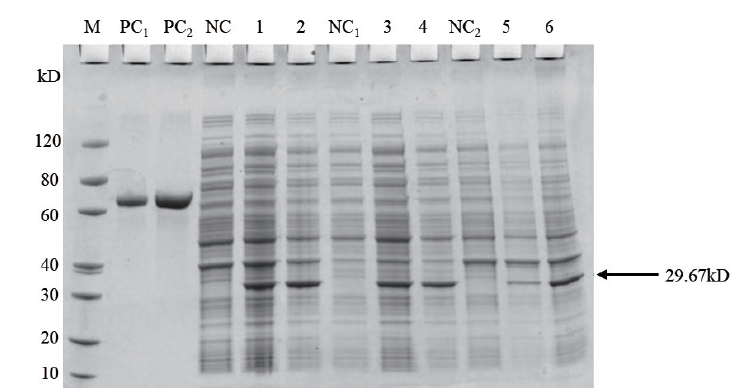
图3 重组质粒pET30a(+)-OsCCoAOMT1不同诱导条件下的SDS-PAGE分析 M:蛋白Marker;PC1:BSA(1 μg);PC2:BSA(2 μg);NC:未经诱导的细胞裂解液;1:在15℃下诱导16 h的细胞裂解液;2:在37℃下诱导4 h的细胞裂解液;NC1:未经诱导的细胞裂解液上清;3:经15℃下16 h诱导的细胞裂解液上清;4:37℃下诱导4 h的细胞裂解液上清;NC2:未诱导的细胞裂解液包涵体;5:15℃下诱导16 h的细胞裂解液包涵体;6:37℃下诱导4 h的细胞裂解液包涵体
Fig.3 SDS-PAGE analysis of recombinant plasmid pET30a(+)-OsCCoAOMT1 under the different inducing condition M:Protein marker;PC1:BSA(1 μg);PC2:BSA(2 μg). NC:Cell lysate without induction. 1:Cell lysate with induction for 16 h at 15℃. 2:Cell lysate with induction for 4 h at 37℃. NC1:Supernatant of cell lysate without induction. 3:Supernatant of cell lysate with induction for 16 h at 15℃. 4:Supernatant of cell lysate with induction for 4 h at 37℃. NC2:Pellet of cell lysate without induction. 5:Pellet of cell lysate with induction for 16 h at 15℃. 6:Pellet of cell lysate with induction for 4 h at 37℃
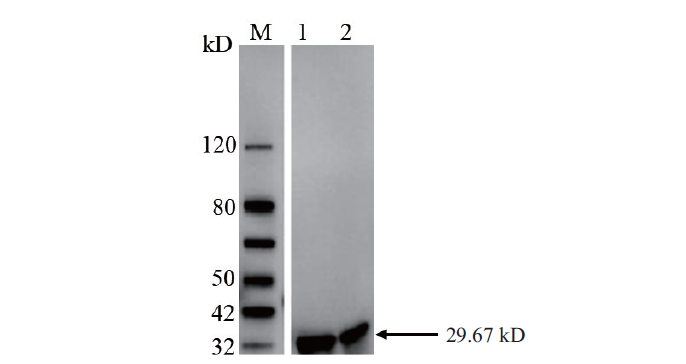
图4 重组质粒pET30a(+)-OsCCoAOMT1不同诱导条件下的Western blot分析 M:Western blot marker;1:经15℃下16 h诱导的细胞裂解液上清;2:37℃下诱导4 h的细胞裂解液上清
Fig.4 Western blot analysis of recombinant plasmid pET30a(+)-OsCCoAOMT1 under the different inducing condition M:Western blot marker. 1:Supernatant of cell lysate induced at 15℃ for 16 h. 2:Supernatant of cell lysate induced at 37℃ for 4 h
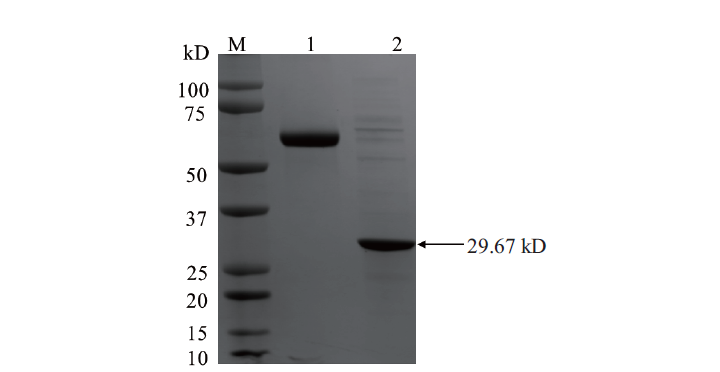
图5 OsCCoAOMT1蛋白的纯化 M:Western blot marker;1:BSA;2:纯化的OsCCoAOMT1蛋白
Fig.5 Purification of OsCCoAOMT1 protein M:Western blot marker. 1:BSA. 2:Purified OsCCoAOMT1 protein
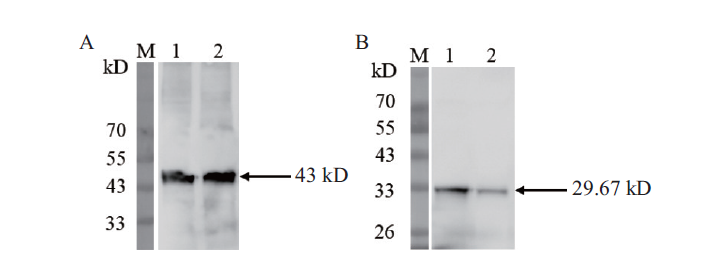
图6 水稻茎、叶中OsCCoAOMT1的Western blot 检测 M:Western blot marker;A:水稻内参抗体(1:水稻茎总蛋白;2:水稻叶总蛋白);B:OsCCoAOMT1抗体(1:水稻茎总蛋白;2:水稻叶总蛋白)
Fig.6 Western blot analysis of OsCCoAOMT1 in rice stem and leaf M:Western blot marker. A:Rice control antibody(1:rice stem protein;2:rice leaf protein). B:OsCCoAOMT1 antibody(1:rice stem protein;2:rice leaf protein)
