








生物技术通报 ›› 2025, Vol. 41 ›› Issue (4): 335-344.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0945
• 研究报告 • 上一篇
刘爽( ), 江洲, 赵帅, 赵雷真, 黄峰, 周佳, 屈建航(
), 江洲, 赵帅, 赵雷真, 黄峰, 周佳, 屈建航( )
)
收稿日期:2024-09-27
出版日期:2025-04-26
发布日期:2025-04-25
通讯作者:
屈建航,女,博士,教授,研究方向 :环境微生物学;E-mail: qjh_bata@163.com作者简介:刘爽,男,硕士研究生,研究方向 :环境微生物学;E-mail: liushuanghaut@outlook.com
基金资助:
LIU Shuang( ), JIANG Zhou, ZHAO Shuai, ZHAO Lei-zhen, HUANG Feng, ZHOU Jia, QU Jian-hang(
), JIANG Zhou, ZHAO Shuai, ZHAO Lei-zhen, HUANG Feng, ZHOU Jia, QU Jian-hang( )
)
Received:2024-09-27
Published:2025-04-26
Online:2025-04-25
摘要:
目的 从酸菜汁中分离筛选产蛋白酶细菌,明确菌株种属分类地位,优化发酵产酶工艺,以丰富蛋白酶菌种资源,提高发酵产酶效能。 方法 以某酸菜作坊大白菜酸菜汁为分离源,采用10倍梯度稀释法通过酪蛋白平板初筛,福林酚法进一步复筛获得产蛋白酶菌株,结合形态学观察和16S rRNA基因系统发育分析完成种属鉴定;单因素试验优化发酵培养基成分及条件,响应面法进一步优化产酶工艺条件,提高产酶效能。 结果 初筛共得到15株蛋白酶产生菌,福林酚法复筛得到同时具备酸性、中性和碱性蛋白酶能力的菌株P-133,其酸性、中性和碱性蛋白酶活力分别为1.00、52.50和48.50 U/mL,经鉴定为假单胞菌(Pseudomonas sp.);单因素及响应面试验结果表明,菌株P-133最佳产酶工艺条件为可溶性淀粉5.0 g/L、菜粕5.9 g/L、NaCl 3.3 g/L、K2HPO4 5.0 g/L,pH 7.8,温度28℃,接种量5%及装液量75 mL/250 mL,该条件下酸性、中性和碱性蛋白酶活力分别为4.14、185.19和177.30 U/mL,是优化前的4.14、3.53和3.66倍。 结论 从酸菜汁分离筛选得到同时具备产酸性、中性和碱性蛋白酶的假单胞菌P-133,发酵产酶工艺优化后产酸性、中性和碱性蛋白酶活性分别是优化前的4.14、3.53和3.66倍。
刘爽, 江洲, 赵帅, 赵雷真, 黄峰, 周佳, 屈建航. 一株产蛋白酶细菌的筛选、鉴定及发酵工艺优化[J]. 生物技术通报, 2025, 41(4): 335-344.
LIU Shuang, JIANG Zhou, ZHAO Shuai, ZHAO Lei-zhen, HUANG Feng, ZHOU Jia, QU Jian-hang. Screening, Identification, and Fermentation Optimization of a Protease-producing Bacterial Strain[J]. Biotechnology Bulletin, 2025, 41(4): 335-344.
