








生物技术通报 ›› 2026, Vol. 42 ›› Issue (1): 161-169.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0747
收稿日期:2025-07-11
出版日期:2026-01-26
发布日期:2026-02-04
通讯作者:
金雪花,女,教授,研究方向 :园林植物;E-mail: xhkim2021@163.com作者简介:吕呈聪,男,硕士研究生,研究方向 :园林植物;E-mail: lvccong@126.com
基金资助:
LYU Cheng-cong( ), HENG Meng, CHEN Si-qi, JIN Xue-hua(
), HENG Meng, CHEN Si-qi, JIN Xue-hua( )
)
Received:2025-07-11
Published:2026-01-26
Online:2026-02-04
摘要:
目的 GSTF转运蛋白参与植物花青素苷的转运过程,研究其对彩色马蹄莲(Zantedeschia hybrida)佛焰苞着色和花青素苷积累的作用及其分子机制,为彩色马蹄莲花青素苷转运机制解析提供理论依据,为新品种选育提供有效基因资源。 方法 基于课题组前期对彩色马蹄莲转录组数据的分析,筛选并克隆彩色马蹄莲佛焰苞花青素苷转运相关基因ZhGSTF,分析其进化关系和序列特征,并测定在不同品种佛焰苞中的基因表达量和花青素苷含量。同时利用病毒诱导的基因沉默(virus induced gene silencing, VIGS)技术构建ZhGSTF沉默体系,比较沉默后佛焰苞表型、基因表达和3个类黄酮合成途径下游结构基因表达量的变化。 结果 ZhGSTF的CDS长度为666 bp,编码221个氨基酸。系统进化树分析表明,ZhGSTF与其他物种花青素苷转运相关的GST聚为一类;氨基酸序列比对显示,ZhGSTF与紫苏(Perilla frutescens)PfGST1相似性最高。ZhGSTF在玫红色‘佳人’和橙色‘奥迪安’中表达量均显著高于黄色‘金城’,在白色‘范图拉’中显著低表达,ZhGSTF在4个品种中的表达量与其花青素苷积累趋势一致。经VIGS沉默后,ZhGSTF表达量显著下调,佛焰苞表型发生明显褪色,而ZhGSTF的沉默对ZhANS、ZhANR和ZhDFR表达量无显著影响。 结论 彩色马蹄莲ZhGSTF在佛焰苞着色过程中促进花青素苷积累并影响其呈色。
吕呈聪, 衡蒙, 陈思琪, 金雪花. 彩色马蹄莲花青素苷转运相关ZhGSTF的克隆及功能分析[J]. 生物技术通报, 2026, 42(1): 161-169.
LYU Cheng-cong, HENG Meng, CHEN Si-qi, JIN Xue-hua. Cloning and Functional Analysis of ZhGSTF Related to Anthocyanin Transport Zantedeschia hybrida[J]. Biotechnology Bulletin, 2026, 42(1): 161-169.

图1 四个品种彩色马蹄莲佛焰苞(A)和玫红色品种佛焰苞的4个着色阶段(B)S1:佛焰苞未着色;S2:佛焰苞微着色;S3:佛焰苞部分着色;S4:佛焰苞完全着色。下同
Fig. 1 Four varieties of Z. hybrida spatula (A) and four coloring stages of rose spatula (B)S1: The spatula is uncolored; S2: micro-coloring the spatula; S3: partially coloring the spathe; S4: completely coloring the spatula. The same below
引物 Primer | 引物序列 Primer sequence (5′‒3′) | 引物用途 Primer usage |
|---|---|---|
| ZhGSTF-F | TATGCCGTTACGTGTGCGAC | 基因克隆 |
| ZhGSTF-R | GGTTGTAGGACTGCCCCTCT | Gene clone |
| ZhGSTF-F-q | AGATCCGTATCAATCCCT | 基因表达 Gene expression |
| ZhGSTF-R-q | TAAGAGTATGAAAGTGCC | |
| ZhANS-F | GATCCCGTGGTTGACGATGT | |
| ZhANS-R | ATCGTGGACTTCACGTCTGG | |
