








生物技术通报 ›› 2025, Vol. 41 ›› Issue (6): 144-154.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0002
• 研究报告 • 上一篇
收稿日期:2025-01-02
出版日期:2025-06-26
发布日期:2025-06-30
通讯作者:
李艳军,女,博士,教授,研究方向 :棉花分子育种;E-mail: lyj20022002@sina.com.canalyze作者简介:张勇,男,硕士研究生,研究方向 :作物遗传育种;E-mail: 20222012025@stu.shzu.edu.cn
基金资助:
ZHANG Yong( ), SONG Sheng-long, LI Yong-tai, ZHANG Xin-yu, LI Yan-jun(
), SONG Sheng-long, LI Yong-tai, ZHANG Xin-yu, LI Yan-jun( )
)
Received:2025-01-02
Published:2025-06-26
Online:2025-06-30
摘要:
目的 研究GhSWEET9基因与棉花抗黄萎病相关性,为探究棉花抗黄萎病的分子机制及选育抗黄萎病棉花新品种提供理论依据。 方法 利用生物信息学软件分析GhSWEET9基因的序列特征、系统进化关系和亚细胞定位。利用酵母异源互补系统明确GhSWEET9蛋白的糖转运功能。利用病毒诱导的基因沉默技术(virus induced gene silencing, VIGS)分析该基因在抗黄萎病中的功能。 结果 GhSWEET9蛋白有7个跨膜结构域,系统进化树分析显示其属于SWEETs Clade2 亚族成员。亚细胞定位结果显示GhSWEET9定位在细胞质膜上。酵母异源互补实验显示GhSWEET9蛋白可以转运半乳糖、甘露糖、葡萄糖和果糖。利用VIGS技术沉默GhSWEET9,葡萄糖含量测定发现沉默植株(pTRV2:GhSWEET9)根系中的葡萄糖含量明显高于空载体对照(pTRV2:00);在大丽轮枝菌Vd991侵染棉苗2 d和6 d时发现,沉默植株根系中的葡萄糖含量仍明显高于对照植株;侵染14 d后发现,沉默植株叶片黄化和萎蔫程度比对照植株更为严重,维管束褐化更加明显,病情指数更高。 结论 GhSWEET9基因沉默有利于葡萄糖在棉花根系中的积累,这可能促进了大丽轮枝菌对棉花根系的侵染,从而减弱棉花对黄萎病菌的抗性,推测该基因在棉花抗黄萎病过程中可能发挥重要作用。
张勇, 宋盛龙, 李永泰, 张新宇, 李艳军. 陆地棉GhSWEET9基因的克隆及抗黄萎病功能分析[J]. 生物技术通报, 2025, 41(6): 144-154.
ZHANG Yong, SONG Sheng-long, LI Yong-tai, ZHANG Xin-yu, LI Yan-jun. Cloning of GhSWEET9 in Upland Cotton and Functional Analysis of Resistance to Verticillium Wilt[J]. Biotechnology Bulletin, 2025, 41(6): 144-154.
| 引物 Primer | 序列 Primer sequence (5'-3') | 用途 Application |
|---|---|---|
| CDS-GhSWEET9 | ATGGTTTCGCACCTTGTCAC | 基因克隆 |
CDS-GhSWEET9 Y-SWEET9-F Y-SWEET9-R | AGGTGCTGTGCAGTTCTTT aacacgggggactttgcaacATGGTTTCGCACCTTGTCACCACC tcctcgcccttcacgatacaAGGTGCTGTGCAGTTCTTTTTGGGATC | |
| VIGS-GhSWEET9-F | aaggttaccgaattGAACAGTTCTCCCCAGCC | |
| VIGS-GhSWEET9-R | ctcggtaccggatcAGCTATAATTCCGACCACCATG | |
| PDR-GhSWEET9-F | ccagcctcgagcgg ATGGTTTCGCACCTTGTCAC | |