水平 Level | 因素 Factor | ||||
|---|---|---|---|---|---|
| A pH | B菜粕浓度 Rapeseed meal concentration/(g·L-1) | C NaCl浓度 NaCl concentration/(g·L-1) | |||
| -1 | 5 | 1 | 0.5 | ||
| 0 | 7 | 5 | 1 | ||
| 1 | 9 | 10 | 5 | ||
表1 发酵条件优化响应面试验设计
Table 1 Response surface design for the optimization of fermentation conditions
水平 Level | 因素 Factor | ||||
|---|---|---|---|---|---|
| A pH | B菜粕浓度 Rapeseed meal concentration/(g·L-1) | C NaCl浓度 NaCl concentration/(g·L-1) | |||
| -1 | 5 | 1 | 0.5 | ||
| 0 | 7 | 5 | 1 | ||
| 1 | 9 | 10 | 5 | ||
编号 Number | 水解圈直径Diameter of hydrolysis ring (D)/mm | 菌落直径 Diameter of colony (d)/mm | 圈径比Ring diameter ratio (D/d) | 编号 Number | 水解圈直径 Diameter of hydrolysis ring (D)/mm | 菌落直径 Diameter of colony (d)/mm | 圈径比Ring diameter ratio (D/d) |
|---|---|---|---|---|---|---|---|
| P-6 | 20 | 11 | 1.8 | P-18 | 12 | 6 | 2.0 |
| P-7 | 11 | 6 | 1.8 | P-25 | 18 | 7 | 2.6 |
| P-8 | 13 | 6 | 2.7 | P-34 | 24 | 5 | 4.8 |
| P-9 | 12 | 5 | 2.4 | P-35 | 20 | 10 | 2.0 |
| P-12 | 14 | 6 | 2.3 | P-36 | 14 | 7 | 2.0 |
| P-13 | 20 | 5 | 4.0 | P-37 | 10 | 5 | 2.0 |
| P-14 | 12 | 6 | 2.0 | P-133 | 20 | 4 | 5.0 |
| P-15 | 10 | 6 | 1.7 |
表2 产蛋白酶菌株初筛结果
Table 2 Results of preliminary screening of proteinase-producing strains
编号 Number | 水解圈直径Diameter of hydrolysis ring (D)/mm | 菌落直径 Diameter of colony (d)/mm | 圈径比Ring diameter ratio (D/d) | 编号 Number | 水解圈直径 Diameter of hydrolysis ring (D)/mm | 菌落直径 Diameter of colony (d)/mm | 圈径比Ring diameter ratio (D/d) |
|---|---|---|---|---|---|---|---|
| P-6 | 20 | 11 | 1.8 | P-18 | 12 | 6 | 2.0 |
| P-7 | 11 | 6 | 1.8 | P-25 | 18 | 7 | 2.6 |
| P-8 | 13 | 6 | 2.7 | P-34 | 24 | 5 | 4.8 |
| P-9 | 12 | 5 | 2.4 | P-35 | 20 | 10 | 2.0 |
| P-12 | 14 | 6 | 2.3 | P-36 | 14 | 7 | 2.0 |
| P-13 | 20 | 5 | 4.0 | P-37 | 10 | 5 | 2.0 |
| P-14 | 12 | 6 | 2.0 | P-133 | 20 | 4 | 5.0 |
| P-15 | 10 | 6 | 1.7 |
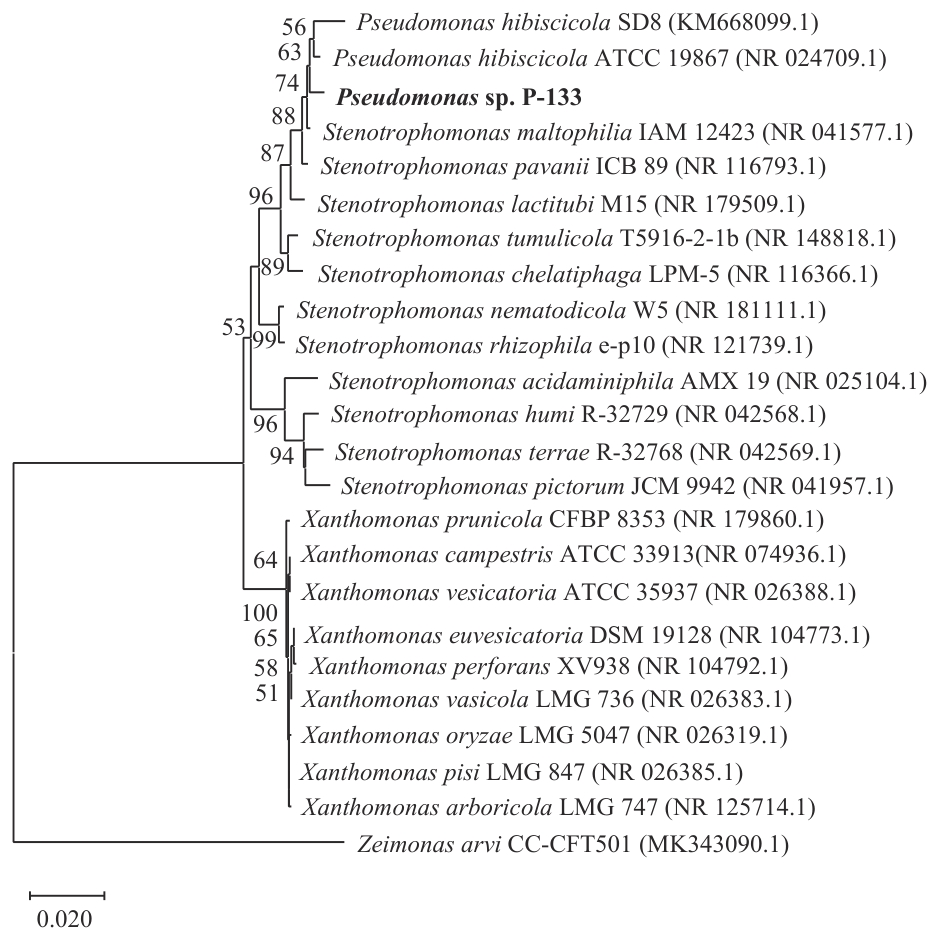
图3 菌株P-133基于16S rRNA基因序列的系统发育树括号内为菌株16S rRNA基因序列的GenBank 登录号;分支节点数字为 Bootstrap 值(低于50 不显示);标尺的数据为进化距离
