








生物技术通报 ›› 2026, Vol. 42 ›› Issue (1): 198-207.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0901
吴翠翠1( ), 陈登科1,2(
), 陈登科1,2( ), 兰刚1, 夏芝1, 李朋波1(
), 兰刚1, 夏芝1, 李朋波1( )
)
收稿日期:2025-08-20
出版日期:2026-01-26
发布日期:2026-02-04
通讯作者:
李朋波,男,博士,研究员,研究方向 :作物遗传育种;E-mail: lipengbo@sxau.edu.cn作者简介:吴翠翠,女,博士,副研究员,研究方向 :作物遗传育种;E-mail: wucuicui19821021@126.com基金资助:
WU Cui-cui1( ), CHEN Deng-ke1,2(
), CHEN Deng-ke1,2( ), LAN Gang1, XIA Zhi1, LI Peng-bo1(
), LAN Gang1, XIA Zhi1, LI Peng-bo1( )
)
Received:2025-08-20
Published:2026-01-26
Online:2026-02-04
摘要:
目的 干旱和盐胁迫是严重影响我国花生产量及品质的环境因素。探究AhHDZ70(Ah12g074600.1)基因结构和功能,揭示其在花生耐旱和耐盐性方面的作用,为花生抗盐和抗旱育种提供重要的基因资源。 方法 利用生物信息学方法对AhHDZ70进行理化性质、基因结构、系统发育、启动子顺式元件、蛋白互作分析;采用荧光定量PCR技术分析AhHDZ70基因在不同组织器官和盐、干旱、高温、低温胁迫下的表达量;利用烟草瞬时转化技术进行AhHDZ70基因的亚细胞定位分析;并采用转基因技术在拟南芥中进行遗传转化,通过盐和干旱胁迫处理进一步验证该基因的生物学功能。 结果 AhHDZ70编码326个氨基酸,是不稳定的亲水蛋白,无信号肽,其二级和三级结构以无规则卷曲为主。结构域分析表明,AhHDZ70包含4个外显子和3个内含子。AhHDZ70启动子区包含2个干旱诱导功能元件(MBS基序)、1个逆境胁迫响应元件(TC-rich repeats基序)和1个低温响应元件(LTR基序)。转录组结合RT-qPCR分析表明,AhHDZ70在种子中的表达量最高,正向响应盐、干旱、高温和低温胁迫。亚定位实验结果显示,AhHDZ70定位于细胞核。AhHDZ70异源过表达拟南芥获得纯合的转基因植株,通过盐、干旱胁迫处理发现,AhHDZ70过表达转基因植株的发芽率均显著高于WT,且根长极显著长于WT。 结论 AhHDZ70属于HD-ZIP Ⅱ转录因子,定位于细胞核。AhHDZ70可能参与对花生多个组织生长发育的调控,并可能正向调控花生对盐和干旱胁迫的响应。
吴翠翠, 陈登科, 兰刚, 夏芝, 李朋波. 花生转录因子AhHDZ70的生物信息学分析及耐盐耐旱性研究[J]. 生物技术通报, 2026, 42(1): 198-207.
WU Cui-cui, CHEN Deng-ke, LAN Gang, XIA Zhi, LI Peng-bo. Bioinformatics Analysis of Peanut Transcription Factor AhHDZ70 and Its Tolerances to Salt and Drought[J]. Biotechnology Bulletin, 2026, 42(1): 198-207.