| PDR-GhSWEET9-R | agctggatccgcgc AGGTGCTGTGCAGTTCTTT | |
| qPCR-GhSWEET9-F | CCATCAATGGCACAGGGACT | 表达量检测 |
| qPCR-GhSWEET9-R | TCGACGTTGAGTGGTGTGAG | |
| UBQ7-F | GAAGGCATTCCACCTGACCAAC | 内参基因扩增 |
| UBQ7-R | CTTGACCTTCTTCTTCTTGTGCTTG |
表1 试验所用引物
Table 1 Primers used in this experiment
| 引物 Primer | 序列 Primer sequence (5'-3') | 用途 Application |
|---|---|---|
| CDS-GhSWEET9 | ATGGTTTCGCACCTTGTCAC | 基因克隆 |
CDS-GhSWEET9 Y-SWEET9-F Y-SWEET9-R | AGGTGCTGTGCAGTTCTTT aacacgggggactttgcaacATGGTTTCGCACCTTGTCACCACC tcctcgcccttcacgatacaAGGTGCTGTGCAGTTCTTTTTGGGATC | |
| VIGS-GhSWEET9-F | aaggttaccgaattGAACAGTTCTCCCCAGCC | |
| VIGS-GhSWEET9-R | ctcggtaccggatcAGCTATAATTCCGACCACCATG | |
| PDR-GhSWEET9-F | ccagcctcgagcgg ATGGTTTCGCACCTTGTCAC | |
| PDR-GhSWEET9-R | agctggatccgcgc AGGTGCTGTGCAGTTCTTT | |
| qPCR-GhSWEET9-F | CCATCAATGGCACAGGGACT | 表达量检测 |
| qPCR-GhSWEET9-R | TCGACGTTGAGTGGTGTGAG | |
| UBQ7-F | GAAGGCATTCCACCTGACCAAC | 内参基因扩增 |
| UBQ7-R | CTTGACCTTCTTCTTCTTGTGCTTG |

图1 黄萎病菌侵染下GhSWEET9基因的表达模式CK:水处理;Vd991:黄萎病菌Vd991侵染。显著性水平(*P<0.05;**P<0.01;***P<0.001,下同)
Fig. 1 Expression pattern of GhSWEET9 gene infected with V. dahliaeCK: Water treatment; Vd991: inoculated with V. dahliae strain Vd991. Significance level (*P<0.05; **P<0.01; ***P<0.001. The same below)
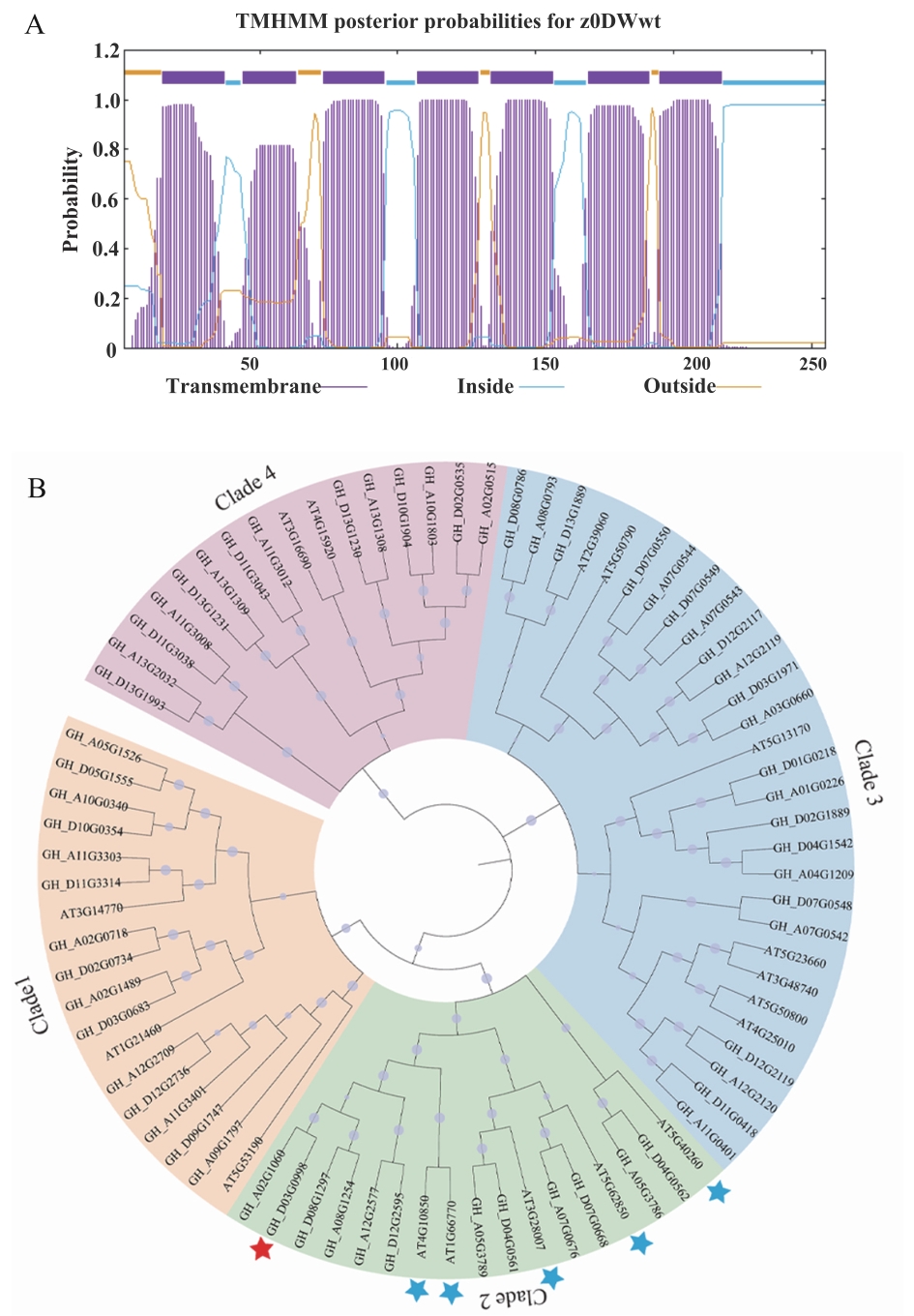
图2 GhSWEET9的生物信息学分析A:GhSWEET9蛋白跨膜结构域预测;B:陆地棉与拟南芥SWEETs蛋白的系统进化树
Fig. 2 Bioinformatics analysis of GhSWEET9A: Prediction of the transmembrane domain of GhSWEET9 protein. B: Phylogenetic tree of SWEETs proteins from upland cotton and Arabidopsis
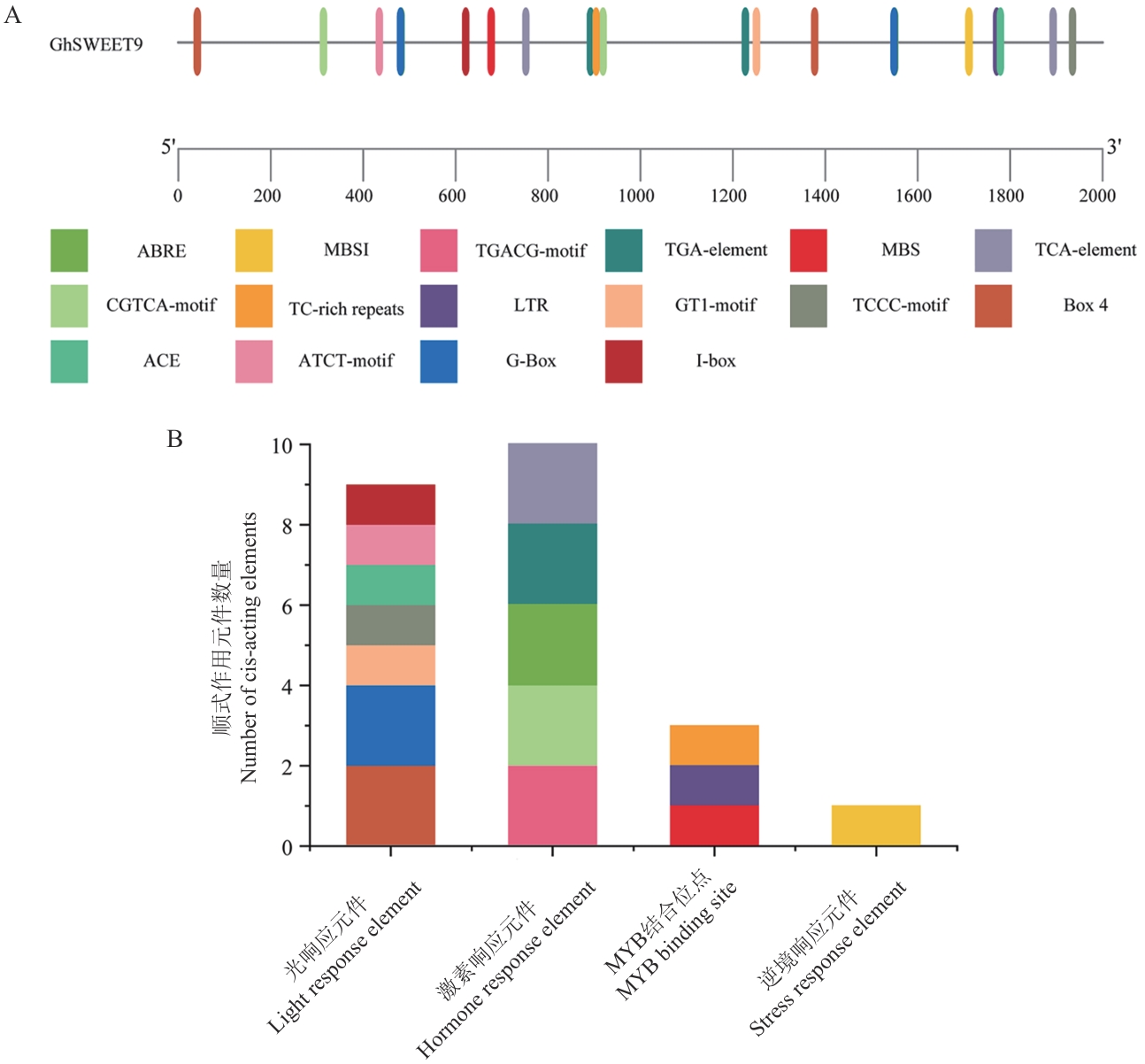
图3 陆地棉GhSWEET9基因启动子顺式作用元件分析A:陆地棉GhSWEET9基因上游2 000 bp启动子区顺式作用元件分析;B:不同类型顺式作用元件数量统计
Fig. 3 Analysis of cis-acting elements in the promoter of GhSWEET9 gene in upland cottonA: Analysis of cis-acting elements in the 2 000 bp promoter region upstream of upland cotton GhSWEET9 gene. B: Number statistics of cis-acting elements in different types