| ZhANR-F | TGGAAGACATAGTCGCAGCC | |
| ZhANR-R | GACGAAGCTCGCTCACCTTA | |
| ZhDFR-F | CGACGAGATGAAAGGACCCA | |
| ZhDFR-R | TGTATCGAAGACGTTGGCGG | |
| ZhActin-F | GAAGGAGAAACTTGCGTATGTAGC | 内参基因 |
| ZhActin-R | GTCCATCTGGCAGCTCATAACT | Reference gene |
| ZhGSTF-Sac I-F | CTCTAGAAGGCCTCCATGGGGATCCGCAGAGGGGCAGTCCTACAA | 载体构建 |
| ZhGSTF-BamH I-R | GCCTCGAGACGCGTGAGCTCGGAGCCTTCTGCTTCTGCA | Vectors construction |
表1 实验所用引物
Table 1 Primers used in the study
引物 Primer | 引物序列 Primer sequence (5′‒3′) | 引物用途 Primer usage |
|---|---|---|
| ZhGSTF-F | TATGCCGTTACGTGTGCGAC | 基因克隆 |
| ZhGSTF-R | GGTTGTAGGACTGCCCCTCT | Gene clone |
| ZhGSTF-F-q | AGATCCGTATCAATCCCT | 基因表达 Gene expression |
| ZhGSTF-R-q | TAAGAGTATGAAAGTGCC | |
| ZhANS-F | GATCCCGTGGTTGACGATGT | |
| ZhANS-R | ATCGTGGACTTCACGTCTGG | |
| ZhANR-F | TGGAAGACATAGTCGCAGCC | |
| ZhANR-R | GACGAAGCTCGCTCACCTTA | |
| ZhDFR-F | CGACGAGATGAAAGGACCCA | |
| ZhDFR-R | TGTATCGAAGACGTTGGCGG | |
| ZhActin-F | GAAGGAGAAACTTGCGTATGTAGC | 内参基因 |
| ZhActin-R | GTCCATCTGGCAGCTCATAACT | Reference gene |
| ZhGSTF-Sac I-F | CTCTAGAAGGCCTCCATGGGGATCCGCAGAGGGGCAGTCCTACAA | 载体构建 |
| ZhGSTF-BamH I-R | GCCTCGAGACGCGTGAGCTCGGAGCCTTCTGCTTCTGCA | Vectors construction |
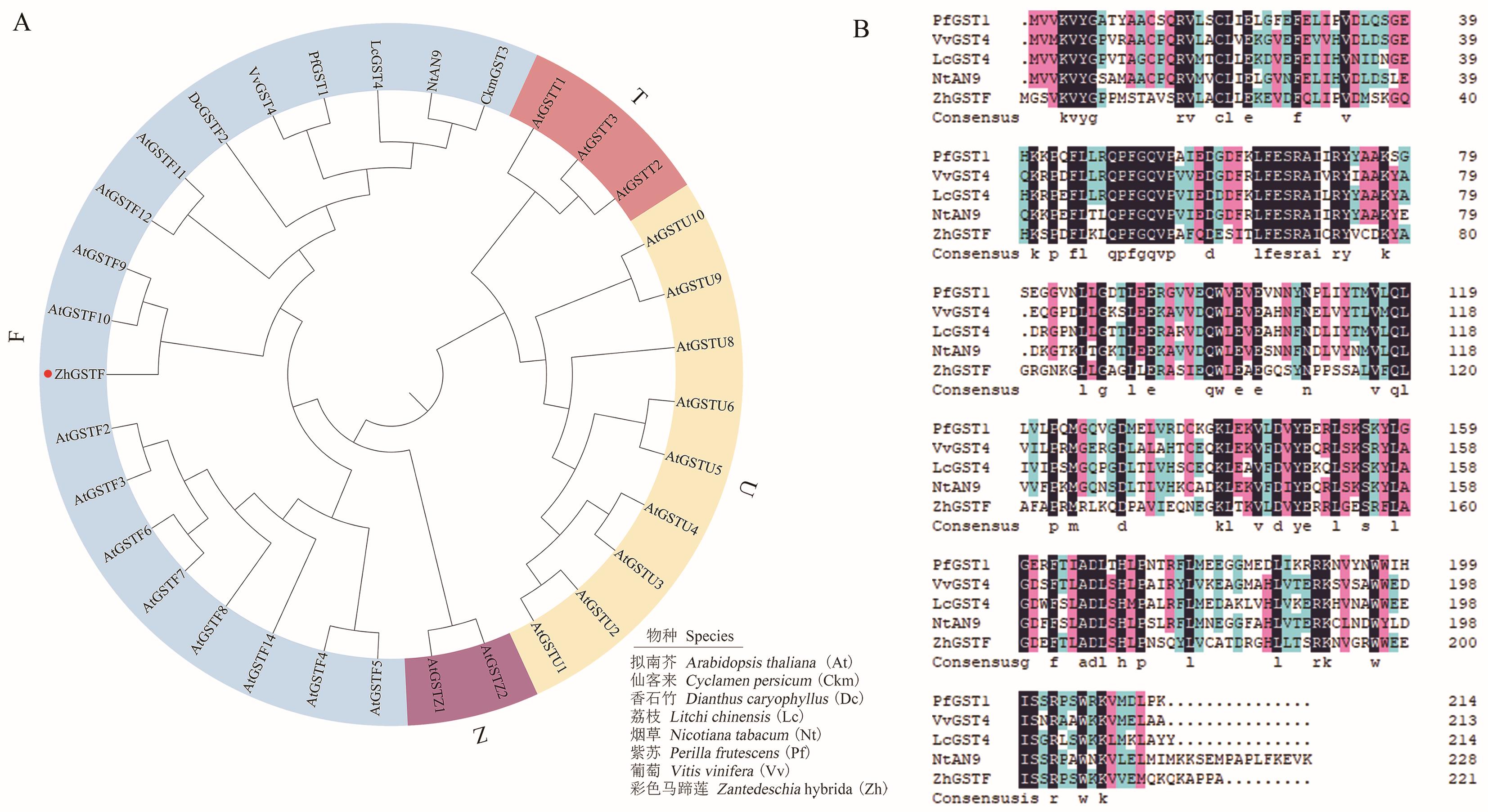
图3 彩色马蹄莲ZhGSTF与其他物种GST系统进化树(A)及氨基酸序列比对(B)
Fig. 3 Phylogenetic tree (A) and amino acid sequence alignment of Z. hybrida ZhGSTF with GST of other species (B)
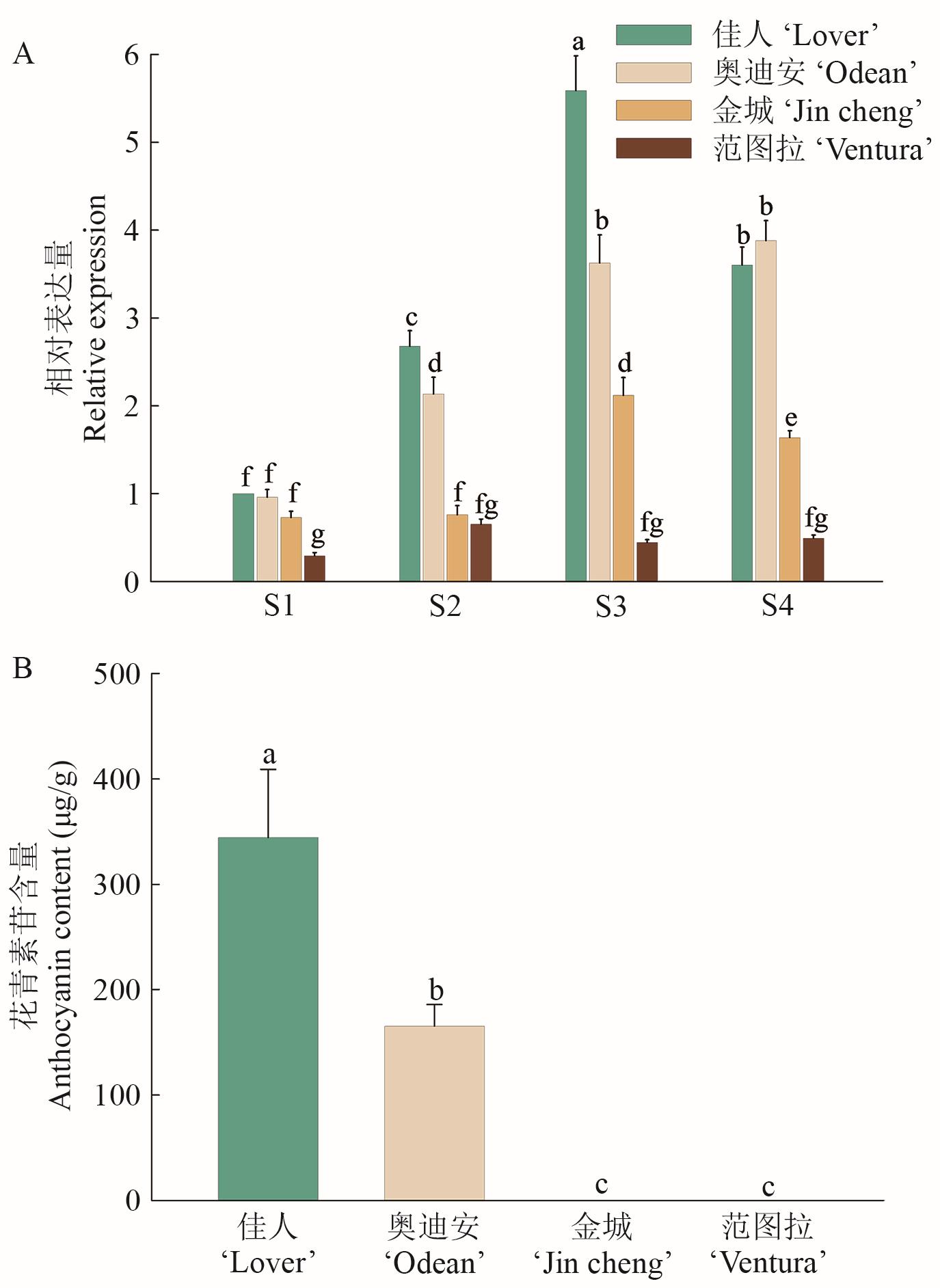
图4 ZhGSTF在4个彩色马蹄莲品种中的表达特性分析A:ZhGSTF在4个彩色马蹄莲品种的4个着色阶段的表达水平;B:4个不同品种彩色马蹄莲在S4阶段的佛焰苞花青素苷含量;不同小写字母代表不同处理间差异显著(P<0.05),下同
Fig. 4 Expressions of ZhGSTF in four coloring stages of four Z. hybrida varietiesA: The expressions of ZhGSTF in four coloring stages of four colored Z. hybrida varieties. B: Anthocyanin content in spatula of four different varieties of Z. hybrida at S4 stage. Different lowercase letters indicate significant differences among different treatments (P<0.05), the same below
图5 TRV2-ZhGSTF菌落PCR检测M:DL 2000 marker;1‒12:ZhGSTF菌落PCR扩增产物