| 基因名称 Gene name | 正向引物 Forward primer (5′‒3′) | 反向引物 Reverse primer (5′‒3′) | 实验类型 Type of experiment |
|---|---|---|---|
| AhHDZ70 | TTGGAGAGAACACGGGGGACGAATTCATGGAATATGCAACATATTCATCAGC | GCCTGCAGGTCGACTCTAGAGGATCCGGACCAAAAGTCCCACCATTG | 过表达实验、亚定位实验、过表达PCR鉴定 |
| β-Actin | CAGGATTTGCCGGTGATGATG | TCTGTTGGCCTTCGGGTTGAG | 内参 |
| AhHDZ70 | AGATGAGTTTGGGTTTGAGT | GAGGTAGAGGCTTCTGGAT | RT-qPCR |
表1 所用相关引物
Table 1 Primers used in the study
| 基因名称 Gene name | 正向引物 Forward primer (5′‒3′) | 反向引物 Reverse primer (5′‒3′) | 实验类型 Type of experiment |
|---|---|---|---|
| AhHDZ70 | TTGGAGAGAACACGGGGGACGAATTCATGGAATATGCAACATATTCATCAGC | GCCTGCAGGTCGACTCTAGAGGATCCGGACCAAAAGTCCCACCATTG | 过表达实验、亚定位实验、过表达PCR鉴定 |
| β-Actin | CAGGATTTGCCGGTGATGATG | TCTGTTGGCCTTCGGGTTGAG | 内参 |
| AhHDZ70 | AGATGAGTTTGGGTTTGAGT | GAGGTAGAGGCTTCTGGAT | RT-qPCR |
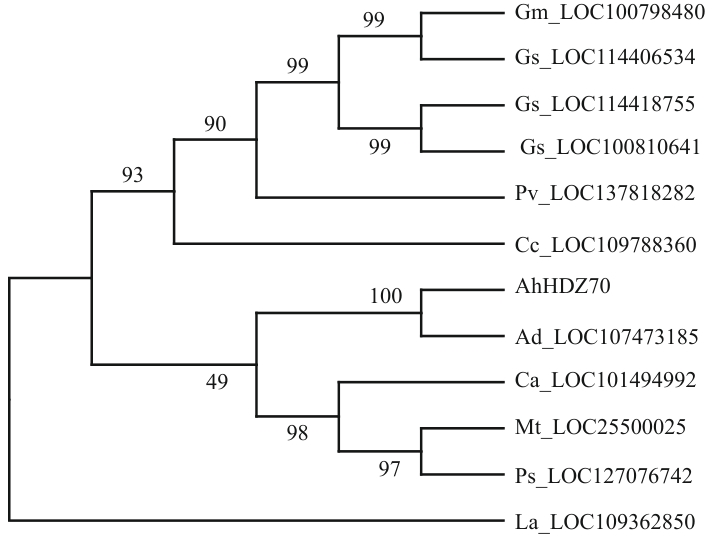
图1 AhHDZ70与其他物种同源蛋白的进化树分析Ah:四倍体花生;Ad:二倍体花生;Ca:鹰嘴豆;Cc:木豆;Gm:栽培大豆;Gs:野生大豆;La:窄叶羽扇豆;Mt:苜蓿;Ps:豌豆;Pv:菜豆
Fig. 1 Phylogenetic tree analysis of homologous proteins between AhHDZ70 and other speciesAh: Arachis hypogaea; Ad: Arachis duranensis; Ca: Cicer arietinum; Cc: Cajanus cajan; Gm: Glycine max; Gs: Glycine soja; La: Lupinus angustifolius; Mt: Medicago truncatula; Ps: Pisum sativum; Pv: Phaseolus vulgaris
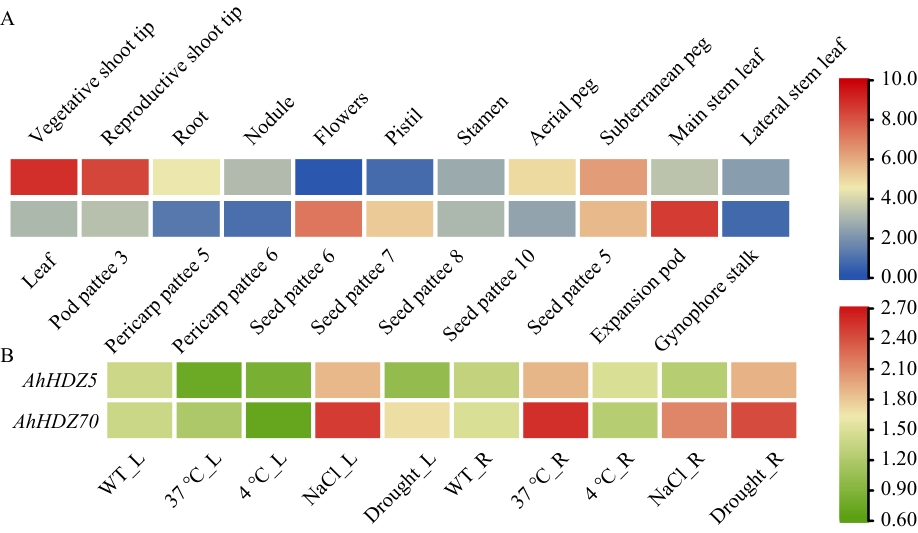
图3 AhHDZ70组织器官和非生物胁迫的表达模式分析A:AhHDZ70在22个组织器官的表达模式;B:AhHDZ70及其同源基因AhHDZ5在非生物胁迫下的组织表达模式。Vegetative shoot tip:主茎营养茎尖;Reproductive shoot tip:第一侧生殖芽尖;Root:根;Nodule:根瘤;Flowers:花;Pistil:雌蕊;Stamen:雄蕊;Aerial peg:果针;Subterranean peg:入土果针;Main stem leaf:主根;Lateral stem leaf:侧枝叶;Leaf:叶;Pod pattee 3:荚果发育期3;Pericarp pattee 5:果皮发育期5;Pericarp pattee 6:果皮发育期6;Seed pattee 6:种子发育期6;Seed pattee 7:种子发育期7;Seed pattee 8:种子发育期8;Seed pattee 10:种子发育期10;Seed pattee5:种子发育期5;Expansion pod:膨大期果针;Gynophore stalk:雌蕊柄。L:叶片;R:根
Fig. 3 Analysis of AhHDZ70 expression patterns in tissues, organs, and abiotic stressA: Expression patterns of AhHDZ70 in 22 tissues and organs. B: Expression patterns of AhHDZ70 under abiotic stress. L: Leaf. R: Root
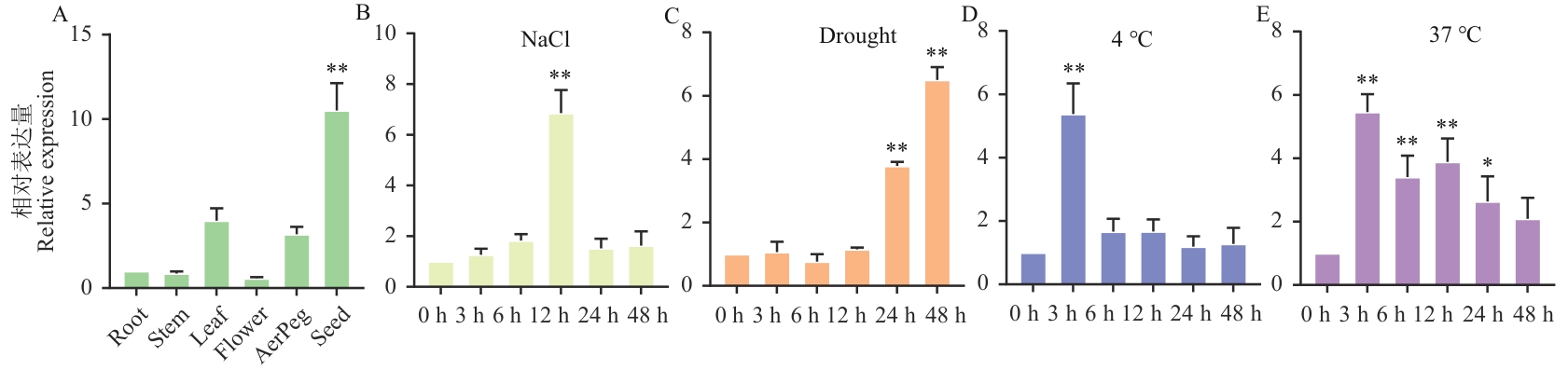
图4 AhHDZ70在花生不同组织器官及非生物胁迫下的RT-qPCR鉴定A:AhHDZ70在花生不同组织器官的RT-qPCR分析;B-E:AhHDZ70在盐、干旱、低温和高温胁迫下的RT-qPCR分析。通过双尾t检验分析差异显著性,误差线代表平均值±SD,*P<0.05, **P<0.01,下同
