








生物技术通报 ›› 2023, Vol. 39 ›› Issue (3): 43-51.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1010
收稿日期:2022-08-22
出版日期:2023-03-26
发布日期:2023-04-10
通讯作者:
郑井元,男,博士,研究员,研究方向:辣椒抗病育种与土壤重金属污染防治;E-mail: zhengjingyuan2004@hunaas.cn作者简介:易希,女,硕士研究生,研究方向:辣椒与病原物互作的分子机制;E-mail: XiY@hnu.edu.cn
基金资助:
YI Xi1,2( ), LIAO Hong-dong3, ZHENG Jing-yuan1,2(
), LIAO Hong-dong3, ZHENG Jing-yuan1,2( )
)
Received:2022-08-22
Published:2023-03-26
Online:2023-04-10
摘要:
根结线虫病是对农作物危害严重且难以防治的病害,并随着我国设施农业的发展日趋严重。常规的化学防治方法因毒性大、破坏生态环境而不适应农业的可持续发展。作为一种能稳定寄生在作物体内的生物防治真菌,内生真菌通过抑制卵的孵化、降低J2期线虫幼虫活力、抑制线虫的入侵、延缓雌虫发育、减少产卵数目、降低作物根中根结和卵块数量,来实现稳定、高效、安全地防治根结线虫病害。近年来,内生真菌的作用机制得到广泛关注和研究,取得了显著进展。本文综述近年来内生真菌生物防治根结线虫机制的研究进展,总结了内生真菌直接攻击、资源竞争、代谢物胁迫、防御激活等4种主要机制,探讨其存在的问题,以期为进一步开发、应用植物内生真菌进行生物防治提供帮助。
易希, 廖红东, 郑井元. 植物内生真菌防治根结线虫研究进展[J]. 生物技术通报, 2023, 39(3): 43-51.
YI Xi, LIAO Hong-dong, ZHENG Jing-yuan. Research Progress in Plant Endophytic Fungi for Root-knot Nematode Control[J]. Biotechnology Bulletin, 2023, 39(3): 43-51.
| 内生真菌种类 Endophytic fungal species | 根结线虫种类 Root-knot nematodes species | 作物 Crop | 对根结线虫的影响 Effect on root-knot nematodes | 参考文献 Reference |
|---|---|---|---|---|
| Fusarium oxysporum 162(Fo162) | 南方根结线虫 Meloidogyne incognita | 番茄Solanum lycopersicum 南瓜Cucurbita moschata 甜瓜Cucumis melo | 减少线虫入侵番茄根系的数量; 阻碍线虫发育; 显著减少雌虫产卵数 | [ |
| Acremonium implicatum | 南方根结线虫 Meloidogyne incognita | 番茄Solanum lycopersicum | 杀死J2期线虫; 抑制卵孵化; 抑制根结的形成; 减少土壤中线虫的数量 | [ |
| Fusarium oxysporum; F. Solani; Trichoderma asperellum | 南方根结线虫 Meloidogyne incognita | 番茄Solanum lycopersicum | 减少线虫的入侵及繁殖; 使根结线虫卵密度降低 | [ |
| Phyllosticta Ph511; Chaetomium Ch1001; Trichoderma Tr882; Paecilomyces Pa972; Acremonium Ac985 | 南方根结线虫 Meloidogyne incognita | 黄瓜Cucumis sativus L. | 减少卵块数量; 产生了影响J2期线虫运动的化合物 | [ |
| Chaetomium globosum strain TAMU 520 | 南方根结线虫 Meloidogyne incognita | 棉花Gossypium spp. | 抑制线虫的入侵; 减少雌虫的繁殖 | [ |
| Trichoderma ; Clonostachys | 南方根结线虫 Meloidogyne incognita | 印加果Plukenetia volubilis | 显著减少了卵块的数量 | [ |
| Fusarium; Trichoderma | 拟禾本科根结线虫 Meloidogyne graminicola | 水稻Oryza sativa L. | 减少根结数量; 使根重增加; 使卵块严重程度下降 | [ |
| Fusarium moniliforme strain Fe14 | 拟禾本科根结线虫 Meloidogyne graminicola | 水稻Oryza sativa L. | 减少线虫入侵; 提高雄虫比雌虫的比例; 延缓幼虫向雌成虫的发育 | [ |
表1 内生真菌在不同作物中对根结线虫的影响