Fig. 5 PCR detection of TRV2-ZhGSTF colonyM: DL 2000 marker; 1‒12: PCR-amplified product of ZhGSTF colony
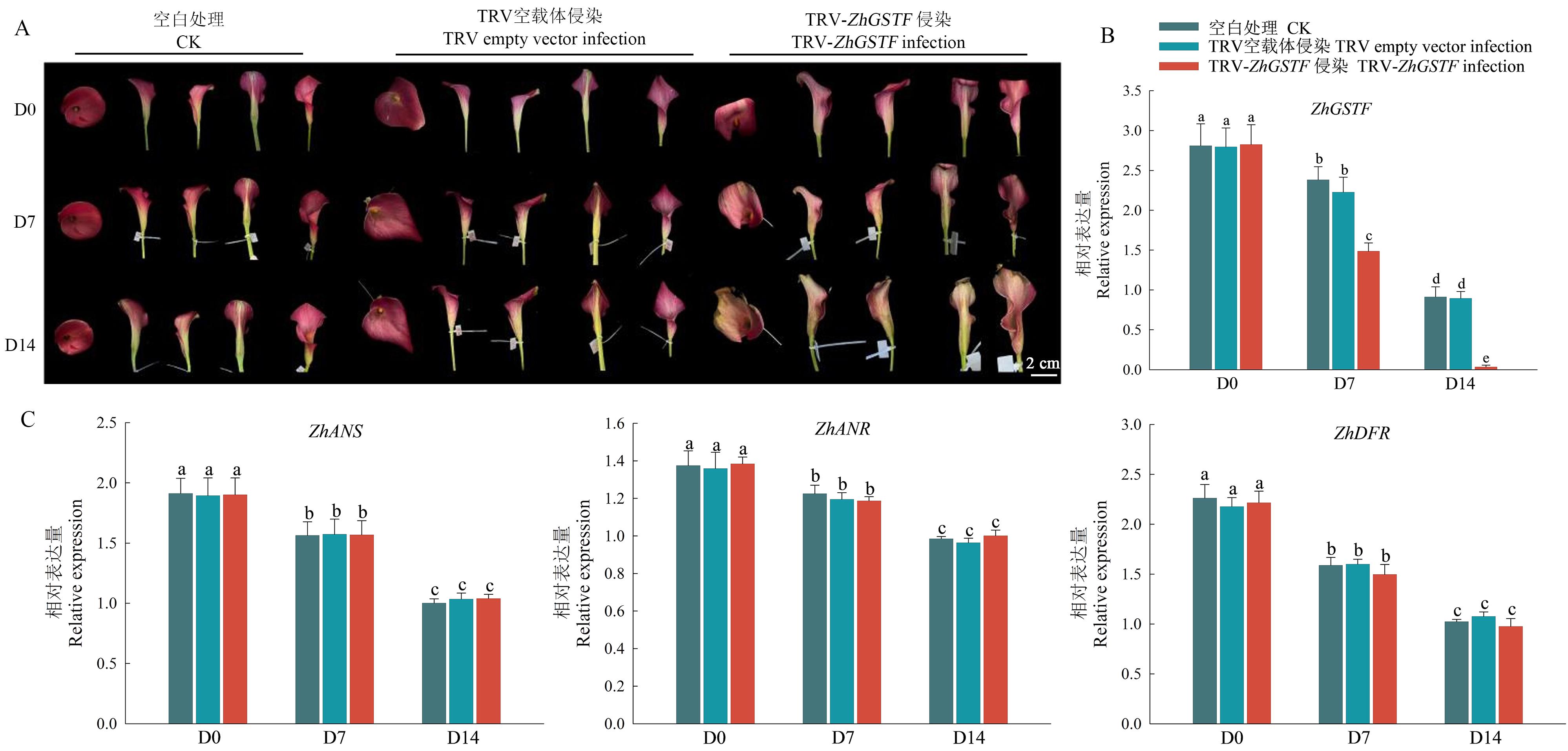
图6 ZhGSTF沉默后佛焰苞的表型及相关基因表达分析A:经VIGS沉默ZhGSTF后佛焰苞的表型;B:经VIGS沉默后ZhGSTF在佛焰苞中的相对表达水平;C:类黄酮合成途径下游3个关键基因在ZhGSTF沉默后佛焰苞中的表达水平;D0:处理第0天;D7:处理第7天;D14:处理第14天
Fig. 6 Phenotype and related gene expression analysis of spathe after ZhGSTF silencingA: Phenotype of spathe after VIGS silencing ZhGSTF. B: Relative expression of ZhGSTF in spathe after VIGS silencing. C: Expressions of three key genes downstream of flavonoid synthesis pathway in spathe after ZhGSTF silencing. D0: 0 d of treatment; D7: 7th day of treatment; D4: 14th day of treatment
| [1] | Wang Y, Yang T, Wang D, et al. Chromosome level genome assembly of colored calla lily (Zantedeschia elliottiana) [J]. Sci Data, 2023, 10(1): 605. |
| [2] | 吴穷, 孙灿, 汤桂钧, 等. 上海地区彩色马蹄莲组织培养快繁及鲜切花周年生产技术 [J]. 上海农业科技, 2022(6): 91-93, 117. |
| Wu Q, Sun C, Tang GJ, et al. Tissue culture and rapid propagation of colored Calla lily and annual production techniques of fresh cut flowers in Shanghai [J]. Shanghai Agric Sci Technol, 2022(6): 91-93, 117. | |
| [3] | 李锐, 胡婷, 陈树溦, 等. 紫苏PfMYB80转录因子正向调控花青素的生物合成 [J]. 生物技术通报, 2025, 41(6): 243-255. |
| Li R, Hu T, Chen SW, et al. Positive regulation of anthocyanin biosynthesis by PfMYB80 transcription factor in Perilla frutescens [J]. Biotechnol Bull, 2025, 41(6): 243-255. | |
| [4] | Smeriglio A, Barreca D, Bellocco E, et al. Chemistry, pharmacology and health benefits of anthocyanins [J]. Phytother Res, 2016, 30(8): 1265-1286. |
| [5] | Cui YM, Fan JW, Lu CF, et al. ScGST3 and multiple R2R3-MYB transcription factors function in anthocyanin accumulation in Senecio cruentus [J]. Plant Sci, 2021, 313: 111094. |
| [6] | Lei T, Song Y, Jin XH, et al. Effects of pigment constituents and their distribution on spathe coloration of Zantedeschia hybrida [J]. HortScience, 2017, 52(12): 1840-1848. |
| [7] | Fang Y, Lei T, Wu YM, et al. Mechanism underlying color variation in calla lily spathes based on transcriptomic analysis [J]. J Amer Soc Hort Sci, 2021, 146(6): 387-398. |
