








生物技术通报 ›› 2025, Vol. 41 ›› Issue (10): 210-221.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0473
李粘前1( ), 李琛1, 李淑婷1, 马菊花1, 景海青1, 孙岩1, 周雅莉1, 薛金爱1,2(
), 李琛1, 李淑婷1, 马菊花1, 景海青1, 孙岩1, 周雅莉1, 薛金爱1,2( ), 李润植1,2(
), 李润植1,2( )
)
收稿日期:2025-05-08
出版日期:2025-10-26
发布日期:2025-10-28
通讯作者:
薛金爱,女,博士,教授,研究方向 :植物分子遗传与基因工程;E-mail: 306214803@qq.com;作者简介:李粘前,男,硕士研究生,研究方向 :作物遗传育种;E-mail: lizhanqian2022@163.com
基金资助:
LI Zhan-qian1( ), LI Chen1, LI Shu-ting1, MA Ju-hua1, JING Hai-qing1, SUN Yan1, ZHOU Ya-li1, XUE Jin-ai1,2(
), LI Chen1, LI Shu-ting1, MA Ju-hua1, JING Hai-qing1, SUN Yan1, ZHOU Ya-li1, XUE Jin-ai1,2( ), LI Run-zhi1,2(
), LI Run-zhi1,2( )
)
Received:2025-05-08
Published:2025-10-26
Online:2025-10-28
摘要:
目的 系统分析油莎豆苹果酸酶(malic enzyme, ME)基因家族成员、挖掘参与油莎豆块茎油脂合成的ME,为全面解析油莎豆块茎富油机制和培育营养器官富油的作物新种质提供科学参考。 方法 应用组学工具全基因组鉴定油莎豆CeME基因家族成员、分析CeME蛋白理化特性;运用实时荧光定量PCR(RT-qPCR)检测CeME基因在块茎不同发育时期的表达谱;构建靶标CeME表达载体,对酵母(Saccharomyces cerevisiae)和烟草(Nicotiana tabacum)进行遗传转化;检测转化体的ME酶活性和油脂代谢。 结果 从油莎豆基因组中共鉴定到6个CeMEs基因家族成员,分布于6条染色体,其中4个NADP类型(CeNADP-ME1-CeNADP-ME4)和2个NAD类型(CeNAD-ME1-CeNAD-ME2)。CeNADP-ME1具有19个内含子,其余5个CeME基因均含有18个内含子。CeME基因启动子含有生长发育、激素和逆境响应等多种顺式作用元件。6个CeME蛋白均具有典型的ME酶蛋白结构域和12个保守基序。RT-qPCR分析显示,CeME基因在油莎豆块茎油脂积累关键时期(播种后80‒120 d)表达上调,其中,NAD-ME类型的CeNAD-ME2表达量最高。CeNAD-ME2编码的酶蛋白定位于线粒体。过表达CeNAD-ME2酵母细胞总脂和棕榈油酸(C16:1)分别比野生型酵母提高5.6%和5%。与野生型烟草相比,转CeNAD-ME2株系ME活性增加1.5‒4倍,烟叶总脂和油酸(C18:1)含量分别提高5.2%和5.6%,而可溶性糖和淀粉含量分别下降2%和5%。 结论 从油莎豆基因组鉴定到6个CeME成员,CeNAD-ME2参与块茎油脂合成。异源表达CeNAD-ME2可驱动碳源更多地流向油脂合成途径,显著提高宿主总脂和单不饱和脂肪酸含量。
李粘前, 李琛, 李淑婷, 马菊花, 景海青, 孙岩, 周雅莉, 薛金爱, 李润植. 油莎豆苹果酸酶(ME)全基因组鉴定及CeNAD-ME2功能分析[J]. 生物技术通报, 2025, 41(10): 210-221.