Table 1 Effects of endophytic fungi on the root-knot nematodes in different crops
| 内生真菌种类 Endophytic fungal species | 根结线虫种类 Root-knot nematodes species | 作物 Crop | 对根结线虫的影响 Effect on root-knot nematodes | 参考文献 Reference |
|---|---|---|---|---|
| Fusarium oxysporum 162(Fo162) | 南方根结线虫 Meloidogyne incognita | 番茄Solanum lycopersicum 南瓜Cucurbita moschata 甜瓜Cucumis melo | 减少线虫入侵番茄根系的数量; 阻碍线虫发育; 显著减少雌虫产卵数 | [ |
| Acremonium implicatum | 南方根结线虫 Meloidogyne incognita | 番茄Solanum lycopersicum | 杀死J2期线虫; 抑制卵孵化; 抑制根结的形成; 减少土壤中线虫的数量 | [ |
| Fusarium oxysporum; F. Solani; Trichoderma asperellum | 南方根结线虫 Meloidogyne incognita | 番茄Solanum lycopersicum | 减少线虫的入侵及繁殖; 使根结线虫卵密度降低 | [ |
| Phyllosticta Ph511; Chaetomium Ch1001; Trichoderma Tr882; Paecilomyces Pa972; Acremonium Ac985 | 南方根结线虫 Meloidogyne incognita | 黄瓜Cucumis sativus L. | 减少卵块数量; 产生了影响J2期线虫运动的化合物 | [ |
| Chaetomium globosum strain TAMU 520 | 南方根结线虫 Meloidogyne incognita | 棉花Gossypium spp. | 抑制线虫的入侵; 减少雌虫的繁殖 | [ |
| Trichoderma ; Clonostachys | 南方根结线虫 Meloidogyne incognita | 印加果Plukenetia volubilis | 显著减少了卵块的数量 | [ |
| Fusarium; Trichoderma | 拟禾本科根结线虫 Meloidogyne graminicola | 水稻Oryza sativa L. | 减少根结数量; 使根重增加; 使卵块严重程度下降 | [ |
| Fusarium moniliforme strain Fe14 | 拟禾本科根结线虫 Meloidogyne graminicola | 水稻Oryza sativa L. | 减少线虫入侵; 提高雄虫比雌虫的比例; 延缓幼虫向雌成虫的发育 | [ |
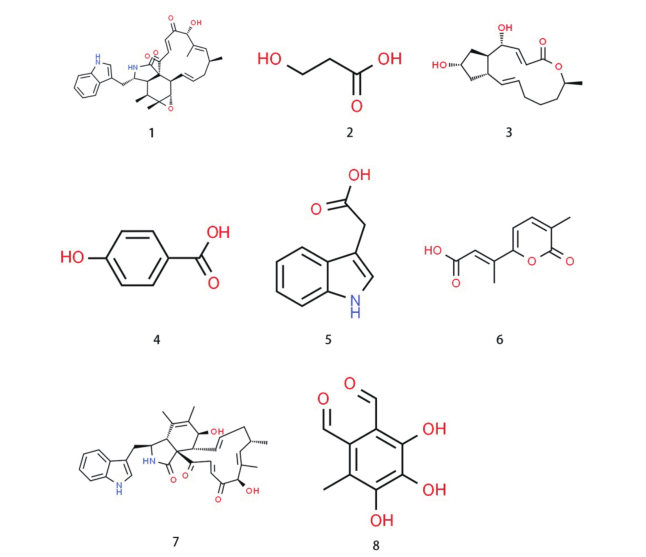
图2 内生真菌产生的具有杀线虫活性的次生代谢物 1:球毛壳菌素A[29];2:3-羟基丙酸[30];3:布雷非德菌素A[31];4:对羟基苯甲酸[32];5:吲哚乙酸[32];6:Gibepyrone D[32];7:球毛壳菌素B[33];8:苯二醛衍生物黄柄曲菌素[33]
Fig. 2 Secondary metabolites with nematicidal activity produced by endophytic fungi 1: chaetoglobosin A[29];2: 3-hydroxypropionie acid[30];3: brefeldin A[31];4: hydroxybenzoic acid[32];5: indole-3-acetic acid[32];6: gibepyrone D[32];7: chaetoglobosin B[33];8: flavipin[33]
| [1] |
陈立杰, 杨帆, 范海燕, 等. 非编码RNA在生防菌-植物线虫-寄主互作中的研究进展[J]. 生物技术通报, 2021, 37(7): 65-70.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0501 |
| Chen LJ, Yang F, Fan HY, et al. Advances of non-coding RNA in interactions among biocontrol bacteria and plant nematodes and host[J]. Biotechnol Bull, 2021, 37(7): 65-70. | |
| [2] |
邓苗苗, 郭晓黎. 植物响应寄生线虫侵染机制的研究进展[J]. 生物技术通报, 2021, 37(7): 25-34.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0669 |