| [8] | 赵婧, 郭茜, 李睿琦, 等. 大豆GmGST基因簇基因序列分析及诱导表达分析 [J]. 生物技术通报, 2025, 41(5): 129-140. |
| Zhao J, Guo Q, Li RQ, et al. Sequence analysis and induced expression analysis of GmGST gene cluster genes in soybean [J]. Biotechnol Bull, 2025, 41(5): 129-140. | |
| [9] | Manzoor MA, Ali Sabir I, Shah IH, et al. Flavonoids: a review on biosynthesis and transportation mechanism in plants [J]. Funct Integr Genomics, 2023, 23(3): 212. |
| [40] | Yang XM, Wang JJ, Wang DL, et al. Functional verification and bioinformatics analysis of cotton GhDMT3 gene [J]. Biotechnol Bull, 2019, 35(1): 11-16. |
| [41] | Tian LL, Liu LQ, Jiang YQ, et al. Tobacco rattle virus-induced VcANS gene silencing in blueberry fruit [J]. Gene, 2023, 852: 147054. |
| [42] | Zhang KJ, Wang XB, Chen XX, et al. Establishment of a homologous silencing system with intact-plant infiltration and minimized operation for studying gene function in herbaceous peonies [J]. Int J Mol Sci, 2024, 25(8): 4412. |
| [43] | 杨君, 孔羽, 刘群录, 等. 遮荫对‘花手鞠’绣球花色和花青素苷组成的影响 [J]. 园艺学报, 2023, 50(7): 1467-1481. |
| Yang J, Kong Y, Liu QL, et al. Effects of shading on the variation of sepal color and pigment composition in Hydrangea macrophylla ‘Hanatemari’ [J]. Acta Hortic Sin, 2023, 50(7): 1467-1481. | |
| [44] | 郑先哲, 赵兴隆, 刘成海, 等. 基于果实颜色特征的蓝靛果忍冬花青素含量预测 [J]. 农业工程学报, 2023, 39(2): 242-251. |
| Zheng XZ, Zhao XL, Liu CH, et al. Prediction of the anthocyanin content of Lonicera edulis based on fruit color characteristics [J]. Trans Chin Soc Agric Eng, 2023, 39(2): 242-251. | |
| [45] | 欧阳莎莎, 曹受金, 熊颖, 等. 不同樱花花瓣色值和花色素测定及抗氧化能力分析 [J]. 中南林业科技大学学报, 2024, 44(2): 166-173, 183. |
| Ouyang SS, Cao SJ, Xiong Y, et al. Measurement of color values and active substances of different flowering cherry petals and analysis of antioxidant capacity [J]. J Cent South Univ For Technol, 2024, 44(2): 166-173, 183. | |
| [46] | Han LL, Zhou L, Zou HZ, et al. PsGSTF3, an anthocyanin-related glutathione S-transferase gene, is essential for petal coloration in tree peony [J]. Int J Mol Sci, 2022, 23(3): 1423. |