Fig. 4 RT-qPCR identification of AhHDZ70 in different tissues and organs of peanut under abiotic stressA: RT-qPCR analysis of AhHDZ70 in different tissues and organs of peanuts. B-E: RT-qPCR analysis of AhHDZ70 under salt, drought, low temperature and high temperature stress. The difference significance was analyzed by two-tailed t-test. Error bars indicate mean ±SD, *P<0.05, **P<0.01,the same below
| [1] | de Carvalho Moretzsohn M, Hopkins MS, Mitchell SE, et al. Genetic diversity of peanut (Arachis hypogaea L.) and its wild relatives based on the analysis of hypervariable regions of the genome [J]. BMC Plant Biol, 2004, 4(1): 11. |
| [2] | Desmae H, Janila P, Okori P, et al. Genetics, genomics and breeding of groundnut (Arachis hypogaea L.) [J]. Plant Breed, 2019, 138(4): 425-444. |
| [3] | Zhuang WJ, Chen H, Yang M, et al. The genome of cultivated peanut provides insight into legume karyotypes, polyploid evolution and crop domestication [J]. Nat Genet, 2019, 51(5): 865-876. |
| [4] | 郑青焕, 李拴柱, 王建玉, 等. 河南省鲜食花生研究现状与发展前景 [J]. 中国种业, 2025(8): 19-23. |
| Zheng QH, Li SZ, Wang JY, et al. Research status and development prospects of fresh peanuts in Henan province [J]. China Seed Ind, 2025(8): 19-23. | |
| [5] | 李博文,赵建军. 挖掘盐碱地改造利用潜力[J].经济日报, 2024, 11, 24. |
| [6] | Wu CC, Hou BG, Wu RL, et al. Genome-wide analysis elucidates the roles of AhLBD genes in different abiotic stresses and growth and development stages in the peanut (Arachis hypogea L.) [J]. Int J Mol Sci, 2024, 25(19): 10561. |
| [7] | Chen XP, Lu Q, Liu H, et al. Sequencing of cultivated peanut, Arachis hypogaea, yields insights into genome evolution and oil improvement [J]. Mol Plant, 2019, 12(7): 920-934. |
| [8] | Bertioli DJ, Jenkins J, Clevenger J, et al. The genome sequence of segmental allotetraploid peanut Arachis hypogaea [J]. Nat Genet, 2019, 51(5): 877-884. |
| [9] | Zhao KK, Xue HZ, Li GW, et al. Pangenome analysis reveals structural variation associated with seed size and weight traits in peanut [J]. Nat Genet, 2025, 57(5): 1250-1261. |
| [10] | Lu Q, Huang L, Liu H, et al. A genomic variation map provides insights into peanut diversity in China and associations with 28 agronomic traits [J]. Nat Genet, 2024, 56(3): 530-540. |
| [11] | Hrmova M, Hussain SS. Plant transcription factors involved in drought and associated stresses [J]. Int J Mol Sci, 2021, 22(11): 5662. |
| [12] | Chen Y, Yin ML, Sun LY, et al. MYB gene family in Magnolia biondii: Identification and functional roles in waterlogging stress response [J]. Plant Physiol Biochem, 2025, 229: 110505. |