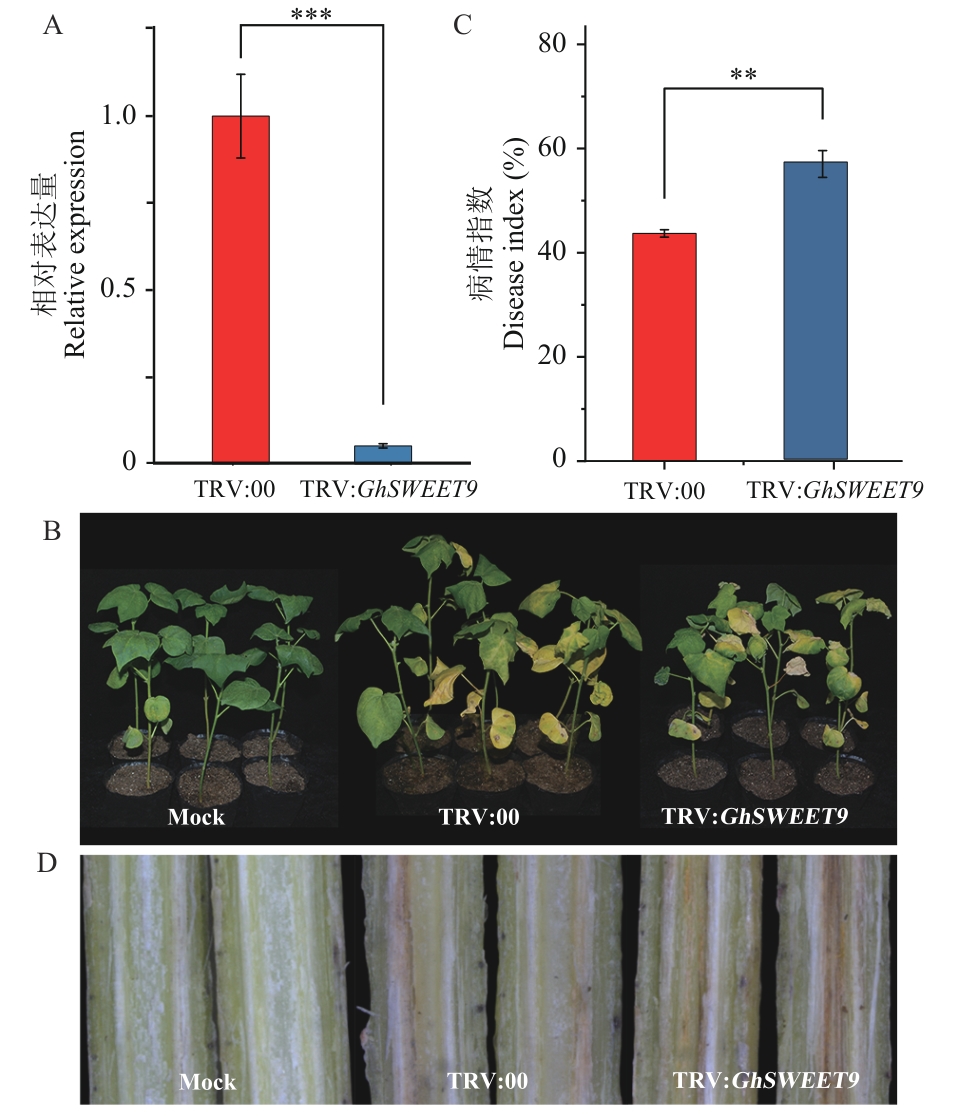
图6 GhSWEET9沉默植株抗病性鉴定A:对照植株和沉默植株中GhSWEET9的表达水平;B:接种黄萎病菌14 d后对照植株和沉默植株的发病症状;C:接种黄萎病菌14 d后对照植株和沉默植株的病情指数;D:接种黄萎病菌14 d后对照植株和沉默植株茎秆维管束褐化情况
Fig. 6 Identification of GhSWEET9-silenced plants’s resistances to diseaseA: Relative expression of GhSWEET9 in control plants and silenced plants at 14 d post infection. B: Disease symptoms of control plants and silenced plants at 14 d post infection.C: Disease index of control plants and silenced plants at 14 d post infection. D: Vascular bundles browning in stem of control plants and silenced plants at 14 d post infection

图7 大丽轮枝菌侵染后对照和VIGS沉默植株根系葡萄糖含量测定A:大丽轮枝菌侵染2 d后棉花根部的葡萄糖含量;B:大丽轮枝菌侵染6 d后棉花根部的葡萄糖含量。CK为水处理,Vd991为黄萎病菌Vd991侵染
Fig. 7 Glucose contents in the roots of control plants and VIGS-silenced plants infected with V. dahliaeA: The glucose content in the roots of cotton plants infected with V. dahliae after 2 d of infection. B: The glucose content in the roots of cotton plants infected with V. dahliae after 6 d of infection. CK: Water treatment; Vd991: inoculated with V. dahliae strain Vd991
| 1 | 刘延财, 唐叶, 吴家和, 等. 生防微生物在棉花黄萎病防治中的研究进展 [J]. 微生物学报, 2025, 65(3): 994-1006. |
| Liu YC, Tan Y, Wu JH, et al. Research progress of biocontrol microbial strains in prevention ofcotton wilt disease [J]. Acta Microbiologica Sinica, 2025, 65(3): 994-1006. | |
| 2 | Zhu YT, Zhao M, Li TT, et al. Interactions between Verticillium dahliae and cotton: pathogenic mechanism and cotton resistance mechanism to Verticillium wilt [J]. Front Plant Sci, 2023, 14: 1174281. |
| 3 | Wilhelm S. Longevity of the Verticillium wilt fungus in the laboratory and field [J]. Phytopathology, 1955, 45(3): 180-181. |
| 4 | 胡丽萍, 张峰, 徐惠, 等. 植物SWEET基因家族结构、功能及调控研究进展 [J]. 生物技术通报, 2017, 33(4): 27-37. |