| [47] | Huang CB, Zhao T, Li JH, et al. Glutathione transferase VvGSTU60 is essential for proanthocyanidin accumulation and cooperates synergistically with MATE in grapes [J]. Plant J, 2025, 121(2): e17197. |
| [10] | Hasan MS, Singh V, Islam S, et al. Genome-wide identification and expression profiling of glutathione S-transferase family under multiple abiotic and biotic stresses in Medicago truncatula L [J]. PLoS One, 2021, 16(2): e0247170. |
| [11] | Dixon DP, Lapthorn A, Edwards R. Plant glutathione transferases [J]. Genome Biol, 2002, 3(3): 3004.1. |
| [12] | Kitamura S, Shikazono N, Tanaka A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis [J]. Plant J, 2004, 37(1): 104-114. |
| [13] | Tasaki K, Yoshida M, Nakajima M, et al. Molecular characterization of an anthocyanin-related glutathione S-transferase gene in Japanese gentian with the CRISPR/Cas9 system [J]. BMC Plant Biol, 2020, 20(1): 370. |
| [14] | Kitamura S, Akita Y, Ishizaka H, et al. Molecular characterization of an anthocyanin-related glutathione S-transferase gene in Cyclamen [J]. J Plant Physiol, 2012, 169(6): 636-642. |
| [15] | Hu B, Zhao JT, Lai B, et al. LcGST4 is an anthocyanin-related glutathione S-transferase gene in Litchi chinensis Sonn [J]. Plant Cell Rep, 2016, 35(4): 831-843. |
| [16] | Pérez-Díaz R, Madrid-Espinoza J, Salinas-Cornejo J, et al. Differential roles for VviGST1, VviGST3, and VviGST4 in proanthocyanidin and anthocyanin transport in Vitis vinífera . [J]. Front Plant Sci, 2016, 7: 1166. |
| [17] | Luo HF, Dai C, Li YP, et al. Reduced anthocyanins in petioles codes for a GST anthocyanin transporter that is essential for the foliage and fruit coloration in strawberry [J]. J Exp Bot, 2018, 69(10): 2595-2608. |
| [18] | Jiang SH, Chen M, He NB, et al. MdGSTF6, activated by MdMYB1, plays an essential role in anthocyanin accumulation in apple [J]. Hortic Res, 2019, 6: 40. |