| Deng MM, Guo XL. Research progress on plants responses to parasitic nematodes infection[J]. Biotechnol Bull, 2021, 37(7): 25-34. | |
| [3] | 彭德良. 植物线虫病害:我国粮食安全面临的重大挑战[J]. 生物技术通报, 2021, 37(7): 1-2. |
| Peng DL. Plant nematode diseases: serious challenges to China's food security[J]. Biotechnol Bull, 2021, 37(7): 1-2. | |
| [4] | Escobar C, Barcala M, Cabrera J, et al. Overview of root-knot Nematodes and giant cells[J]. Adv Bot Res, 2015, 73: 1-32. |
| [5] | Sikandar A, Zhang MY, Wang YY, et al. Review article: Meloidogyne incognita(root-knot nematode)a risk to agriculture[J]. Applied Ecology and Enviromental Research, 2020, 18(1):1679-1690. |
| [6] |
金娜, 王学艳, 刘倩, 等. 土壤生物熏蒸对蔬菜根结线虫及土壤线虫群落的影响[J]. 生物技术通报, 2021, 37(7): 156-163.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0670 |
|
Jin N, Wang XY, Liu Q, et al. Effects of biofumigation on root-knot nematodes and soil nematode community[J]. Biotechnol Bull, 2021, 37(7): 156-163.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0670 |
|
| [7] | Talavera-Rubia M, Vela-Delgado MD, Verdejo-Lucas S. Nematicidal efficacy of milbemectin against root-knot nematodes[J]. Plants(Basel), 2020, 9(7): 839. |
| [8] |
Yue X, Li F, Wang B. Activity of four nematicides against Meloidogyne incognita race 2 on tomato plants[J]. Journal of Phytopathology, 2020, 168(7-8): 399-404.
doi: 10.1111/jph.v168.7-8 URL |
| [9] |
Çetintas R, Kusek M, Fateh SA. Effect of some plant growth-promoting rhizobacteria strains on root-knot nematode, Meloidogyne incognita, on tomatoes[J]. Egypt J Biol Pest Control, 2018, 28: 1-5.
doi: 10.1186/s41938-017-0002-3 |
| [10] |
Poveda J, Abril-Urias P, Escobar C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi[J]. Front Microbiol, 2020, 11: 992.
doi: 10.3389/fmicb.2020.00992 pmid: 32523567 |
| [11] |
Bacon CW, White JF. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants[J]. Symbiosis, 2016, 68: 87-98.
doi: 10.1007/s13199-015-0350-2 URL |
| [12] |
Kumar KK, Dara SK. Fungal and bacterial endophytes as microbial control agents for plant-parasitic nematodes[J]. Int J Environ Res Public Health, 2021, 18(8): 4269.
doi: 10.3390/ijerph18084269 URL |
| [13] |
Sikora R, Dababat A. Influence of the mutualistic endophyte Fusarium oxysporum 162 on Meloidogyne incognita attraction and invasion[J]. Nematology, 2007, 9: 771-776.