LI Zhan-qian, LI Chen, LI Shu-ting, MA Ju-hua, JING Hai-qing, SUN Yan, ZHOU Ya-li, XUE Jin-ai, LI Run-zhi. Genome-wide Identification of the ME Family in Cyperus esculentusis and Functional Analysis of CeNAD-ME2[J]. Biotechnology Bulletin, 2025, 41(10): 210-221.
| 基因 Gene | 正向引物 Forward primer (5′-3′) | 反向引物 Reverse primer (5′-3′) |
|---|---|---|
| CeNADP-ME1 | GAGGAGCTGTTGCCATCTGT | TGTGACAGATGGCGAACGG |
| CeNADP-ME2 | CACTGACGGAGGACGGATT | TCTGCGTGCTTGCCTATTACA |
| CeNADP-ME3 | GCATGAGAAGAGCATCCAGG | CTTCTGCGTGCCTGCCTATTA |
| CeNADP-ME4 | CTTCTGCCTCCTGCTATCGTT | TGGTTCCATCTTCAGGCGTC |
| CeNAD-ME1 | TGGTGACAGATGGAAGCAGGA | CTGTATGTCTCCGCTGCTGG |
| CeNAD-ME2 | AGAATGCTCGGCACTGACC | AGTGGTCAAGAAGGTGAAGCC |
| CeActin | TCGGTGGTATTGGAACTG | AAGCACTGGAGCGTAGCC |
| pBI121-CeNAD-ME2 | aacctgcaggtcgac | ggtttaaacgagctc |
| pYES2.0-CeNAD-ME2 | attaagcttggtacc | tacatgatgcggccc |
| 1300-CeNAD-ME2 | atacaccaaatcgac | cgatcggggaaattc |
表1 引物序列
Table 1 Primer sequences
| 基因 Gene | 正向引物 Forward primer (5′-3′) | 反向引物 Reverse primer (5′-3′) |
|---|---|---|
| CeNADP-ME1 | GAGGAGCTGTTGCCATCTGT | TGTGACAGATGGCGAACGG |
| CeNADP-ME2 | CACTGACGGAGGACGGATT | TCTGCGTGCTTGCCTATTACA |
| CeNADP-ME3 | GCATGAGAAGAGCATCCAGG | CTTCTGCGTGCCTGCCTATTA |
| CeNADP-ME4 | CTTCTGCCTCCTGCTATCGTT | TGGTTCCATCTTCAGGCGTC |
| CeNAD-ME1 | TGGTGACAGATGGAAGCAGGA | CTGTATGTCTCCGCTGCTGG |
| CeNAD-ME2 | AGAATGCTCGGCACTGACC | AGTGGTCAAGAAGGTGAAGCC |
| CeActin | TCGGTGGTATTGGAACTG | AAGCACTGGAGCGTAGCC |
| pBI121-CeNAD-ME2 | aacctgcaggtcgac | ggtttaaacgagctc |
| pYES2.0-CeNAD-ME2 | attaagcttggtacc | tacatgatgcggccc |
| 1300-CeNAD-ME2 | atacaccaaatcgac | cgatcggggaaattc |

图1 油莎豆ME家族蛋白氨基酸序列比对(A、B)及与其他植物ME蛋白的进化树分析(C)I‒V:苹果酸酶的5个保守结构域。Ce:油莎豆;At:拟南芥;Zm:玉米;Ta:小麦;Os:水稻;St:马铃薯;Gm:大豆
Fig. 1 Amino acid sequence alignment of the CeME family proteins (A, B) and the evolutionary tree analysis (C) of CeMEs with ME proteins from other plantsI‒V: Five conserved domains of malic enzyme. Ce: Cyperus esculentusis; At: Arabidopsis thaliana; Zm: Zea mays (maize). Ta: Triticum aestivum (wheat); Os: Oryza sativa (rice); St: Solanum tuberosum (potato); Gm: Glycine max (soybean)
基因 Gene | 基因 ID Gene ID | 氨基酸数 Number of amino acids | 相对分子质量 Molecular weight (kD) | 理论等电点 pI | 不稳定系数 Instability factor | 亲水性 Hydrophilicity | 亚细胞定位预测 Prediction of subcellu-lar location |
|---|---|---|---|---|---|---|---|
| CeNADP-ME1 | CESC_03761 | 650 | 71.11 | 7.00 | 37.79 | -0.206 | 叶绿体 |
| CeNADP-ME2 | CESC_08481 | 577 | 64.01 | 5.97 | 41.44 | -0.112 | 叶绿体 |
| CeNADP-ME3 | CESC_16067 | 641 | 71.43 | 6.73 | 44.48 | -0.153 | 叶绿体 |
| CeNADP-ME4 | CESC_16827 | 662 | 73.62 | 6.78 | 49.33 | -0.121 | 叶绿体 |
| CeNAD-ME1 | CESC_15235 | 623 | 68.87 | 5.59 | 43.08 | -0.112 | 线粒体 |
| CeNAD-ME2 | CESC_17435 | 609 | 67.51 | 7.98 | 36.58 | -0.119 | 线粒体 |
表2 油莎豆CeME家族蛋白理化性质分析
Table 2 Analysis of the physiochemical properties of the ME family proteins in C. yperus esculentusis L.