| [13] | Yu YA, Wu YX, He LY. A wheat WRKY transcription factor TaWRKY17 enhances tolerance to salt stress in transgenic Arabidopsis and wheat plant [J]. Plant Mol Biol, 2023, 113(4/5): 171-191. |
| [14] | Li L, Zhao ZZ, Li WQ, et al. The bHLH transcription factor PdbUNE12 functions as a positive regulator of the salt stress response in Populus davidiana × Populus bolleana [J]. Physiol Plant, 2025, 177(5): e70531. |
| [15] | Qiang ZQ, Zeng Z, Ma DF, et al. NAC transcription factor LpNAC22 positively regulates drought tolerance in perennial ryegrass [J]. Plant Cell Environ, 2025, 48(10): 7256-7270. |
| [16] | Chen N, Pan LJ, Yang Z, et al. A MYB-related transcription factor from peanut, AhMYB30, improves freezing and salt stress tolerance in transgenic Arabidopsis through both DREB/CBF and ABA-signaling pathways [J]. Front Plant Sci, 2023, 14: 1136626. |
| [17] | Yuan CL, Li CJ, Lu XD, et al. Comprehensive genomic characterization of NAC transcription factor family and their response to salt and drought stress in peanut [J]. BMC Plant Biol, 2020, 20(1): 454. |
| [18] | Zhao XB, Wang Q, Yan CX, et al. The bHLH transcription factor AhbHLH121 improves salt tolerance in peanut [J]. Int J Biol Macromol, 2024, 256: 128492. |
| [19] | Elhiti M, Stasolla C. Structure and function of homodomain-leucine zipper (HD-Zip) proteins [J]. Plant Signal Behav, 2009, 4(2): 86-88. |
| [20] | Shao JX, Haider I, Xiong LZ, et al. Functional analysis of the HD-Zip transcription factor genes Oshox12 and Oshox14 in rice [J]. PLoS One, 2018, 13(7): e0199248. |
| [21] | Lin ZF, Hong YG, Yin MG, et al. A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening [J]. Plant J, 2008, 55(2): 301-310. |
| [22] | Jiao P, Jiang ZZ, Wei XT, et al. Overexpression of the homeobox-leucine zipper protein ATHB-6 improves the drought tolerance of maize (Zea mays L.) [J]. Plant Sci, 2022, 316: 111159. |
| [23] | Ren AP, Wen TY, Xu X, et al. Cotton HD-Zip I transcription factor GhHB4-like regulates the plant response to salt stress [J]. Int J Biol Macromol, 2024, 278: 134857. |
| [24] | Possenti M, Sessa G, Alfè A, et al. HD-Zip II transcription factors control distal stem cell fate in Arabidopsis roots by linking auxin signaling to the FEZ/SOMBRERO pathway [J]. Development, 2024, 151(8): dev202586. |
| [25] | Zhao YQ, Han Q, Kang XK, et al. The HAT1 transcription factor regulates photomorphogenesis and skotomorphogenesis via phytohormone levels [J]. Plant Physiol, 2024, 197(1): kiae542. |