| Hu LP, Zhang F, Xu H, et al. Research advances in the structure, function and regulation of SWEET gene family in plants [J]. Biotechnol Bull, 2017, 33(4): 27-37. | |
| 5 | Julius BT, Leach KA, Tran TM, et al. Sugar transporters in plants: new insights and discoveries [J]. Plant Cell Physiol, 2017, 58(9): 1442-1460. |
| 6 | Eom JS, Chen LQ, Sosso D, et al. SWEETs, transporters for intracellular and intercellular sugar translocation [J]. Curr Opin Plant Biol, 2015, 25: 53-62. |
| 7 | Chen LQ, Hou BH, Lalonde S, et al. Sugar transporters for intercellular exchange and nutrition of pathogens [J]. Nature, 2010, 468: 527-532. |
| 8 | Chen LQ, Qu XQ, Hou BH, et al. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport [J]. Science, 2012, 335(6065): 207-211. |
| 9 | Yuan M, Wang SP. Rice MtN3/saliva/SWEET family genes and their homologs in cellular organisms [J]. Mol Plant, 2013, 6(3): 665-674. |
| 10 | Zhang XS, Feng CY, Wang MN, et al. Plasma membrane-localized SlSWEET7a and SlSWEET14 regulate sugar transport and storage in tomato fruits [J]. Hortic Res, 2021, 8(1): 186. |
| 11 | Valifard M, Le Hir R, Müller J, et al. Vacuolar fructose transporter SWEET17 is critical for root development and drought tolerance [J]. Plant Physiol, 2021, 187(4): 2716-2730. |
| 12 | Chen LQ, Winnie Lin I, Qu XQ, et al. A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo [J]. Plant Cell, 2015, 27(3): 607-619. |
| 13 | Wang J, Xue XY, Zeng HQ, et al. Sucrose rather than GA transported by AtSWEET13 and AtSWEET14 supports pollen fitness at late anther development stages [J]. New Phytol, 2022, 236(2): 525-537. |
| 14 | Fakher B, Arif Ashraf M, Wang LL, et al. Pineapple SWEET10 is a glucose transporter [J]. Hortic Res, 2023, 10(10): uhad175. |
| 15 | Feng CY, Han JX, Han XX, et al. Genome-wide identification, phylogeny, and expression analysis of the SWEET gene family in tomato [J]. Gene, 2015, 573(2): 261-272. |