基因 Gene | 基因 ID Gene ID | 氨基酸数 Number of amino acids | 相对分子质量 Molecular weight (kD) | 理论等电点 pI | 不稳定系数 Instability factor | 亲水性 Hydrophilicity | 亚细胞定位预测 Prediction of subcellu-lar location |
|---|---|---|---|---|---|---|---|
| CeNADP-ME1 | CESC_03761 | 650 | 71.11 | 7.00 | 37.79 | -0.206 | 叶绿体 |
| CeNADP-ME2 | CESC_08481 | 577 | 64.01 | 5.97 | 41.44 | -0.112 | 叶绿体 |
| CeNADP-ME3 | CESC_16067 | 641 | 71.43 | 6.73 | 44.48 | -0.153 | 叶绿体 |
| CeNADP-ME4 | CESC_16827 | 662 | 73.62 | 6.78 | 49.33 | -0.121 | 叶绿体 |
| CeNAD-ME1 | CESC_15235 | 623 | 68.87 | 5.59 | 43.08 | -0.112 | 线粒体 |
| CeNAD-ME2 | CESC_17435 | 609 | 67.51 | 7.98 | 36.58 | -0.119 | 线粒体 |
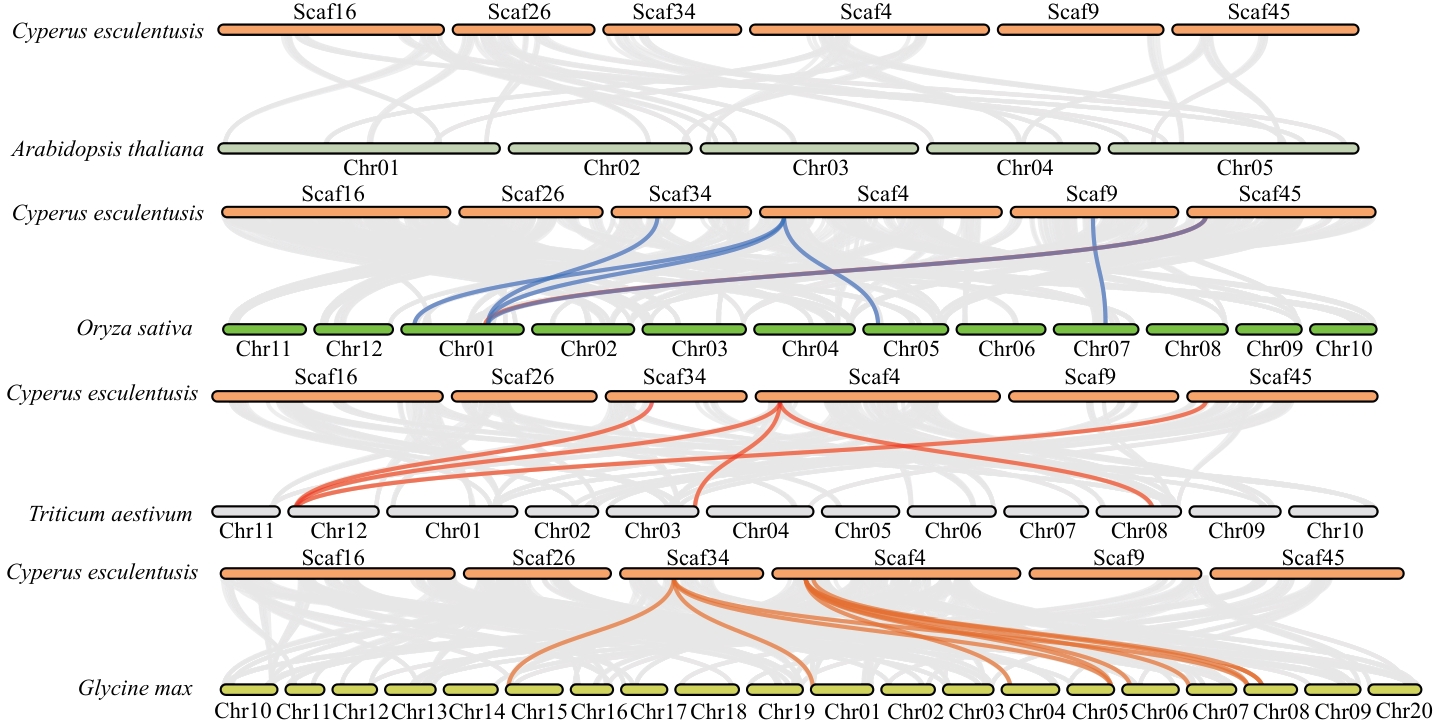
图3 油莎豆CeMEs基因家族与4种典型植物(拟南芥、小麦、水稻和大豆)的共线性分析
Fig. 3 Collinear analysis of CeMEs gene family with 4 other plants (Arabidopsis thaliana, wheat, rice and soybean)

图4 油莎豆CeMEs家族蛋白的保守基序/结构域分析(A)和基因内含子/外显子组成分析(B)
Fig. 4 Analysis of the conserved motifs and domains among CeMEs family (A)and the inron/exon organization of CeME genes (B) in C. esculentusis

图7 油莎豆块茎发育不同时期ME基因表达量的定量分析不同字母表示在P<0.05水平差异显著。下同
Fig. 7 Quantitative analysis of ME gene expression in different stages of C. esculentusis tuber developmentDifferent letters indicate significant differences at P<0.05. The same below
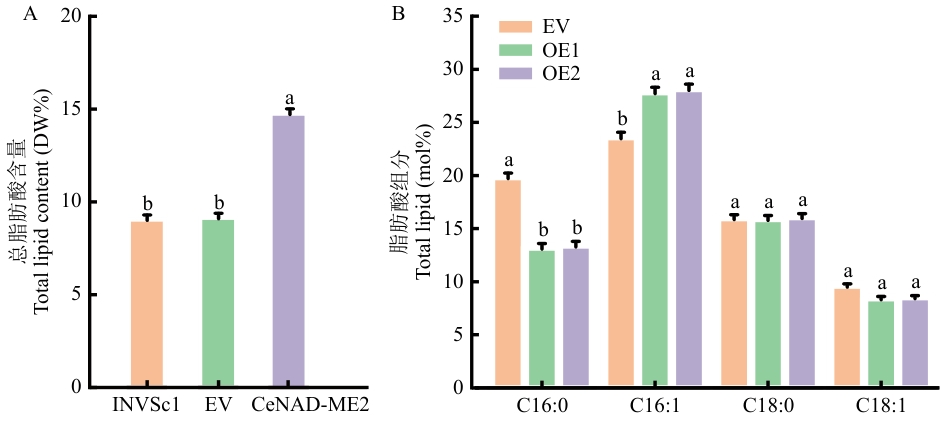
图9 转基因酵母总脂肪酸含量和转基因酵母脂肪酸组分INVSc1:野生型对照;EV:空载对照;OE:过表达株系
Fig. 9 Total fatty acid content and fatty acid profiles of the transgenic yeastINVSc1: Wild contro; EV: empty vector control; OE: overexpressed line
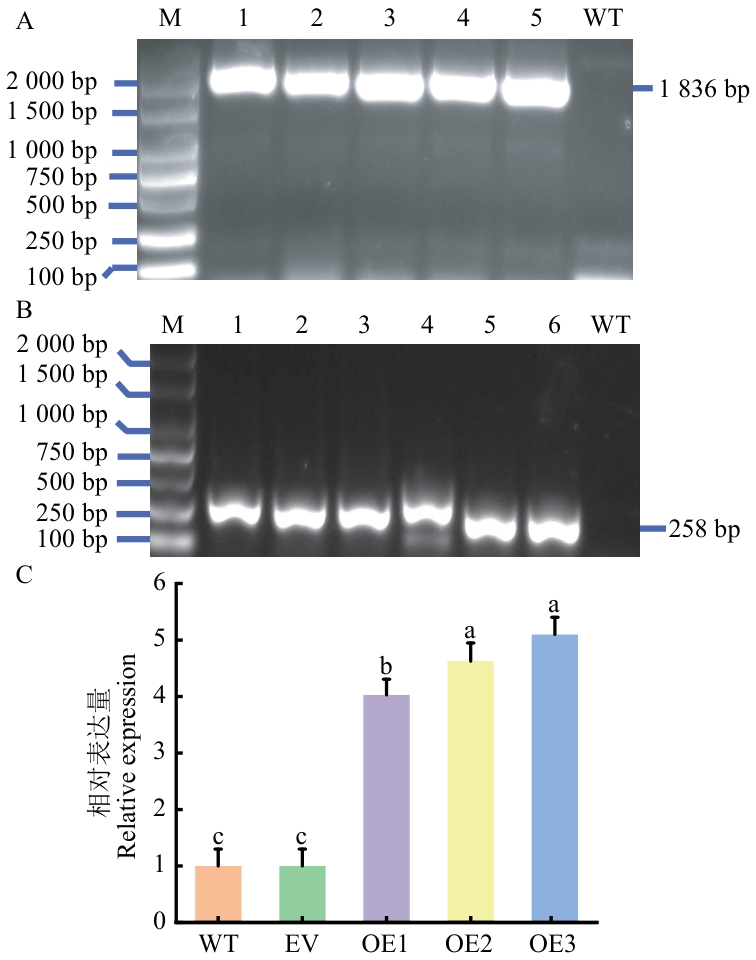
图11 转CeNAD-ME2烟草植株的DNA分子鉴定(A)、RNA分子鉴定(B)及相对表达量(C)M:DL2000 marker;1‒6:转PBI121-CeNAD-ME2阳性条带;WT:野生对照;EV:空载对照:OE:转PBI121-CeNAD-ME2阳性植株。下同
Fig. 11 DNA molecular identification (A), RNA molecule identification (B) and relative expression (C) of CeNAD-ME2-transgenic tobacco plantsM: DL2000 marker; 1‒6: transfer PBI121-CeNAD-ME2 positive band; WT: wild control; EV: empty vector control; OE: transgenic PBI121-CeNAD-ME2 positive plants. The same below