Fig. 3 Phylogenetic tree of strain P-133 based on the 16S rRNA gene sequencesCode in parentheses is GenBank accession number of the strain. The numbers of branch nodes are bootstrap values (<50 not displayed). The data of the scale is the evolutionary distance

图5 菌株P-133的发酵培养基优化不同小写字母、大写字母、数字,分别代表酸性蛋白酶活性、中性蛋白酶活性和碱性蛋白酶活性的显著差异(P<0.05),下同
Fig. 5 Optimization of fermentation medium for strain P-133Different lowercase letters, uppercase letters and numbers, indicate significant differences in acid protease activity, neutral protease activity and alkaline protease activity (P<0.05). The same below
方差来源 Source of variance | 平方和 Sum of squares | 自由度 Freedom | 均方 Mean | F值 F value | P值 P value | 显著性 Significance |
|---|---|---|---|---|---|---|
| pH | 64 659.717 | 4 | 16 164.929 | 1 349.359 | 1.30×10-13 | ** |
| 菜粕浓度 | 21 586.241 | 6 | 3 597.707 | 203.065 | 8.62×10-13 | ** |
| NaCl浓度 | 20 247.864 | 6 | 3 374.644 | 126.434 | 2.24×10-11 | ** |
| 装液量 | 2 923.535 | 5 | 584.707 | 208.34 | 3.15×10-11 | ** |
| 温度 | 53 335.824 | 4 | 13 333.96 | 349.142 | 1.08×10-10 | ** |
| 接种量 | 1 205.128 | 4 | 301.282 | 64.771 | 4.12×10-7 | ** |
| 可溶性淀粉浓度 | 3 331.388 | 6 | 555.231 | 4.269 | 0.012 | * |
表3 IBM SPSS Statistics 方差分析
Table 3 Analysis of variance in IBM SPSS statistics
方差来源 Source of variance | 平方和 Sum of squares | 自由度 Freedom | 均方 Mean | F值 F value | P值 P value | 显著性 Significance |
|---|---|---|---|---|---|---|
| pH | 64 659.717 | 4 | 16 164.929 | 1 349.359 | 1.30×10-13 | ** |
| 菜粕浓度 | 21 586.241 | 6 | 3 597.707 | 203.065 | 8.62×10-13 | ** |
| NaCl浓度 | 20 247.864 | 6 | 3 374.644 | 126.434 | 2.24×10-11 | ** |
| 装液量 | 2 923.535 | 5 | 584.707 | 208.34 | 3.15×10-11 | ** |
| 温度 | 53 335.824 | 4 | 13 333.96 | 349.142 | 1.08×10-10 | ** |
| 接种量 | 1 205.128 | 4 | 301.282 | 64.771 | 4.12×10-7 | ** |
| 可溶性淀粉浓度 | 3 331.388 | 6 | 555.231 | 4.269 | 0.012 | * |
| Test number | A | B | C | Y/(U·mL-1) |
|---|---|---|---|---|
| 1 | 1 | -1 | 0 | 38.43 |
| 2 | -1 | 1 | 0 | 129.26 |
| 3 | 0 | 0 | 0 | 144.55 |
| 4 | 1 | 0 | 1 | 145.14 |
| 5 | 0 | 0 | 0 | 170.45 |
| 6 | 1 | 1 | 0 | 115.7 |
| 7 | 0 | 0 | 0 | 159.06 |
| 8 | -1 | 0 | -1 | 89.69 |
| 9 | 0 | 0 | 0 | 149.34 |
| 10 | -1 | 0 | 1 | 81.90 |
| 11 | -1 | -1 | 0 | 17.51 |