doi: 10.1163/156854107782331225 URL |
| [14] |
Martinuz A, Schouten A, Sikora RA. Post-infection development of Meloidogyne incognita on tomato treated with the endophytes Fusarium oxysporum strain Fo162 and Rhizobium etli strain G12[J]. BioControl, 2013, 58(1): 95-104.
doi: 10.1007/s10526-012-9471-1 URL |
| [15] |
Menjivar RD, Hagemann MH, Kranz J, et al. Biological control of Meloidogyne incognita on cucurbitaceous crops by the non-pathogenic endophytic fungus Fusarium oxysporum strain 162[J]. Int J Pest Manag, 2011, 57: 249-253.
doi: 10.1080/09670874.2011.590239 URL |
| [16] |
Tian XL, Yao YR, Chen GH, et al. Suppression of Meloidogyne incognita by the endophytic fungus Acremonium implicatum from tomato root galls[J]. Int J Pest Manag, 2014, 60(4): 239-245.
doi: 10.1080/09670874.2014.958604 URL |
| [17] |
Bogner CW, Kariuki GM, Elashry A, et al. Fungal root endophytes of tomato from Kenya and their nematode biocontrol potential[J]. Mycol Prog, 2016, 15(3): 1-17.
doi: 10.1007/s11557-015-1147-7 URL |
| [18] |
Yan XN, Sikora RA, Zheng JW. Potential use of cucumber(Cucumis sativus L.)endophytic fungi as seed treatment agents against root-knot nematode Meloidogyne incognita[J]. J Zhejiang Univ Sci B, 2011, 12(3): 219-225.
doi: 10.1631/jzus.B1000165 URL |
| [19] |
Zhou WQ, Starr JL, Krumm JL, et al. The fungal endophyte Chaetomium globosum negatively affects both above- and belowground herbivores in cotton[J]. FEMS Microbiol Ecol, 2016, 92(10): fiw158.
doi: 10.1093/femsec/fiw158 URL |
| [20] | Márquez-Dávila K, Arévalo-López L, Gonzáles R, et al. Trichoderma and Clonostachys as biocontrol agents against Meloidogyne incognita in Sacha inchi[J]. Pesq Agropec Trop, 2020, 55: e60890. |
| [21] |
le HTT, Padgham JL, Sikora RA. Biological control of the rice root-knot nematode Meloidogyne Graminicola on rice, using endophytic and rhizosphere fungi[J]. Int J Pest Manag, 2009, 55(1): 31-36.
doi: 10.1080/09670870802450235 URL |
| [22] |
Le H, Padgham J, Hagemann MH, et al. Developmental and behavioural effects of the endophytic Fusarium moniliforme Fe14 towards Meloidogyne Graminicola in rice[J]. Ann Appl Biol, 2016, 169: 134-143.
doi: 10.1111/aab.2016.169.issue-1 URL |
| [23] |
Schouten A. Mechanisms involved in nematode control by endophytic fungi[J]. Annu Rev Phytopathol, 2016, 54: 121-142.
doi: 10.1146/annurev-phyto-080615-100114 pmid: 27296146 |
| [24] |
Escudero N, Lopez-Llorca LV. Effects on plant growth and root-knot nematode infection of an endophytic GFP transformant of the nematophagous fungus Pochonia chlamydosporia[J]. Symbiosis, 2012, 57(1): 33-42.
doi: 10.1007/s13199-012-0173-3 URL |
| [25] |
Yao YR, Tian XL, Shen BM, et al. Transformation of the endophytic fungus Acremonium implicatum with GFP and evaluation of its biocontrol effect against Meloidogyne incognita[J]. World J Microbiol Biotechnol, 2015, 31(4): 549-556.
doi: 10.1007/s11274-014-1781-2 URL |
| [26] |
Hofmann J, Hess PH, Szakasits D, et al. Diversity and activity of sugar transporters in nematode-induced root syncytia[J]. J Exp Bot, 2009, 60(11): 3085-3095.
doi: 10.1093/jxb/erp138 pmid: 19487386 |
| [27] |
Chen LQ, Hou BH, Lalonde S, et al. Sugar transporters for intercellular exchange and nutrition of pathogens[J]. Nature, 2010, 468(7323): 527-532.