| [26] | Baek W, Bae Y, Lim CW, et al. Pepper homeobox abscisic acid signalling-related transcription factor 1, CaHAT1, plays a positive role in drought response [J]. Plant Cell Environ, 2023, 46(7): 2061-2077. |
| [27] | Ariel FD, Manavella PA, Dezar CA, et al. The true story of the HD-Zip family [J]. Trends Plant Sci, 2007, 12(9): 419-426. |
| [28] | Li SJ, Yu ML, Qanmber GL, et al. GhHB14_D10 and GhREV_D5, two HD-ZIP III transcription factors, play a regulatory role in cotton fiber secondary cell wall biosynthesis [J]. Plant Cell Rep, 2024, 43(3): 76. |
| [29] | Mabuchi A, Soga K, Wakabayashi K, et al. Phenotypic screening of Arabidopsis T-DNA insertion lines for cell wall mechanical properties revealed ANTHOCYANINLESS2, a cell wall-related gene [J]. J Plant Physiol, 2016, 191: 29-35. |
| [30] | Ren WB, Wu CC, Wang T, et al. GhHDZ50 regulates cotton fiber elongation in Gossypium hirsutum L. through control of fatty acid biosynthesis [J]. Plant Sci, 2025, 359: 112641. |
| [31] | Clevenger J, Chu Y, Scheffler B, et al. A developmental transcriptome map for allotetraploid Arachis hypogaea [J]. Front Plant Sci, 2016, 7: 1446. |
| [32] | Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method [J]. Nat Protoc, 2008, 3(6): 1101-1108. |
| [33] | Chen CJ, Chen H, Zhang Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data [J]. Mol Plant, 2020, 13(8): 1194-1202. |
| [34] | Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana [J]. Plant J, 1998, 16(6): 735-743. |
| [35] | Wang Q, Wang YY, Zhang FH, et al. Genome-wide characterisation of HD-Zip transcription factors and functional analysis of PbHB24 during stone cell formation in Chinese white pear (Pyrus bretschneideri) [J]. BMC Plant Biol, 2024, 24(1): 444. |
| [36] | 刘育佼, 闫小玲, 畅欣, 等. 毛竹HD-Zip转录因子Pehox14基因的克隆及其在干旱和盐胁迫下的功能分析 [J]. 农业生物技术学报, 2025, 33(7): 1476-1489. |
| Liu YJ, Yan XL, Chang X, et al. Cloning of HD-zip transcription factor Pehox14 gene in moso bamboo (Phyllostachys edulis) and its functional analysis under drought and salt stress [J]. J Agric Biotechnol, 2025, 33(7): 1476-1489. | |
| [37] | Huang H, Wang H, Tong Y, et al. Identification and characterization of HD-Zip genes reveals their roles in stresses responses and facultative crassulacean acid metabolism in Dendrobium catenatum [J]. Sci Hortic, 2021, 285: 110058. |