| [19] | Zhao YW, Wang CK, Huang XY, et al. Genome-wide analysis of the glutathione S-transferase (GST) genes and functional identification of MdGSTU12 reveals the involvement in the regulation of anthocyanin accumulation in apple [J]. Genes, 2021, 12(11): 1733. |
| [20] | Zhao Y, Dong WQ, Zhu YC, et al. PpGST1, an anthocyanin-related glutathione S-transferase gene, is essential for fruit coloration in peach [J]. Plant Biotechnol J, 2020, 18(5): 1284-1295. |
| [21] | Lu ZH, Cao HH, Pan L, et al. Two loss-of-function alleles of the glutathione S-transferase (GST) gene cause anthocyanin deficiency in flower and fruit skin of peach (Prunus persica) [J]. Plant J, 2021, 107(5): 1320-1331. |
| [22] | Zhang T, Wu H, Sun YJ, et al. Identification of the GST gene family and functional analysis of RcGSTF2 related to anthocyanin in Rosa chinensis ‘old blush’ [J]. Plants, 2025, 14(6): 932. |
| [23] | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 -ΔΔCT method [J]. Methods, 2001, 25(4): 402-408. |
| [24] | 孙卫, 李崇晖, 王亮生, 等. 花青苷成分对瓜叶菊花色的影响 [J]. 园艺学报, 2009, 36(12): 1775-1782. |
| Sun W, Li CH, Wang LS, et al. Anthocyanins present in flowers of Senecio cruentus with different colors [J]. Acta Hortic Sin, 2009, 36(12): 1775-1782. | |
| [25] | 胡可, 韩科厅, 戴思兰. 环境因子调控植物花青素苷合成及呈色的机理 [J]. 植物学报, 2010, 45(3): 307-317. |
| Hu K, Han KT, Dai SL. Regulation of plant anthocyanin synthesis and pigmentation by environmental factors [J]. Chin Bull Bot, 2010, 45(3): 307-317. | |
| [26] | Saito K, Yonekura-Sakakibara K, Nakabayashi R, et al. The flavonoid biosynthetic pathway in Arabidopsis: structural and genetic diversity [J]. Plant Physiol Biochem, 2013, 72: 21-34. |
| [27] | Gomez C, Conejero G, Torregrosa L, et al. In vivo grapevine anthocyanin transport involves vesicle-mediated trafficking and the contribution of anthoMATE transporters and GST [J]. Plant J, 2011, 67(6): 960-970. |
| [28] | 尹雨钦, 徐欢欢, 唐丽萍, 等. 不结球白菜GST基因家族的全基因组鉴定及花青素相关基因BcGSTF6的功能分析 [J]. 中国农业科学, 2024, 57(16): 3234-3249. |