doi: 10.1038/nature09606 |
| [28] |
Singh A, Singh DK, Kharwar RN, et al. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: insights, avenues, and challenges[J]. Microorganisms, 2021, 9(1): 197.
doi: 10.3390/microorganisms9010197 URL |
| [29] |
Hu Y, Zhang W, Zhang P, et al. Nematicidal activity of chaetoglobosin A poduced by Chaetomium globosum NK102 against Meloidogyne incognita[J]. J Agric Food Chem, 2013, 61(1): 41-46.
doi: 10.1021/jf304314g URL |
| [30] |
Schwarz M, Köpcke B, Weber RWS, et al. 3-hydroxypropionic acid as a nematicidal principle in endophytic fungi[J]. Phytochemistry, 2004, 65(15): 2239-2245.
pmid: 15587708 |
| [31] | Betina V. Biological effects of the antibiotic brefeldin A(decumbin, cyanein, ascotoxin, synergisidin): a retrospective[J]. Folia Microbiol(Praha), 1992, 37(1): 3-11. |
| [32] |
Bogner CW, Kamdem RST, Sichtermann G, et al. Bioactive secondary metabolites with multiple activities from a fungal endophyte[J]. Microb Biotechnol, 2017, 10(1): 175-188.
doi: 10.1111/1751-7915.12467 pmid: 27990770 |
| [33] |
Khan B, Yan W, Wei S, et al. Nematicidal metabolites from endophytic fungus Chaetomium globosum YSC5[J]. FEMS Microbiol Lett, 2019, 366(14): fnz169.
doi: 10.1093/femsle/fnz169 URL |
| [34] |
Miao GP, Han J, Zhang KG, et al. Protection of melon against Fusarium wilt-root knot nematode complex by endophytic fungi Penicillium brefeldianum HS-1[J]. Symbiosis, 2019, 77(1): 83-89.
doi: 10.1007/s13199-018-0565-0 |
| [35] |
Sikora RA, Pocasangre L, Felde AZ, et al. Mutualistic endophytic fungi and in-planta suppressiveness to plant parasitic Nema-todes[J]. Biol Control, 2008, 46(1): 15-23.
doi: 10.1016/j.biocontrol.2008.02.011 URL |
| [36] |
Backman PA, Sikora RA. Endophytes: an emerging tool for biological control[J]. Biol Control, 2008, 46(1): 1-3.
doi: 10.1016/j.biocontrol.2008.03.009 URL |
| [37] |
Martínez-Medina A, Fernandez I, Lok GB, et al. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita[J]. New Phytol, 2017, 213(3): 1363-1377.
doi: 10.1111/nph.14251 pmid: 27801946 |
| [38] |
Pieterse CMJ, Leon-Reyes A, van der Ent S, et al. Networking by small-molecule hormones in plant immunity[J]. Nat Chem Biol, 2009, 5(5): 308-316.
doi: 10.1038/nchembio.164 pmid: 19377457 |
| [39] |
Bhattarai KK, Xie QG, Mantelin S, et al. Tomato susceptibility to root-knot nematodes requires an intact jasmonic acid signaling pathway[J]. Mol Plant Microbe Interact, 2008, 21(9): 1205-1214.
doi: 10.1094/MPMI-21-9-1205 URL |
| [40] |
Fujimoto T, Tomitaka Y, Abe H, et al. Expression profile of jasmonic acid-induced genes and the induced resistance against the root-knot nematode(Meloidogyne incognita)in tomato plants(Solanum lycopersicum)after foliar treatment with methyl jasmonate[J]. J Plant Physiol, 2011, 168(10): 1084-1097.
doi: 10.1016/j.jplph.2010.12.002 URL |
| [41] |
Hamamouch N, Li CY, Seo PJ, et al. Expression of Arabidopsis pathogenesis-related genes during nematode infection[J]. Mol Plant Pathol, 2011, 12(4): 355-364.
doi: 10.1111/mpp.2011.12.issue-4 URL |
| [42] |
Nahar K, Kyndt T, de Vleesschauwer D, et al. The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice[J]. Plant Physiol, 2011, 157(1): 305-316.