| 12 | 0 | 1 | 1 | 147.61 |
| 13 | 0 | 0 | 0 | 140.501 |
| 14 | 0 | 1 | -1 | 152.94 |
| 15 | 1 | 0 | -1 | 95.91 |
| 16 | 0 | -1 | -1 | 72.67 |
| 17 | 0 | -1 | 1 | 75.88 |
表4 Box-Behnken 试验设计及结果
Table 4 Box-Behnken design and test results
| Test number | A | B | C | Y/(U·mL-1) |
|---|---|---|---|---|
| 1 | 1 | -1 | 0 | 38.43 |
| 2 | -1 | 1 | 0 | 129.26 |
| 3 | 0 | 0 | 0 | 144.55 |
| 4 | 1 | 0 | 1 | 145.14 |
| 5 | 0 | 0 | 0 | 170.45 |
| 6 | 1 | 1 | 0 | 115.7 |
| 7 | 0 | 0 | 0 | 159.06 |
| 8 | -1 | 0 | -1 | 89.69 |
| 9 | 0 | 0 | 0 | 149.34 |
| 10 | -1 | 0 | 1 | 81.90 |
| 11 | -1 | -1 | 0 | 17.51 |
| 12 | 0 | 1 | 1 | 147.61 |
| 13 | 0 | 0 | 0 | 140.501 |
| 14 | 0 | 1 | -1 | 152.94 |
| 15 | 1 | 0 | -1 | 95.91 |
| 16 | 0 | -1 | -1 | 72.67 |
| 17 | 0 | -1 | 1 | 75.88 |
| 来源Source | 平方和 Sum of square | 自由度 Freedom | 均方 Mean | F值 F value | P值 P value | 显著性Significance |
|---|---|---|---|---|---|---|
| 模型 | 31 171.60 | 9 | 3 463.51 | 26.81 | 0.000 1 | ** |
| A | 1 462.82 | 1 | 1 462.82 | 11.32 | 0.012 0 | * |
| B | 10 789.03 | 1 | 10 789.03 | 83.50 | <0.000 1 | ** |
| C | 171.86 | 1 | 171.86 | 1.33 | 0.286 6 | |
| AB | 308.14 | 1 | 308.14 | 2.38 | 0.166 4 | |
| AC | 1 216.09 | 1 | 1 216.09 | 9.41 | 0.018 1 | * |
| BC | 122.90 | 1 | 122.90 | 0.951 2 | 0.361 9 | |
| A2 | 7 969.72 | 1 | 7 969.72 | 61.68 | 0.000 1 | ** |
| B2 | 6 347.18 | 1 | 6 347.18 | 49.12 | 0.000 2 | ** |
| C2 | 326.71 | 1 | 326.71 | 2.53 | 0.155 8 | |
| 残差 | 904.44 | 7 | 129.21 | |||
| 失拟项 | 322.52 | 3 | 107.51 | 0.739 0 | 0.581 5 | 不显著 |
| 纯误差 | 581.91 | 4 | 145.48 | |||
| 总和 | 32 076.04 | 16 |
表5 二次回归方程方差分析
Table 5 Analysis of variance via the quadratic regression equation
| 来源Source | 平方和 Sum of square | 自由度 Freedom | 均方 Mean | F值 F value | P值 P value | 显著性Significance |
|---|---|---|---|---|---|---|
| 模型 | 31 171.60 | 9 | 3 463.51 | 26.81 | 0.000 1 | ** |
| A | 1 462.82 | 1 | 1 462.82 | 11.32 | 0.012 0 | * |
| B | 10 789.03 | 1 | 10 789.03 | 83.50 | <0.000 1 | ** |
| C | 171.86 | 1 | 171.86 | 1.33 | 0.286 6 | |
| AB | 308.14 | 1 | 308.14 | 2.38 | 0.166 4 | |
| AC | 1 216.09 | 1 | 1 216.09 | 9.41 | 0.018 1 | * |