| [38] | 黄丹, 彭兵阳, 张盼盼, 等. 油茶HD-Zip基因家族鉴定及其在非生物胁迫下的表达分析 [J]. 生物技术通报, 2025, 41(6): 191-207. |
| Huang D, Peng BY, Zhang PP, et al. Identification of HD-Zip gene family in Camellia oleifera and analysis of its expression under abiotic stress [J]. Biotechnol Bull, 2025, 41(6): 191-207. | |
| [39] | Li W, Dong JY, Cao MX, et al. Genome-wide identification and characterization of HD-ZIP genes in potato [J]. Gene, 2019, 697: 103-117. |
| [40] | 刘梦梦, 刘晓, 尤倩, 等. 半夏HD-Zip基因家族全基因组鉴定及表达分析 [J]. 农业生物技术学报, 2024, 32(11): 2540-2551. |
| Liu MM, Liu X, You Q, et al. Genome identification and expression analysis of HD-Zip gene family in Pinellia ternata [J]. J Agric Biotechnol, 2024, 32(11): 2540-2551. | |
| [41] | 吴翠翠, 肖水平. 陆地棉HD-Zip家族全基因组鉴定及响应非生物胁迫的表达分析 [J]. 生物技术通报, 2024, 40(2): 130-145. |
| Wu CC, Xiao SP. Genome-wide identification of HD-Zip gene family in Gossypium hirsutum L. and expression analysis in response to abiotic stress [J]. Biotechnol Bull, 2024, 40(2): 130-145. | |
| [42] | Zhang QY, Chen T, Wang X, et al. Genome-wide identification and expression analyses of homeodomain-leucine zipper family genes reveal their involvement in stress response in apple (Malus×domestica) [J]. Hortic Plant J, 2022, 8(3): 261-278. |
| [43] | Shen W, Li H, Teng RM, et al. Genomic and transcriptomic analyses of HD-Zip family transcription factors and their responses to abiotic stress in tea plant (Camellia sinensis) [J]. Genomics, 2019, 111(5): 1142-1151. |
| [44] | Xie LH, Yan TX, Li L, et al. An HD-ZIP-MYB complex regulates glandular secretory trichome initiation in Artemisia annua [J]. New Phytol, 2021, 231(5): 2050-2064. |
| [45] | Romani F, Ribone PA, Capella M, et al. A matter of quantity: Common features in the drought response of transgenic plants overexpressing HD-Zip I transcription factors [J]. Plant Sci, 2016, 251: 139-154. |
| [46] | He G, Liu P, Zhao H, Sun J. The HD-ZIP II transcription factors regulate plant architecture through the auxin pathway [J]. Int J Mol Sci, 2020, 21(9): 3250. |
| [47] | Scarpella E, Boot KJ, Rueb S, et al. The procambium specification gene Oshox1 promotes polar auxin transport capacity and reduces its sensitivity toward inhibition [J]. Plant Physiol, 2002, 130: 1349-60. |
| [48] | Raza A. Eco-physiological and biochemical responses of rapeseed (Brassica napus L.) to abiotic stresses: consequences and mitigation strategies [J]. J Plant Growth Regul, 2021, 40(4): 1368-1388. |