| Yin YQ, Xu HH, Tang LP, et al. Genome-wide identification of GST gene family and functional analysis of the BcGSTF6 gene related to anthocyanin in pak choi [J]. Sci Agric Sin, 2024, 57(16): 3234-3249. | |
| [29] | Marrs KA, Alfenito MR, Lloyd AM, et al. A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2 [J]. Nature, 1995, 375(6530): 397-400. |
| [30] | Yamazaki M, Shibata M, Nishiyama Y, et al. Differential gene expression profiles of red and green forms of Perilla frutescens leading to comprehensive identification of anthocyanin biosynthetic genes [J]. FEBS J, 2008, 275(13): 3494-3502. |
| [31] | Matsui K, Tomatsu T, Kinouchi S, et al. Identification of a gene encoding glutathione S-transferase that is related to anthocyanin accumulation in buckwheat (Fagopyrum esculentum) [J]. J Plant Physiol, 2018, 231: 291-296. |
| [32] | Lin YX, Zhang LX, Zhang JH, et al. Identification of anthocyanins-related glutathione S-transferase (GST) genes in the genome of cultivated strawberry (Fragaria × Ananassa) [J]. Int J Mol Sci, 2020, 21(22): 8708. |
| [33] | Xue L, Huang XR, Zhang ZH, et al. An anthocyanin-related glutathione S-transferase, MrGST1, plays an essential role in fruit coloration in Chinese bayberry (Morella rubra) [J]. Front Plant Sci, 2022, 13: 903333. |
| [34] | Qiu LK, Chen K, Pan J, et al. Genome-wide analysis of glutathione S-transferase genes in four Prunus species and the function of PmGSTF2, activated by PmMYBa1, in regulating anthocyanin accumulation in Prunus mume [J]. Int J Biol Macromol, 2024, 281: 136506. |
| [35] | Chen HX, Lei PH, Zhang HJ, et al. HmGST9, an anthocyanin-related glutathione S-transferase gene, is essential for sepals coloration in Hydrangea macrophylla [J]. J Plant Physiol, 2025, 307: 154466. |
| [36] | Zhang Z, Tian CP, Zhang Y, et al. Transcriptomic and metabolomic analysis provides insights into anthocyanin and procyanidin accumulation in pear [J]. BMC Plant Biol, 2020, 20(1): 129. |
| [37] | Grotewold E. The genetics and biochemistry of floral pigments [J]. Annu Rev Plant Biol, 2006, 57: 761-780. |