| BC | 122.90 | 1 | 122.90 | 0.951 2 | 0.361 9 | |
| A2 | 7 969.72 | 1 | 7 969.72 | 61.68 | 0.000 1 | ** |
| B2 | 6 347.18 | 1 | 6 347.18 | 49.12 | 0.000 2 | ** |
| C2 | 326.71 | 1 | 326.71 | 2.53 | 0.155 8 | |
| 残差 | 904.44 | 7 | 129.21 | |||
| 失拟项 | 322.52 | 3 | 107.51 | 0.739 0 | 0.581 5 | 不显著 |
| 纯误差 | 581.91 | 4 | 145.48 | |||
| 总和 | 32 076.04 | 16 |
| 1 | Research QY. 2024年全球蛋白酶行业总体规模、主要企业国内外市场占有率及排名 [M]. 北京: 恒州博智, 2024. |
| Research QY. The overall scale of the global protease industry, the domestic and foreign market share and ranking of major companies in 2024 [M]. Beijing: QYResearch, 2024. | |
| 2 | Song P, Zhang X, Wang SH, et al. Microbial proteases and their applications [J]. Front Microbiol, 2023, 14: 1236368. |
| 3 | Razzaq A, Shamsi S, Ali A, et al. Microbial proteases applications [J]. Front Bioeng Biotechnol, 2019, 7: 110. |
| 4 | 张雯姝. 产蛋白酶菌株的ARTP诱变及其降解蛋白质代谢通路分析 [D]. 大连: 辽宁师范大学, 2023. |
| Zhang WS. Mutation of protease-producing strain by ARTP and analysis of its metabolic pathway for degrading protein [D]. Dalian: Liaoning Normal University, 2023. | |
| 5 | Jisha VN, Smitha RB, Pradeep S, et al. Versatility of microbial proteases [J]. Adv Enzyme Res, 2013, 1(3): 39-51. |
| 6 | Banerjee S, Maiti TK, Roy RN. Enzyme producing insect gut microbes: an unexplored biotechnological aspect [J]. Crit Rev Biotechnol, 2022, 42(3): 384-402. |
| 7 | Matkawala F, Nighojkar S, Kumar A, et al. Microbial alkaline serine proteases: Production, properties and applications [J]. World J Microbiol Biotechnol, 2021, 37(4): 63. |
| 8 | Hasan MJ, Haque P, Rahman MM. Protease enzyme based cleaner leather processing: a review [J]. J Clean Prod, 2022, 365: 132826. |
| 9 | 赵华, 任青霞, 张敏, 等. 酒曲中高产蛋白酶菌株的筛选及其发酵培养基优化 [J]. 中国酿造, 2023, 42(5): 157-164. |
| Zhao H, Ren QX, Zhang M, et al. Screening and fermentation medium optimization of high-yield protease-producing strain from Jiuqu [J]. China Brew, 2023, 42(5): 157-164. | |
| 10 | 贾仲昕, 赵佳男, 季芳, 等. 高产中性蛋白酶芽孢杆菌的筛选鉴定及酶学性质研究 [J]. 黑龙江畜牧兽医, 2023(1): 106-112, 132. |