| 16 | Gao Y, Wang ZY, Kumar V, et al. Genome-wide identification of the SWEET gene family in wheat [J]. Gene, 2018, 642: 284-292. |
| 17 | Zhao LJ, Yao JB, Chen W, et al. A genome-wide analysis of SWEET gene family in cotton and their expressions under different stresses [J]. J Cotton Res, 2018, 1(1): 7. |
| 18 | Xu ZY, Xu XM, Gong Q, et al. Engineering broad-spectrum bacterial blight resistance by simultaneously disrupting variable TALE-binding elements of multiple susceptibility genes in rice [J]. Mol Plant, 2019, 12(11): 1434-1446. |
| 19 | Liu XZ, Zhang Y, Yang C, et al. AtSWEET4, a hexose facilitator, mediates sugar transport to axial sinks and affects plant development [J]. Sci Rep, 2016, 6: 24563. |
| 20 | Chen HY, Huh JH, Yu YC, et al. The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection [J]. Plant J, 2015, 83(6): 1046-1058. |
| 21 | Meteier E, Camera SL, Goddard ML, et al. Overexpression of the VvSWEET4 transporter in grapevine hairy roots increases sugar transport and contents and enhances resistance to Pythium irregulare, a soilborne pathogen [J]. Front Plant Sci, 2019, 10: 884. |
| 22 | Singh J, Das S, Jagadis Gupta K, et al. Physiological implications of SWEETs in plants and their potential applications in improving source-sink relationships for enhanced yield [J]. Plant Biotechnol J, 2023, 21(8): 1528-1541. |
| 23 | Chong JL, Piron MC, Meyer S, et al. The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea [J]. J Exp Bot, 2014, 65(22): 6589-6601. |
| 24 | Sun MX, Zhang ZQ, Ren ZY, et al. The GhSWEET42 glucose transporter participates in Verticillium dahliae infection in cotton [J]. Front Plant Sci, 2021, 12: 690754. |
| 25 | Zhang SL, Dong LJ, Zhang X, et al. The transcription factor GhWRKY70 from Gossypium hirsutum enhances resistance to Verticillium wilt via the jasmonic acid pathway [J]. BMC Plant Biol, 2023, 23(1): 141. |