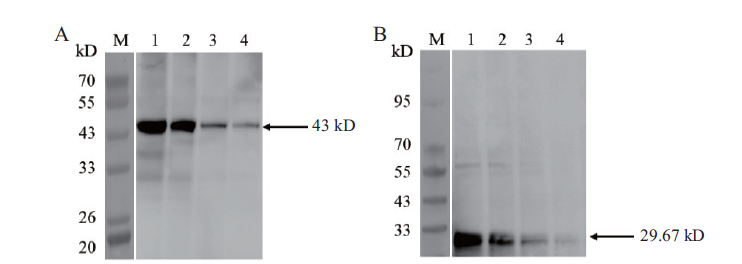
图7 水稻不同蛋白浓度Western blot检测 M:Western blot marker;A:水稻内参抗体(1:100 μg;2:50 μg;3:25 μg;4:12.5 μg);B:OsCCoAOMT1抗体(1:100 μg;2:50 μg;3:25 μg;4:12.5 μg)
Fig.7 Western blot analysis of different protein concentra-tions in rice M:Western blot marker;A:rice control antibody(1:100 μg;2:50 μg;3:25 μg;4:12.5 μg). B:OsCCoAOMT1 antibody(1:100 μg;2:50 μg;3:25 μg;4:12.5 μg)

图9 OsCCoAOMT1蛋白在水稻细胞质和细胞核中的表达量分析 M:Western blot marker;1:水稻茎细胞质蛋白;2:水稻茎细胞核蛋白
Fig. 9 Expression level analysis the OsCCoAOMT1 protein in rice cytoplasm and nucleus M:Western blot marker;1:rice stem cytoplasmic protein;2:rice stem nucleoprotein
| [1] |
Bonawitz ND, Chapple C. The genetics of lignin biosynthesis:connecting genotype to phenotype[J]. Annu Rev Genet, 2010, 44:337-363.
doi: 10.1146/annurev-genet-102209-163508 URL |
| [2] | Engelhardt J. Sources, industrial derivatives and commercial application of cellulose[J]. Carbohydrate Eur, 1995, 12:5-14. |
| [3] |
Ma QH, Luo HR. Biochemical characterization of caffeoyl coenzyme A 3-O-methyltransferase from wheat[J]. Planta, 2015, 242(1):113-122.
doi: 10.1007/s00425-015-2295-3 URL |
| [4] |
Ralph J, Lundquist K, Brunow G, et al. Lignins:Natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids[J]. Phytochem Rev, 2004, 3(1/2):29-60.
doi: 10.1023/B:PHYT.0000047809.65444.a4 URL |
| [5] | 魏建华, 宋艳茹. 木质素生物合成途径及调控的研究进展[J]. 植物学报, 2001, 43(8):771-779. |
| Wei JH, Song YR. Recent advances in study of lignin biosynthesis and manipulation[J]. Acta Bot Sin, 2001, 43(8):771-779. | |
| [6] | 陈冰玉, 邸明伟. 木质素解聚研究新进展[J]. 高分子材料科学与工程, 2019, 35(6):157-164. |
| Chen BY, Di MW. Progress on research of depolymerization of lignin[J]. Polym Mater Sci Eng, 2019, 35(6):157-164. | |
| [7] |
Ye ZH, Kneusel RE, Matern U, et al. An alternative methylation pathway in lignin biosynthesis in Zinnia[J]. Plant Cell, 1994, 6(10):1427-1439.