图13 转基因烟草叶片淀粉、可溶性糖、蛋白、总脂肪酸、叶绿素和脂肪酸组分测定
Fig. 13 Contents of starch, soluble sugar, protein, total fatty acid, chlorophyll,and fatty acid profiles of the transgenic tobacco leaves
| [1] | Sánchez-Zapata E, Fernández-López J, Angel Pérez-Alvarez J. Tiger nut (Cyperus esculentus) commercialization: health aspects, composition, properties, and food applications [J]. Compr Rev Food Sci Food Saf, 2012, 11(4): 366-377. |
| [2] | Bullen JJ, Rogers HJ, Spalding PB, et al. Natural resistance, iron and infection: a challenge for clinical medicine [J]. J Med Microbiol, 2006, 55(Pt 3): 251-258. |
| [3] | 瞿萍梅, 程治英, 龙春林, 等. 油莎豆资源的综合开发利用 [J]. 中国油脂, 2007, 32(9): 61-63. |
| Qu PM, Cheng ZY, Long CL, et al. Comprehensive development of chufa (Cyperus esculentus L. var. sativus) [J]. China Oils Fats, 2007, 32(9): 61-63. | |
| [4] | Makareviciene V, Gumbyte M, Yunik A, et al. Opportunities for the use of chufa sedge in biodiesel production [J]. Ind Crops Prod, 2013, 50: 633-637. |
| [5] | Li T, Sun Y, Chen Y, et al. Characterisation of two novel genes encoding Δ9 fatty acid desaturases (CeSADs) for oleic acid accumulation in the oil-rich tuber of Cyperus esculentus [J]. Plant Sci, 2022, 319: 111243. |
| [6] | McCullough W, Roberts CF. The role of malic enzyme in Aspergillus nidulans [J]. FEBS Lett, 1974, 41(2): 238-242. |
| [7] | Boles E, de Jong-Gubbels P, Pronk JT. Identification and characterization of MAE1 the Saccharomyces cerevisiae structural gene encoding mitochondrial malic enzyme [J]. J Bacteriol, 1998, 180(11): 2875-2882. |
| [8] | Thelen JJ, Ohlrogge JB. Metabolic engineering of fatty acid biosynthesis in plants [J]. Metab Eng, 2002, 4(1): 12-21. |
| [9] | 周滈, 杨传平, 柳参奎. 植物苹果酸酶在抵御逆境中的作用 [J]. 林业科技, 2011, 36(6): 44-47. |
| Zhou H, Yang CP, Liu SK. The role of plant malic enzyme in defense against stress environments [J]. For Sci Technol, 2011, 36(6): 44-47. | |