| 26 | Xu L, Zhang WW, He X, et al. Functional characterization of cotton genes responsive to Verticillium dahliae through bioinformatics and reverse genetics strategies [J]. J Exp Bot, 2014, 65(22): 6679-6692. |
| 27 | Li YT, Li YJ, Yang QW, et al. Dual transcriptome analysis reveals the changes in gene expression in both cotton and Verticillium dahliae during the infection process [J]. J Fungi, 2024, 10(11): 773. |
| 28 | Li W, Ren ZY, Wang ZY, et al. Evolution and stress responses of Gossypium hirsutum SWEET genes [J]. Int J Mol Sci, 2018, 19(3): 769. |
| 29 | Zhang Y, Wang XF, Rong W, et al. Histochemical analyses reveal that stronger intrinsic defenses in Gossypium barbadense than in G. hirsutum are associated with resistance to Verticillium dahliae [J]. Mol Plant Microbe Interact, 2017, 30(12): 984-996. |
| 30 | Gao XQ, Wheeler T, Li ZH, et al. Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt [J]. Plant J, 2011, 66(2): 293-305. |
| 31 | Mo HJ, Wang XF, Zhang Y, et al. Cotton polyamine oxidase is required for spermine and camalexin signalling in the defence response to Verticillium dahliae [J]. Plant J, 2015, 83(6): 962-975. |
| 32 | Breia R, Conde A, Pimentel D, et al. VvSWEET7 is a mono- and disaccharide transporter up-regulated in response to Botrytis cinerea infection in grape berries [J]. Front Plant Sci, 2020, 10: 1753. |
| 33 | Liu QS, Yuan M, Zhou Y, et al. A paralog of the MtN3/saliva family recessively confers race-specific resistance to Xanthomonas oryzae in rice [J]. Plant Cell Environ, 2011, 34(11): 1958-1969. |
| 34 | Cao ZH, Li Z, Meng L, et al. Genome-wide characterization of pyrabactin resistance 1-like (PYL) family genes revealed AhPYL6 confer the resistance to Ralstonia solanacearum in peanut [J]. Plant Physiol Biochem, 2024, 217: 109295. |