doi: 10.1104/pp.111.177576 pmid: 21715672 |
| [43] |
Molinari S, Fanelli E, Leonetti P. Expression of tomato salicylic acid(SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes[J]. Mol Plant Pathol, 2014, 15(3): 255-264.
doi: 10.1111/mpp.12085 pmid: 24118790 |
| [44] |
Fan JW, Hu CL, Zhang LN, et al. Jasmonic acid mediates tomato's response to root knot nematodes[J]. J Plant Growth Regul, 2015, 34(1): 196-205.
doi: 10.1007/s00344-014-9457-6 URL |
| [45] | Gleason C, Leelarasamee N, Meldau D, et al. OPDA has key role in regulating plant susceptibility to the root-knot nematode Meloidogyne hapla in Arabidopsis[J]. Front Plant Sci, 2016, 7: 1565. |
| [46] |
Bali SG, Kaur P, Sharma A, et al. Jasmonic acid-induced tolerance to root-knot nematodes in tomato plants through altered photosynthetic and antioxidative defense mechanisms[J]. Protoplasma, 2018, 255(2): 471-484.
doi: 10.1007/s00709-017-1160-6 pmid: 28905119 |
| [47] |
Bali SG, Kaur P, Jamwal VL, et al. Seed priming with jasmonic acid counteracts root knot nematode infection in tomato by modulating the activity and expression of antioxidative enzymes[J]. Biomolecules, 2020, 10(1): 98.
doi: 10.3390/biom10010098 URL |
| [48] |
Zinovieva S, Udalova ZV, Seiml-Buchinger V, et al. Gene expression of protease inhibitors in tomato plants with invasion by root-knot nematode Meloidogyne incognita and modulation of their activity with salicylic and jasmonic acids[J]. Biol Bull, 2021, 48: 130-139.
doi: 10.1134/S1062359021020175 |
| [49] |
Sahebani N, Hadavi N. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum[J]. Soil Biology and Biochemistry, 2008, 40(8): 2016-2020.
doi: 10.1016/j.soilbio.2008.03.011 URL |
| [50] | Jin R, Liao HD, Liu XM, et al. Identification and characterization of a fungal strain with lignin and cellulose hydrolysis activities[J]. African Journal of Microbiology Research, 2012, 6(36): 6545-6550. |
| [51] |
Xiong XQ, Liao HD, Ma JS, et al. Isolation of a rice endophytic bacterium, Pantoea sp. Sd-1, with ligninolytic activity and characterization of its rice straw degradation ability[J]. Lett Appl Microbiol, 2014, 58(2): 123-129.
doi: 10.1111/lam.12163 pmid: 24111687 |
| [52] |
Ma JS, Zhang KK, Liao HD, et al. Genomic and secretomic insight into lignocellulolytic system of an endophytic bacterium Pantoea ananatis Sd-1[J]. Biotechnol Biofuels, 2016, 9: 25.
doi: 10.1186/s13068-016-0439-8 URL |
| [53] | 王真真, 徐婷, 袁珊珊, 等. 水稻内生放线菌OsiRt-1的分离鉴定及对稻瘟病的防治作用[J]. 微生物学通报, 2016, 43(5): 1009-1018. |
| Wang ZZ, Xu T, Yuan SS, et al. Identification of an endophytic actinomyce OsiRt-1 isolated from rice and its effect against rice blast disease[J]. Microbiol China, 2016, 43(5): 1009-1018. | |
| [54] |
Xu T, Li Y, Zeng XD, et al. Isolation and evaluation of endophytic Streptomyces endus OsiSh-2 with potential application for biocontrol of rice blast disease[J]. J Sci Food Agric, 2017, 97(4): 1149-1157.