| [10] | Rao XL, Dixon RA. The differences between NAD-ME and NADP-ME subtypes of C4 photosynthesis: more than decarboxylating enzymes [J]. Front Plant Sci, 2016, 7: 1525. |
| [11] | Wheeler MC, Tronconi MA, Drincovich MF, et al. A comprehensive analysis of the NADP-malic enzyme gene family of Arabidopsis [J]. Plant Physiol, 2005, 139(1): 39-51. |
| [12] | Tronconi MA, Fahnenstich H, Gerrard Weehler MC, et al. Arabidopsis NAD-malic enzyme functions as a homodimer and heterodimer and has a major impact on nocturnal metabolism [J]. Plant Physiol, 2008, 146(4): 1540-1552. |
| [13] | Alvarez CE, Saigo M, Margarit E, et al. Kinetics and functional diversity among the five members of the NADP-malic enzyme family from Zea mays, a C4 species [J]. Photosynth Res, 2013, 115(1): 65-80. |
| [14] | Ratledge C. The role of malic enzyme as the provider of NADPH in oleaginous microorganisms: a reappraisal and unsolved problems [J]. Biotechnol Lett, 2014, 36(8): 1557-1568. |
| [15] | Zhu BH, Zhang RH, Lv NN, et al. The role of malic enzyme on promoting total lipid and fatty acid production in Phaeodactylum tricornutum [J]. Front Plant Sci, 2018, 9: 826. |
| [16] | Zhang Y, Adams IP, Ratledge C. Malic enzyme: the controlling activity for lipid production overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation [J]. Microbiology, 2007, 153(Pt 7): 2013-2025. |
| [17] | Wynn JP, Ratledge C. Malic enzyme is a major source of NADPH for lipid accumulation by Aspergillus nidulans [J]. Microbiology, 1997, 143(1): 253-257. |
| [18] | Gerrard Wheeler MC, Arias CL, Righini S, et al. Differential contribution of malic enzymes during soybean and castor seeds maturation [J]. PLoS One, 2016, 11(6): e0158040. |
| [19] | Morley SA, Ma FF, Alazem M, et al. Expression of malic enzyme reveals subcellular carbon partitioning for storage reserve production in soybeans [J]. New Phytol, 2023, 239(5): 1834-1851. |