| [49] | Himmelbach A, Hoffmann T, Leube M, et al. Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis [J]. EMBO J, 2002, 21(12): 3029-3038. |
| [50] | Valdés AE, Overnäs E, Johansson H, et al. The homeodomain-leucine zipper (HD-Zip) class I transcription factors ATHB7 and ATHB12 modulate abscisic acid signalling by regulating protein phosphatase 2C and abscisic acid receptor gene activities [J]. Plant Mol Biol, 2012, 80(4/5): 405-418. |
| [51] | Zhang SX, Haider I, Kohlen W, et al. Function of the HD-Zip Ⅰ gene Oshox22 in ABA-mediated drought and salt tolerances in rice [J]. Plant Mol Biol, 2012, 80(6): 571-585. |
| [52] | Dai MQ, Hu YF, Ma Q, et al. Functional analysis of rice HOMEOBOX4 (Oshox4) gene reveals a negative function in gibberellin responses [J]. Plant Mol Biol, 2008, 66(3): 289-301. |
| [1] | 龙林茜, 曾银萍, 王茜, 邓玉萍, 葛敏茜, 陈彦灼, 李鑫娟, 杨军, 邹建. 向日葵GH3基因家族鉴定及其在花发育中的功能分析[J]. 生物技术通报, 2026, 42(1): 125-138. |
| [2] | 曾厅, 张兰, 罗睿. 转录因子MpR2R3-MYB17调控地钱胞芽发育的功能研究[J]. 生物技术通报, 2026, 42(1): 208-217. |
| [3] | 贺启琛, 杨扬, 阿丽亚·外力, 唐新月, 李忠喜, 陈永坤, 陈凌娜. 薰衣草CuAO基因家族特征及LaCuAO1降解生物胺功能研究[J]. 生物技术通报, 2026, 42(1): 114-124. |
| [4] | 吕呈聪, 衡蒙, 陈思琪, 金雪花. 彩色马蹄莲花青素苷转运相关ZhGSTF的克隆及功能分析[J]. 生物技术通报, 2026, 42(1): 161-169. |
| [5] | 杨娟, 冯慧, 吉乃喆, 孙丽萍, 王赟, 张佳楠, 赵世伟. 月季AP2/ERF转录因子RcERF4和RcRAP2-12的克隆及功能分析[J]. 生物技术通报, 2026, 42(1): 150-160. |
| [6] | 张驰昊, 刘晋囡, 晁跃辉. 蒺藜苜蓿bZIP转录因子MtbZIP29的克隆及功能分析[J]. 生物技术通报, 2026, 42(1): 241-250. |
| [7] | 刘佳丽, 宋经荣, 赵文宇, 张馨元, 赵子洋, 曹一博, 张凌云. 蓝莓R2R3-MYB基因鉴定及类黄酮调控基因表达分析[J]. 生物技术通报, 2025, 41(9): 124-138. |
| [8] | 李玉珍, 李梦丹, 张蔚, 彭婷. 基于月季扩展蛋白基因家族鉴定的野蔷薇RmEXPB2基因功能研究[J]. 生物技术通报, 2025, 41(9): 182-194. |
| [9] | 王斌, 林冲, 袁晓, 蒋园园, 王玉昆, 肖艳辉. bHLH转录因子UNE10克隆及其在丁香罗勒挥发性化合物合成调控中的功能[J]. 生物技术通报, 2025, 41(9): 207-218. |
| [10] | 陈强, 于璎霏, 张颖, 张冲. 茉莉酸甲酯对薄皮甜瓜‘绿宝石’采后冷害的调控[J]. 生物技术通报, 2025, 41(9): 105-114. |
| [11] | 程婷婷, 刘俊, 王利丽, 练从龙, 魏文君, 郭辉, 吴尧琳, 杨晶凡, 兰金旭, 陈随清. 杜仲查尔酮异构酶基因家族全基因组鉴定及其表达模式分析[J]. 生物技术通报, 2025, 41(9): 242-255. |
| [12] | 徐小萍, 杨成龙, 和兴, 郭文杰, 吴健, 方少忠. 百合LoAPS1克隆及其在休眠解除过程的功能分析[J]. 生物技术通报, 2025, 41(9): 195-206. |
| [13] | 张超超, 韩开元, 王彤, 陈仲. 毛白杨PtoYABBY2和PtoYABBY12的克隆及功能分析[J]. 生物技术通报, 2025, 41(9): 256-264. |
| [14] | 史发超, 姜永华, 刘海伦, 文英杰, 严倩. 荔枝LcTFL1基因的克隆与功能分析[J]. 生物技术通报, 2025, 41(9): 159-167. |
| [15] | 董向向, 缪百灵, 许贺娟, 陈娟娟, 李亮杰, 龚守富, 朱庆松. 森林草莓FveBBX20基因的生物信息学分析及开花调控功能[J]. 生物技术通报, 2025, 41(9): 115-123. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||