| Jia ZX, Zhao JN, Ji F, et al. Study on screening, identification and enzymatic properties of high neutral protease-producing Bacillus [J]. Heilongjiang Anim Sci Vet Med, 2023(1): 106-112, 132. | |
| 11 | 刘晓艳, 封健, 韩傲, 等. 高产中/碱性蛋白酶的枯草芽孢杆菌W1菌株的筛选及条件优化 [J]. 现代食品科技, 2020, 36(4): 157-163. |
| Liu XY, Feng J, Han A, et al. Isolation and optimization of Bacillus subtilis W1 strain with high neutral/alkaline protease activity [J]. Mod Food Sci Technol, 2020, 36(4): 157-163. | |
| 12 | Cui HX, Yang MY, Wang LP, et al. Identification of a new marine bacterial strain SD8 and optimization of its culture conditions for producing alkaline protease [J]. PLoS One, 2015, 10(12): e0146067. |
| 13 | 李娜, 附俊杰, 刘军, 等. 一株产中性蛋白酶菌株的筛选及其发酵产酶条件优化 [J]. 食品工业科技, 2023, 44(1): 189-199. |
| Li N, Fu JJ, Liu J, et al. Screening of A neutral protease-producing strain and optimization of fermentation conditions [J]. Sci Technol Food Ind, 2023, 44(1): 189-199. | |
| 14 | 朱祥杰, 王震, 苑志欣, 等. 海洋芽孢杆菌N11-8产蛋白酶的发酵条件优化 [J]. 渔业科学进展, 2018, 39(6): 155-163. |
| Zhu XJ, Wang Z, Yuan ZX, et al. Optimization of fermentation conditions of Bacillus sp. N11-8 on the production of protease PBN11-8 [J]. Prog Fish Sci, 2018, 39(6): 155-163. | |
| 15 | Reasoner DJ, Geldreich EE. A new medium for the enumeration and subculture of bacteria from potable water [J]. Appl Environ Microbiol, 1985, 49(1): 1-7. |
| 16 | 侯泽林. 从土壤中筛选碱性蛋白酶产生菌及产酶条件优化研究 [D]. 哈尔滨: 东北农业大学, 2021. |
| Hou ZL. Screening alkaline protease-producing bacteria from soil and optimization of enzyme-producing conditions [D]. Harbin: Northeast Agricultural University, 2021. | |
| 17 | 冯璨, 马香, 刘柱, 等. 海南近海水域产碱性蛋白酶菌株的分离筛选及发酵条件优化 [J]. 微生物学通报, 2022, 49(10): 4291-4304. |
| Feng C, Ma X, Liu Z, et al. Isolation and screening of alkaline protease-producing strains from Hainan offshore areas and optimization of the fermentation conditions [J]. Microbiol China, 2022, 49(10): 4291-4304. | |
| 18 | Qu JH, Fu YH, Li XD, et al. Brevundimonas lutea sp. nov., isolated from lake sediment [J]. Int J Syst Evol Microbiol, 2019, 69(5): 1417-1422. |
| 19 | Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms [J]. Mol Biol Evol, 2018, 35(6): 1547-1549. |