| [20] | Ji HY, Liu DT, Yang ZL. High oil accumulation in tuber of yellow nutsedge compared to purple nutsedge is associated with more abundant expression of genes involved in fatty acid synthesis and triacylglycerol storage [J]. Biotechnol Biofuels, 2021, 14(1): 54. |
| [21] | Gao Y, Sun Y, Gao HL, et al. Correction to: Ectopic overexpression of a type-II DGAT (CeDGAT2-2) derived from oil-rich tuber of Cyperus esculentus enhances accumulation of oil and oleic acid in tobacco leaves [J]. Biotechnol Biofuels, 2021, 14(1): 139. |
| [22] | 李秀峰, 张欣欣, 高野哲夫, 等. 水稻(Oryza sativa L.)苹果酸酶(OsNADP-ME3)基因在逆境下的表达特性研究 [J]. 基因组学与应用生物学, 2012, 31(4): 327-332. |
| Li XF, Zhang XX, Takano T, et al. Expression characteristics of rice (Oryza sativa L.) malic enzyme (OsNADP-ME3) gene under environmental stress [J]. Genom Appl Biol, 2012, 31(4): 327-332. | |
| [23] | 刘增辉. 小麦NADP-苹果酸酶与逆境关系的初步研究 [D]. 青岛: 青岛科技大学, 2010. |
| Liu ZH. Preliminary study on the relationg of NADP-malic enzyme and stresses [D]. Qingdao: Qingdao University of Science & Technology, 2010. | |
| [24] | Kawaoka A, Kawamoto T, Sekine M, et al. A cis-acting element and a trans-acting factor involved in the wound-induced expression of a horseradish peroxidase gene [J]. Plant J, 1994, 6(1): 87-97. |
| [25] | Ni M, Tepperman JM, Quail PH. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein [J]. Cell, 1998, 95(5): 657-667. |
| [26] | 张玉慧, 王玉兰, 夏春兰, 等. 松叶猪毛菜NADP-苹果酸酶基因家族及启动子克隆分析 [J]. 西北植物学报, 2022, 42(5): 760-769. |
| Zhang YH, Wang YL, Xia CL, et al. Cloning and analysis of NADP-ME gene family and promoters in Salsola laricifolia [J]. Acta Bot Boreali Occidentalia Sin, 2022, 42(5): 760-769. | |
| [27] | 史先飞, 高宇, 黄旭升, 等. 油莎豆丙酮酸激酶基因的鉴定及其在块茎发芽和幼苗形态建成时期的表达分析 [J]. 激光生物学报, 2022, 31(6): 533-541. |
| Shi XF, Gao Y, Huang XS, et al. Identification of pyruvate kinase genes and their expression analysis during Cyperus esculentus tuber germination and seedling establishment [J]. Acta Laser Biol Sin, 2022, 31(6): 533-541. | |