pmid: 7994176 |
| [8] |
Pakusch AE, Kneusel RE, Matern U. S-adenosyl-L-methionine:trans-caffeoyl-coenzyme A 3-O-methyltransferase from elicitor-treated parsley cell suspension cultures[J]. Arch Biochem Biophys, 1989, 271(2):488-494.
pmid: 2499260 |
| [9] |
Pinçon G, Maury S, Hoffmann L, et al. Repression of O-methyltransferase genes in transgenic tobacco affects lignin synthesis and plant growth[J]. Phytochemistry, 2001, 57(7):1167-1176.
pmid: 11430989 |
| [10] | 林楠, 王彦珍, 李桢, 等. 抑制咖啡酰-辅酶A甲基转移酶(CCoAOMT)表达的转基因多年生杨树检测与分析[J]. 农业生物技术学报, 2009, 17(6):1121-1122. |
| Lin N, Wang YZ, Li Z, et al. Analysis and evaluation of multiple-year-old transgenic poplar with antisense caffeoyl-CoA-O-methyltransferase(CCoAOMT)[J]. J Agric Biotechnol, 2009, 17(6):1121-1122. | |
| [11] |
Zhong R, Morrison WH 3rd, Himmelsbach DS, et al. Essential role of caffeoyl coenzyme A O-methyltransferase in lignin biosynthesis in woody poplar plants[J]. Plant Physiol, 2000, 124(2):563-578.
pmid: 11027707 |
| [12] |
Bhuiyan NH, Selvaraj G, Wei YD, et al. Role of lignification in plant defense[J]. Plant Signal Behav, 2009, 4(2):158-159.
doi: 10.4161/psb.4.2.7688 pmid: 19649200 |
| [13] | 宫世龙. 甜菜M14品系咖啡酰辅酶A-O-甲基转移酶基因功能的研究[D]. 哈尔滨: 黑龙江大学, 2013. |
| Gong SL. Study on the function of caffeoyl-CoA-O-methyltransferase gene in sugar beet M14[D]. Harbin: Helongjiang University, 2013. | |
| [14] | Chun HJ, Lim LH, Cheong MS, et al. Arabidopsis CCoAOMT1 plays a role in drought stress response via ROS- and ABA-dependent manners[J]. Plants(Basel), 2021, 10(5):831. |
| [15] | 赵华燕, 沈庆喜, 吕世友, 等. 水稻咖啡酰辅酶A-O-甲基转移酶基因(CCoAOMT)表达特性分析[J]. 科学通报, 2004, 49(14):1390-1394. |
|
Zhao HY, Shen QX, Lv SY, et al. Analysis of expression characteristics of rice caffeoyl-CoA-O-methyltransferase gene(CCoAOMT)[J]. Chin Sci Bull, 2004, 49(14):1390-1394.
doi: 10.1360/csb2004-49-14-1390 URL |
|
| [16] |
Wang YJ, Mao QZ, Liu WW, et al. Localization and distribution of wheat dwarf virus in its vector leafhopper, Psammotettix alienus[J]. Phytopathology, 2014, 104(8):897-904.
doi: 10.1094/PHYTO-09-13-0251-R URL |
| [17] | 程彦伟, 李亮, 沈嵘, 等. 水稻LRR型类受体蛋白激酶胞外区的原核表达及多克隆抗体制备[J]. 生物化学与生物物理进展, 2008, 35(9):1077-1083. |
| Cheng YW, Li L, Shen R, et al. Prokaryotic expression and polyclonal antibody preparation of the extracellular domain about rice LRR receptor-like protein kinase[J]. Prog Biochem Biophys, 2008, 35(9):1077-1083. | |
| [18] | 王增, 代茹, 张江巍, 等. 拟南芥WUSCHEL蛋白的原核表达、亲和纯化和多克隆抗体制备[J]. 生物工程学报, 2009, 25(9):1409-1416. |
| Wang Z, Dai R, Zhang JW, et al. Induced expression of Arabidopsis thaliana WUSCHEL in Escherichia coli, affinity protein purification and polyclonal antibody preparation[J]. Chin J Biotechnol, 2009, 25(9):1409-1416. | |
| [19] | 秦伟, 黄昆仑, 贺晓云, 等. 水稻密码子优化的cry2A*基因在大肠杆菌中的表达及其表达产物的纯化[J]. 食品科学, 2008, 29(7):267-271. |
| Qin W, Huang KL, He XY, et al. Expression of rice Codon optimized cry2A* gene in Escherichia coli and purification of its expressed proteins[J]. Food Sci, 2008, 29(7):267-271. | |
| [20] |
Chai HZ, Wu SB, Deng JF, et al. Preparation and identification of polyclonal antibody against human Cytomegalovirus encoding protein UL23[J]. Protein Expr Purif, 2019, 161:78-83.