doi: 10.1002/jsfa.2017.97.issue-4 URL |
| [55] | 刘雨晴, 许奕涵, 刘选明, 等. 内生白色链霉菌OsiSh-10对拟南芥根系结构的影响[J]. 基因组学与应用生物学, 2018, 37(8): 3503-3509. |
| Liu YQ, Xu YH, Liu XM, et al. Effects of endophytic Streptomyces albus OsiSh-10 on Arabidopsis thaliana root architecture[J]. Genom Appl Biol, 2018, 37(8): 3503-3509. |
| [1] | 江润海, 姜冉冉, 朱城强, 侯秀丽. 微生物强化植物修复铅污染土壤的机制研究进展[J]. 生物技术通报, 2023, 39(8): 114-125. |
| [2] | 褚睿, 李昭轩, 张学青, 杨东亚, 曹行行, 张雪艳. 黄瓜枯萎病拮抗芽孢杆菌的筛选、鉴定及其生防潜力[J]. 生物技术通报, 2023, 39(8): 262-271. |
| [3] | 方澜, 黎妍妍, 江健伟, 成胜, 孙正祥, 周燚. 盘龙参内生真菌胞内细菌7-2H的分离鉴定和促生特性研究[J]. 生物技术通报, 2023, 39(8): 272-282. |
| [4] | 王天依, 王荣焕, 王夏青, 张如养, 徐瑞斌, 焦炎炎, 孙轩, 王继东, 宋伟, 赵久然. 玉米矮秆基因与矮秆育种研究[J]. 生物技术通报, 2023, 39(8): 43-51. |
| [5] | 张蓓, 任福森, 赵洋, 郭志伟, 孙强, 刘贺娟, 甄俊琦, 王童童, 程相杰. 辣椒响应热胁迫机制的研究进展[J]. 生物技术通报, 2023, 39(7): 37-47. |
| [6] | 李典典, 粟元, 李洁, 许文涛, 朱龙佼. 抗菌适配体的筛选与应用进展[J]. 生物技术通报, 2023, 39(6): 126-132. |
| [7] | 张和臣, 袁欣, 高杰, 王校晨, 王慧娟, 李艳敏, 王利民, 符真珠, 李保印. 植物花瓣呈色机理及花色分子育种[J]. 生物技术通报, 2023, 39(5): 23-31. |
| [8] | 任沛东, 彭健玲, 刘圣航, 姚姿婷, 朱桂宁, 陆光涛, 李瑞芳. 沙福芽孢杆菌GX-H6的分离鉴定及对水稻细菌性条斑病的防病效果[J]. 生物技术通报, 2023, 39(5): 243-253. |
| [9] | 章乐乐, 王冠, 柳凤, 胡汉桥, 任磊. 芒果炭疽病拮抗菌分离、鉴定及生防机制研究[J]. 生物技术通报, 2023, 39(4): 277-287. |
| [10] | 王伟宸, 赵进, 黄薇颐, 郭芯竹, 李婉颖, 张卓. 芽胞杆菌代谢产物防治三种常见植物病原真菌的研究进展[J]. 生物技术通报, 2023, 39(3): 59-68. |
| [11] | 崔吉洁, 蔡文波, 庄庆辉, 高爱平, 黄建峰, 陈亚辉, 宋志忠. 杧果Fe-S簇装配基因MiISU1的生物学功能[J]. 生物技术通报, 2023, 39(2): 139-146. |
| [12] | 杨东亚, 祁瑞雪, 李昭轩, 林薇, 马慧, 张雪艳. 黄瓜茄病镰刀菌拮抗芽孢杆菌的筛选、鉴定及促生效果[J]. 生物技术通报, 2023, 39(2): 211-220. |
| [13] | 李凯航, 王浩臣, 程可心, 杨艳, 金一, 何晓青. 全基因组关联分析研究植物与微生物组的互作遗传机制[J]. 生物技术通报, 2023, 39(2): 24-34. |
| [14] | 罗宁, 焦阳, 茆振川, 李惠霞, 谢丙炎. 木霉菌对根结线虫和孢囊线虫防治机理研究进展[J]. 生物技术通报, 2023, 39(2): 35-50. |
| [15] | 陈广霞, 李秀杰, 蒋锡龙, 单雷, 张志昌, 李勃. 植物小分子信号肽参与非生物逆境胁迫应答的研究进展[J]. 生物技术通报, 2023, 39(11): 61-73. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||