| 35 | Xiao W, Zhang Y, Wang Y, et al. The transcription factor TGA2 orchestrates salicylic acid signal to regulate cold-induced proline accumulation in Citrus [J]. Plant Cell, 2024, 37(1): koae290. |
| 36 | Tan ZW, Lu DD, Li L, et al. Comprehensive analysis of safflower R2R3-MYBs reveals the regulation mechanism of CtMYB76 on flavonol biosynthesis [J]. Ind Crops Prod, 2025, 227: 120795. |
| 37 | Liu HB, Liu JX, Si XH, et al. Sugar transporter HmSWEET8 cooperates with HmSTP1 to enhance powdery mildew susceptibility in Heracleum moellendorffii hance [J]. Plants, 2024, 13(16): 2302. |
| 38 | Sosso D, van der Linde K, Bezrutczyk M, et al. Sugar partitioning between Ustilago maydis and its host Zea mays L during infection [J]. Plant Physiol, 2019, 179(4): 1373-1385. |
| 39 | Walerowski P, Gündel A, Yahaya N, et al. Clubroot disease stimulates early steps of phloem differentiation and recruits SWEET sucrose transporters within developing galls [J]. Plant Cell, 2018, 30(12): 3058-3073. |
| 40 | An JY, Zeng T, Ji CY, et al. A Medicago truncatula SWEET transporter implicated in arbuscule maintenance during arbuscular mycorrhizal symbiosis [J]. New Phytol, 2019, 224(1): 396-408. |
| 41 | Chang Q, Liu J, Wang QL, et al. The effect of Puccinia striiformis f.sp. tritici on the levels of water-soluble carbohydrates and the photosynthetic rate in wheat leaves [J]. Physiol Mol Plant Pathol, 2013, 84: 131-137. |
| 42 | Fatima U, Anjali A, Senthil-Kumar M. AtSWEET11 and AtSWEET12: the twin traders of sucrose [J]. Trends Plant Sci, 2022, 27(10): 958-960. |
| 43 | Gao Y, Xue CY, Liu JM, et al. Sheath blight resistance in rice is negatively regulated by WRKY53 via SWEET2a activation [J]. Biochem Biophys Res Commun, 2021, 585: 117-123. |
| 44 | Gao Y, Zhang C, Han X, et al. Inhibition of OsSWEET11 function in mesophyll cells improves resistance of rice to sheath blight disease [J]. Mol Plant Pathol, 2018, 19(9): 2149-2161. |