| 20 | Berkman SJ, Roscoe EM, Bourret JC. Comparing self-directed methods for training staff to create graphs using Graphpad Prism [J]. J Appl Behav Anal, 2019, 52(1): 188-204. |
| 21 | 陈茏. 产蛋白酶菌株的筛选及酶学性质的研究 [D]. 南昌: 南昌大学, 2020. |
| Chen L. Screening of protease-producing strains and study on enzymatic properties [D]. Nanchang: Nanchang University, 2020. | |
| 22 | Zhang Y, Hu JM, Zhang Q, et al. Enhancement of alkaline protease production in recombinant Bacillus licheniformis by response surface methodology [J]. Bioresour Bioprocess, 2023, 10(1): 27. |
| 23 | 强济宇. 碱性蛋白酶耐盐芽孢杆菌DS5的筛选、鉴定和性质研究 [D]. 太原: 山西大学, 2023. |
| Qiang JY. Screening, identification and characterization of Bacillus halotolerans DS5 with alkaline protease production [D]. Taiyuan: Shanxi University, 2023. | |
| 24 | 李雪, 蔡丹, 沈月, 等. 微生物来源蛋白酶的研究进展 [J]. 食品科技, 2019, 44(1): 32-36. |
| Li X, Cai D, Shen Y, et al. Research progress of microbial protease [J]. Food Sci Technol, 2019, 44(1): 32-36. | |
| 25 | 农业农村部办公厅. 关于印发《饲用豆粕减量替代三年行动方案》的通知: 农办牧 [2023] 9号 [EB/OL]. (2023-04-12)[2024-09-27]. . |
| General Office of the Ministry of Agriculture and Rural Affairs. Notice on the issuance of the "Three-year Action Plan for Reducing and Replacing Soybean Meal for Feed": Nongbanmu [2023]No.9 [EB/OL]. (2023-04-12)[2024-09-27]. . | |
| 26 | 付晓琪, 商振达, 谭占坤, 等. 发酵菜籽粕替代豆粕在动物生产中的应用研究进展 [J]. 中国畜牧杂志, 2022, 58(10): 125-129. |
| Fu XQ, Shang ZD, Tan ZK, et al. Research progress on application of replacing soybean meal with fermented rapeseed meal in animal production [J]. Chin J Anim Sci, 2022, 58(10): 125-129. | |
| 27 | Liya SM, Umesh M, Nag A, et al. Optimized production of keratinolytic proteases from Bacillus tropicus LS27 and its application as a sustainable alternative for dehairing, destaining and metal recovery [J]. Environ Res, 2023, 221: 115283. |
| 28 | 陈菲. 一株蛋白酶产生菌的鉴定、酶性质研究和表达载体构建 [D]. 郑州: 河南工业大学, 2018. |
| Chen F. Identification, characterization and expression vector construction of a protease-producing strain [D]. Zhengzhou: Henan University of Technology, 2018. | |
| 29 | 张宇洁, 王丽军, 李梦, 等. 一株产低温β-半乳糖苷酶微杆菌的筛选鉴定、产酶条件及其酶学特性 [J]. 微生物学通报, 2019, 46(3): 609-617. |