| [28] | Dao O, Kuhnert F, Weber APM, et al. Physiological functions of malate shuttles in plants and algae [J]. Trends Plant Sci, 2022, 27(5): 488-501. |
| [29] | Wedding RT. Malic enzymes of higher plants: characteristics, regulation, and physiological function [J]. Plant Physiol, 1989, 90(2): 367-371. |
| [30] | Bonnerot C, Galle AM, Jolliot A, et al. Purification and properties of plant cytochrome b5 [J]. Biochem J, 1985, 226(1): 331-334. |
| [31] | Kumar R, Tran LP, Neelakandan AK, et al. Higher plant cytochrome b5 polypeptides modulate fatty acid desaturation [J]. PLoS One, 2012, 7(2): e31370. |
| [32] | Schultz CJ, Coruzzi GM. The aspartate aminotransferase gene family of Arabidopsis encodes isoenzymes localized to three distinct subcellular compartments [J]. Plant J, 1995, 7(1): 61-75. |
| [33] | Allen DK, Young JD. Carbon and nitrogen provisions alter the metabolic flux in developing soybean embryos [J]. Plant Physiol, 2013, 161(3): 1458-1475. |
| [34] | Eastmond PJ, Dennis DT, Rawsthorne S. Evidence that a malate/inorganic phosphate exchange translocator imports carbon across the leucoplast envelope for fatty acid synthesis in developing castor seed endosperm [J]. Plant Physiol, 1997, 114(3): 851-856. |
| [35] | 李秀婷. 现代啤酒生产工艺 [M]. 北京: 中国农业大学出版社, 2013. |
| Li XT. Modern technology of beer production [M]. Beijing: China Agricultural University Press, 2013. | |
| [36] | 荆美玲. 油莎豆油脂合成基因CePDAT3基因的克隆及功能研究 [D]. 长春: 吉林农业大学, 2022. |
| Jing ML. Cloning and functional study of CePDAT3 gene for oil synthesis fromcyperus esculentusis [D]. Changchun: Jilin Agricultural University, 2022. | |
| [37] | Cramer CL, Weissenborn DL, Oishi KK, et al. Bioproduction of human enzymes in transgenic tobacco [J]. Ann N Y Acad Sci, 1996, 792(1): 62-71. |
| [38] | 吉夏洁. 过表达蓖麻RcWRI1提高烟叶油脂积累 [D]. 太谷: 山西农业大学, 2018. |