| 45 | Kim P, Xue CY, Song HD, et al. Tissue-specific activation of DOF11 promotes rice resistance to sheath blight disease and increases grain weight via activation of SWEET14 [J]. Plant Biotechnol J, 2021, 19(3): 409-411. |
| [1] | 樊玥妮, 仙保山, 师艺萍, 任梦圆, 徐佳慧, 魏绍巍, 许晓敬, 罗晓峰, 舒凯. SPINDLY和SECRET AGENT介导的蛋白糖基化调控植物发育与逆境响应[J]. 生物技术通报, 2025, 41(4): 1-8. |
| [2] | 杨代毅, 樊杨, 屠焰, 徐志宇, 薛颖昊, 孙元丰, 王进, 郝小燕, 马涛. 不同处理对油菜秸秆养分、纤维结构和硫苷含量的影响[J]. 生物技术通报, 2024, 40(6): 172-179. |
| [3] | 王娟, 王新, 田琴, 马晓梅, 周小凤, 李保成, 董承光. 陆地棉主要株型性状关联分析及优异等位基因挖掘[J]. 生物技术通报, 2024, 40(3): 146-154. |
| [4] | 杨伟成, 孙岩, 杨倩, 王壮琳, 马菊花, 薛金爱, 李润植. 陆地棉FAX家族的全基因组鉴定及GhFAX1的功能分析[J]. 生物技术通报, 2024, 40(3): 155-169. |
| [5] | 吴翠翠, 肖水平. 陆地棉HD-Zip家族全基因组鉴定及响应非生物胁迫的表达分析[J]. 生物技术通报, 2024, 40(2): 130-145. |
| [6] | 华炫, 田博雯, 周欣彤, 江梓涵, 王诗琦, 黄倩慧, 张健, 陈艳红. 旱柳SmERF B3-45的克隆及耐盐功能研究[J]. 生物技术通报, 2024, 40(12): 124-135. |
| [7] | 张怡, 张心如, 张金珂, 胡利宗, 上官欣欣, 郑晓红, 胡娟娟, 张聪聪, 穆桂清, 李成伟. 小麦镉胁迫响应基因TaMYB1的功能分析[J]. 生物技术通报, 2024, 40(1): 194-206. |
| [8] | 赵光绪, 杨合同, 邵晓波, 崔志豪, 刘红光, 张杰. 一株高效溶磷产红青霉培养条件优化及其溶磷特性[J]. 生物技术通报, 2023, 39(9): 71-83. |
| [9] | 李文辰, 刘鑫, 康越, 李伟, 齐泽铮, 于璐, 王芳. TRV病毒诱导大豆基因沉默体系优化及应用[J]. 生物技术通报, 2023, 39(7): 143-150. |
| [10] | 董聪, 高庆华, 王玥, 罗同阳, 王庆庆. 基于联合策略提高FAD依赖的葡萄糖脱氢酶的酵母表达[J]. 生物技术通报, 2023, 39(6): 316-324. |
| [11] | 王一帆, 候林慧, 常永春, 杨亚杰, 陈天, 赵祝跃, 荣二花, 吴玉香. 陆地棉与拟似棉异源六倍体的合成与性状鉴定[J]. 生物技术通报, 2023, 39(5): 168-176. |
| [12] | 高凯月, 郭雨婷, 杜奕谋, 郑小梅, 马欣荣, 赵伟, 郑平, 孙际宾. 黑曲霉葡萄糖吸收定量检测的方法建立及其在MstC功能研究中的应用[J]. 生物技术通报, 2023, 39(12): 71-80. |
| [13] | 张君, 张虹, 张芮, 路国栋, 雍婧姣, 郎思睿, 陈任. 甜菊醇糖苷生物合成关键基因的导入和鉴定分析[J]. 生物技术通报, 2023, 39(1): 214-223. |
| [14] | 李秀青, 胡子曜, 雷建峰, 代培红, 刘超, 邓嘉辉, 刘敏, 孙玲, 刘晓东, 李月. 棉花黄萎病抗性相关基因GhTIFY9的克隆与功能分析[J]. 生物技术通报, 2022, 38(8): 127-134. |
| [15] | 牛宇辉, 李向茸, 吴贝, 李洪珊, 李殿玉, 陈磊, 魏锁成, 冯若飞. 葡萄糖和丁酸钠对CHO-rHSA工程细胞株中rHSA产量的影响[J]. 生物技术通报, 2022, 38(7): 278-286. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||