| Zhang YJ, Wang LJ, Li M, et al. Isolation and identification of a Microbacterium sp. LW106 strain producing cold-active β-galactosidase, and study on its enzyme production conditions and enzymatic properties [J]. Microbiol China, 2019, 46(3): 609-617. | |
| 30 | 梁安健, 石沁兰, 王金丽, 等. 产蛋白酶波茨坦短芽孢杆菌的鉴定及产酶条件优化 [J]. 现代食品科技, 2024, 40(5): 73-83. |
| Liang AJ, Shi QL, Wang JL, et al. Identification of a protease producing Brevibacillus borstelensis strain and optimization of enzyme production conditions [J]. Mod Food Sci Technol, 2024, 40(5): 73-83. |
| [1] | 李文兰, 侯辛未, 李燕, 赵瑞君, 孟昭东, 岳润清. 转基因抗虫耐除草剂玉米LD05纯杂合植株的鉴定及抗性检测[J]. 生物技术通报, 2025, 41(4): 123-133. |
| [2] | 孙天国, 衣兰, 秦旭洋, 乔梦雪, 谷新颖, 韩艺, 沙伟, 张梅娟, 马天意. 大白菜DABB基因家族的全基因组鉴定及盐碱胁迫下的表达分析[J]. 生物技术通报, 2025, 41(4): 156-165. |
| [3] | 王田田, 常雪瑞, 黄婉洋, 黄嘉欣, 苗如意, 梁燕平, 王静. 辣椒GASA基因家族的鉴定及分析[J]. 生物技术通报, 2025, 41(4): 166-175. |
| [4] | 刘丽, 王辉, 关天舒, 李柏宏, 于舒怡. 葡萄脱落酸受体VvPYL4互作蛋白的筛选及互作蛋白基因表达[J]. 生物技术通报, 2025, 41(4): 188-197. |
| [5] | 宋佳怡, 苏芸丽, 郑兴艳, 夏文念, 杨冬梅, 胡慧贞. 金鱼草Expansin基因家族鉴定及其抗核盘菌相关基因筛选[J]. 生物技术通报, 2025, 41(4): 227-242. |
| [6] | 黄金恒, 黄茜, 张家燕, 周新裕, 廖沛然, 杨全. 广金钱草C3H基因家族鉴定及不同品种表达分析[J]. 生物技术通报, 2025, 41(4): 243-255. |
| [7] | 宋姝熠, 蒋开秀, 刘欢艳, 黄亚成, 刘林娅. ‘红阳’猕猴桃TCP基因家族鉴定及其在果实中的表达分析[J]. 生物技术通报, 2025, 41(3): 190-201. |
| [8] | 陈海敏, 孙菲, 袁源, 吴佳雯, 江红, 周剑. 紫外诱变选育巴弗洛霉素A1高产菌株及其培养基优化[J]. 生物技术通报, 2025, 41(3): 51-61. |
| [9] | 马天意, 许家佳, 路文婧, 吴艳, 沙伟, 张梅娟, 彭疑芳. ‘金小童’大白菜BrcGASA3基因在盐碱胁迫下的表达分析及抗性鉴定[J]. 生物技术通报, 2025, 41(2): 127-138. |
| [10] | 项波卡, 周钻钻, 冯佳卉, 夏琛, 李奇, 陈春. 一株烟叶霉变真菌的分离鉴定及其致霉因素研究[J]. 生物技术通报, 2025, 41(2): 321-330. |
| [11] | 何财林, 卢晶, 郭会会, 李小安, 吴琪. 藜麦MADS-box基因家族的全基因组鉴定和表达分析[J]. 生物技术通报, 2025, 41(1): 157-172. |
| [12] | 刘倩, 马连杰, 张慧, 王冬, 范茂, 廖敦秀, 赵正武, 卢文才. 辣椒炭疽病生防菌株TN2的筛选鉴定与抑菌效果[J]. 生物技术通报, 2025, 41(1): 287-297. |
| [13] | 谭景轩, 邢德勋, 何天锦, 刘占英. 荧光假单胞菌蛋白表达系统研究进展[J]. 生物技术通报, 2025, 41(1): 49-61. |
| [14] | 张亚亚, 李盼盼, 高惠惠, 贾晨波, 徐春燕. 基于根表真菌群落与病原菌鉴定探究‘宁杞5号’枸杞根腐病的发生机制[J]. 生物技术通报, 2024, 40(9): 238-248. |
| [15] | 袁兰, 黄娅楠, 张贝妮, 熊雨萌, 王洪洋. 基于流式细胞仪鉴定马铃薯倍性的高通量样品制备方法[J]. 生物技术通报, 2024, 40(9): 141-147. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||