| Ji XJ. Overexpression of Ricinus communis RcWRI1 improves oil accumulation in tobacco leaves [D]. Taigu: Shanxi Agricultural University, 2018. | |
| [39] | Wilson RS, Thelen JJ. In vivo quantitative monitoring of subunit stoichiometry for metabolic complexes [J]. J Proteome Res, 2018, 17(5): 1773-1783. |
| [1] | 程婷婷, 刘俊, 王利丽, 练从龙, 魏文君, 郭辉, 吴尧琳, 杨晶凡, 兰金旭, 陈随清. 杜仲查尔酮异构酶基因家族全基因组鉴定及其表达模式分析[J]. 生物技术通报, 2025, 41(9): 242-255. |
| [2] | 李凯月, 邓晓霞, 殷缘, 杜亚彤, 徐元静, 王竞红, 于耸, 蔺吉祥. 蓖麻LEA基因家族的鉴定和铝胁迫响应分析[J]. 生物技术通报, 2025, 41(7): 128-138. |
| [3] | 龚钰涵, 陈兰, 尚方慧子, 郝灵颖, 刘硕谦. 茶树TRB基因家族鉴定及表达模式分析[J]. 生物技术通报, 2025, 41(7): 214-225. |
| [4] | 程珊, 王会伟, 陈晨, 朱雅婧, 李春鑫, 别海, 王树峰, 陈献功, 张向歌. 油莎豆MYB转录因子基因CeMYB154克隆及耐盐功能分析[J]. 生物技术通报, 2025, 41(6): 218-228. |
| [5] | 孙天国, 衣兰, 秦旭洋, 乔梦雪, 谷新颖, 韩艺, 沙伟, 张梅娟, 马天意. 大白菜DABB基因家族的全基因组鉴定及盐碱胁迫下的表达分析[J]. 生物技术通报, 2025, 41(4): 156-165. |
| [6] | 宋姝熠, 蒋开秀, 刘欢艳, 黄亚成, 刘林娅. ‘红阳’猕猴桃TCP基因家族鉴定及其在果实中的表达分析[J]. 生物技术通报, 2025, 41(3): 190-201. |
| [7] | 何财林, 卢晶, 郭会会, 李小安, 吴琪. 藜麦MADS-box基因家族的全基因组鉴定和表达分析[J]. 生物技术通报, 2025, 41(1): 157-172. |
| [8] | 崔原瑗, 王昭懿, 白双宇, 任毓昭, 豆飞飞, 刘彩霞, 刘凤楼, 王掌军, 李清峰. 大麦非特异性磷脂酶C基因家族全基因组鉴定及苗期胁迫表达分析[J]. 生物技术通报, 2024, 40(8): 74-82. |
| [9] | 臧文蕊, 马明, 砗根, 哈斯阿古拉. 甜瓜BZR转录因子家族基因的全基因组鉴定及表达模式分析[J]. 生物技术通报, 2024, 40(7): 163-171. |
| [10] | 阿丽亚·外力, 陈永坤, 克拉热木·克里木江, 王宝庆, 陈凌娜. 核桃SPL基因家族的系统进化和表达分析[J]. 生物技术通报, 2024, 40(6): 180-189. |
| [11] | 龚丽丽, 余花, 杨杰, 陈天池, 赵双滢, 吴月燕. 葡萄CYP707A基因家族的鉴定及对果实成熟的功能验证[J]. 生物技术通报, 2024, 40(2): 160-171. |
| [12] | 路喻丹, 刘晓驰, 冯新, 陈桂信, 陈义挺. 猕猴桃BBX基因家族成员鉴定与转录特征分析[J]. 生物技术通报, 2024, 40(2): 172-182. |
| [13] | 张路阳, 韩文龙, 徐晓雯, 姚健, 李芳芳, 田效园, 张智强. 烟草TCP基因家族的鉴定及表达分析[J]. 生物技术通报, 2023, 39(6): 248-258. |
| [14] | 赖瑞联, 冯新, 高敏霞, 路喻丹, 刘晓驰, 吴如健, 陈义挺. 猕猴桃过氧化氢酶基因家族全基因组鉴定与表达分析[J]. 生物技术通报, 2023, 39(4): 136-147. |
| [15] | 段敏杰, 李怡斐, 杨小苗, 王春萍, 黄启中, 黄任中, 张世才. 辣椒锌指蛋白DnaJ-Like基因家族鉴定及对高温胁迫的表达响应[J]. 生物技术通报, 2023, 39(1): 187-198. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||