| [38] | Liu YH, Li YM, Liu Z, et al. Integrated transcriptomic and metabolomic analysis revealed altitude-related regulatory mechanisms on flavonoid accumulation in potato tubers [J]. Food Res Int, 2023, 170: 112997. |
| [39] | Rössner C, Lotz D, Becker A. VIGS goes viral: how VIGS transforms our understanding of plant science [J]. Annu Rev Plant Biol, 2022, 73: 703-728. |
| [40] | 杨笑敏, 王俊娟, 王德龙, 等. 棉花GhDMT3的功能验证及生物信息学分析 [J]. 生物技术通报, 2019, 35(1): 11-16. |
| [1] | 杨娟, 冯慧, 吉乃喆, 孙丽萍, 王赟, 张佳楠, 赵世伟. 月季AP2/ERF转录因子RcERF4和RcRAP2-12的克隆及功能分析[J]. 生物技术通报, 2026, 42(1): 150-160. |
| [2] | 吴翠翠, 陈登科, 兰刚, 夏芝, 李朋波. 花生转录因子AhHDZ70的生物信息学分析及耐盐耐旱性研究[J]. 生物技术通报, 2026, 42(1): 198-207. |
| [3] | 程婷婷, 刘俊, 王利丽, 练从龙, 魏文君, 郭辉, 吴尧琳, 杨晶凡, 兰金旭, 陈随清. 杜仲查尔酮异构酶基因家族全基因组鉴定及其表达模式分析[J]. 生物技术通报, 2025, 41(9): 242-255. |
| [4] | 徐小萍, 杨成龙, 和兴, 郭文杰, 吴健, 方少忠. 百合LoAPS1克隆及其在休眠解除过程的功能分析[J]. 生物技术通报, 2025, 41(9): 195-206. |
| [5] | 董向向, 缪百灵, 许贺娟, 陈娟娟, 李亮杰, 龚守富, 朱庆松. 森林草莓FveBBX20基因的生物信息学分析及开花调控功能[J]. 生物技术通报, 2025, 41(9): 115-123. |
| [6] | 李珊, 马登辉, 马红义, 姚文孔, 尹晓. 葡萄SKP1基因家族鉴定与表达分析[J]. 生物技术通报, 2025, 41(9): 147-158. |
| [7] | 巩慧玲, 邢玉洁, 马俊贤, 蔡霞, 冯再平. 马铃薯LAC基因家族的鉴定及盐胁迫下表达分析[J]. 生物技术通报, 2025, 41(9): 82-93. |
| [8] | 腊贵晓, 赵玉龙, 代丹丹, 余永亮, 郭红霞, 史贵霞, 贾慧, 杨铁钢. 红花质膜H+-ATPase基因家族成员鉴定及响应低氮低磷胁迫的表达分析[J]. 生物技术通报, 2025, 41(8): 220-233. |
| [9] | 豆飞飞, 任毓昭, 王石磊, 刘春颖, 王晓东, 王昭懿, 刘彩霞, 刘凤楼, 王掌军, 李清峰. 宁春4号小麦EMS突变体库的构建及表型变异分析[J]. 生物技术通报, 2025, 41(8): 92-101. |
| [10] | 赖诗雨, 梁巧兰, 魏列新, 牛二波, 陈应娥, 周鑫, 杨思正, 王博. NbJAZ3在苜蓿花叶病毒侵染本氏烟过程中的作用[J]. 生物技术通报, 2025, 41(8): 186-196. |
| [11] | 李亚, 蒋林, 徐闯, 王苏慧, 马曌, 王亮. 莱茵衣藻响应重金属胁迫的分子防御机制研究进展[J]. 生物技术通报, 2025, 41(8): 53-64. |
| [12] | 郭秀娟, 冯宇, 吴瑞香, 王利琴, 杨建春. Ca2+处理对胡麻种子萌发影响的转录组分析[J]. 生物技术通报, 2025, 41(7): 139-149. |
| [13] | 王芳, 乔帅, 宋伟, 崔鹏娟, 廖安忠, 谭文芳, 杨松涛. 甘薯IbNRT2基因家族全基因组鉴定和表达分析[J]. 生物技术通报, 2025, 41(7): 193-204. |
| [14] | 张学琼, 潘素君, 李魏, 戴良英. 植物磷酸盐转运蛋白在胁迫响应中的研究进展[J]. 生物技术通报, 2025, 41(7): 28-36. |
| [15] | 张勇, 宋盛龙, 李永泰, 张新宇, 李艳军. 陆地棉GhSWEET9基因的克隆及抗黄萎病功能分析[J]. 生物技术通报, 2025, 41(6): 144-154. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||