doi: 10.1016/j.pep.2019.04.008 URL |
| [21] |
Yang Q, He YJ, Kabahuma M, et al. A gene encoding maize caffeoyl-CoA O-methyltransferase confers quantitative resistance to multiple pathogens[J]. Nat Genet, 2017, 49(9):1364-1372.
doi: 10.1038/ng.3919 pmid: 28740263 |
| [22] |
Wang YG, Zhan YN, Wu C, et al. Cloning of a cystatin gene from sugar beet M14 that can enhance plant salt tolerance[J]. Plant Sci, 2012, 191/192:93-99.
doi: 10.1016/j.plantsci.2012.05.001 URL |
| [1] | 梅欢, 李玥, 刘可蒙, 刘吉华. 小檗碱桥酶高效原核表达及生物合成l-SLR的研究[J]. 生物技术通报, 2023, 39(7): 277-287. |
| [2] | 郭文博, 路杨, 隋丽, 赵宇, 邹晓威, 张正坤, 李启云. 球孢白僵菌真菌病毒BbPmV-4外壳蛋白多克隆抗体制备及应用[J]. 生物技术通报, 2023, 39(10): 58-67. |
| [3] | 覃雪晶, 王雨涵, 曹一博, 张凌云. 青杄PwHAP5基因原核表达及多克隆抗体制备[J]. 生物技术通报, 2022, 38(8): 142-149. |
| [4] | 王光丽, 范婵, 王辉, 卢惠芳, 夏灵尹, 黄健, 闵迅. 霍乱弧菌溶血素HlyA的原核表达、纯化及多克隆抗体制备与鉴定[J]. 生物技术通报, 2022, 38(7): 269-277. |
| [5] | 汪巧菊, 胡雨萌, 温亚亚, 宋丽, 孟闯, 潘志明, 焦新安. 新型冠状病毒S1蛋白的表达及活性鉴定[J]. 生物技术通报, 2022, 38(3): 157-163. |
| [6] | 沈俊强, 张莉萍, 于瑞明, 王永录, 潘丽, 刘霞, 刘新生. 猪嵴病毒结构蛋白VP0与VP1原核表达及间接ELISA方法的建立[J]. 生物技术通报, 2022, 38(10): 243-253. |
| [7] | 山草梅, 叶蕾, 张连虎, 况卫刚, 孙晓棠, 马建, 崔汝强. 水稻抗潜根线虫基因OsRAI1的克隆及功能分析[J]. 生物技术通报, 2021, 37(7): 146-155. |
| [8] | 曾福源, 苏泽辉, 周诗慧, 谢妙, 庞欢瑛. 溶藻弧菌PEPCK蛋白原核表达及其乙酰化、琥珀酰化修饰的鉴定[J]. 生物技术通报, 2021, 37(5): 84-91. |
| [9] | 张西西, 张怡青, 李玉林, 韩笑, 王国强, 王晓军, 王旭东, 王云龙. 新型冠状病毒(SARS-CoV-2)N蛋白C端重组蛋白的原核表达、纯化及应用[J]. 生物技术通报, 2021, 37(5): 92-97. |
| [10] | 白福美, 李至敏, 王小琴, 胡紫微, 鲍玲玲, 李志敏. 集胞藻PCC6803中N-乙酰鸟氨酸转氨酶的生化表征及结构分析[J]. 生物技术通报, 2021, 37(5): 98-107. |
| [11] | 瞿欢, 李成, 陈汭, 廖艺杰, 曹三杰, 文翼平, 颜其贵, 黄小波. 猪δ冠状病毒S1-CTD的截短表达及间接ELISA抗体方法的建立[J]. 生物技术通报, 2021, 37(5): 273-280. |
| [12] | 彭利忠, 张鹏, 周雯雯, 曾旭辉, 张小宁. 精子特异性蛋白Cabs1多克隆抗体的制备及多用途验证[J]. 生物技术通报, 2021, 37(3): 261-270. |
| [13] | 贺扬, 余巧玲, 王均, 覃川杰, 李华涛. 罗非鱼原核表达基因研究进展[J]. 生物技术通报, 2021, 37(2): 195-202. |
| [14] | 付强, 郭妍婷, 陈俊贞, 王金泉, 史慧君. 牛病毒性腹泻病毒离子孔道蛋白p7多肽多克隆抗体的制备和鉴定[J]. 生物技术通报, 2021, 37(10): 137-142. |
| [15] | 唐禄, 董丽平, 尹茉莉, 刘磊, 董媛, 王会岩. 成纤维细胞生长因子20单克隆抗体的制备及鉴定[J]. 生物技术通报, 2021, 37(10): 179-185. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||