








生物技术通报 ›› 2021, Vol. 37 ›› Issue (7): 146-155.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0362
山草梅( ), 叶蕾, 张连虎, 况卫刚, 孙晓棠, 马建, 崔汝强(
), 叶蕾, 张连虎, 况卫刚, 孙晓棠, 马建, 崔汝强( )
)
收稿日期:2021-03-22
出版日期:2021-07-26
发布日期:2021-08-13
作者简介:山草梅,女,硕士研究生,研究方向:植物病理学;E-mail: 基金资助:
SHAN Cao-mei( ), YE Lei, ZHANG Lian-hu, KUANG Wei-gang, SUN Xiao-tang, MA Jian, CUI Ru-qiang(
), YE Lei, ZHANG Lian-hu, KUANG Wei-gang, SUN Xiao-tang, MA Jian, CUI Ru-qiang( )
)
Received:2021-03-22
Published:2021-07-26
Online:2021-08-13
摘要:
水稻潜根线虫病是江西省水稻生产上危害严重的病害之一。前期通过对水稻细尖潜根线虫侵染抗/感病水稻品种的根部组织进行比较转录组分析,筛选出差异表达上调基因OsRAI1。本研究克隆获得OsRAI1全长,通过蛋白结构预测、亚细胞定位对其功能进行分析,通过IPTG诱导,对OsRAI1蛋白表达条件进行了优化。结果表明,OsRAI1基因ORF全长1 065 bp,编码354个氨基酸,属于bHLH转录因子家族。该蛋白为亲水性蛋白,分子量37.90 kD,pI值4.86,脂肪系数68.33,总平均亲水性(GRAVY)-0.415,为非跨膜蛋白。蛋白互作预测显示该蛋白可能是通过两个蛋白间HLH区域相互结合作用。亚细胞定位结果表明该蛋白在细胞核中表达。可溶性蛋白在0.6 mmol/L IPTG 16℃诱导18 h后表达量最高。本研究结果为进一步阐明水稻抗潜根线虫分子机制奠定基础。
山草梅, 叶蕾, 张连虎, 况卫刚, 孙晓棠, 马建, 崔汝强. 水稻抗潜根线虫基因OsRAI1的克隆及功能分析[J]. 生物技术通报, 2021, 37(7): 146-155.
SHAN Cao-mei, YE Lei, ZHANG Lian-hu, KUANG Wei-gang, SUN Xiao-tang, MA Jian, CUI Ru-qiang. Cloning,and Functional Analysis of Gene OsRAI1 Resistant to Hirschmanniella mucronate in Rice[J]. Biotechnology Bulletin, 2021, 37(7): 146-155.
| 名称Name | 序列 Sequences(5'-3') | 作用 Function |
|---|---|---|
| OsRAI1-F | ATGGAGCTTGACGAG | 基因克隆 Gene cloning |
| OsRAI1-R | CAGACACCTTCCGCC | |
| T7 | CCTATAGTGAGTCGTATTA | 原核表达载体鉴定 Identification of prokaryotic expression vectors |
| T7ter | CTAGCATAACCCCTTGGGGC | |
| pET28a-OsRAI1-F | CAGCAAATGGGTCGCGGATCCATGGAGCTTGACGAG | 添加酶切位点 Adding enzyme cutting site |
| pET28a-OsRAI1-R | ACTCGAGCACCACCAGCGGCCGCCAGACACCTTCCGCC | |
| 1302-OsRAI1-F | ACCATGGTAGATCTGACTAGTATGGAGCTTGACGAGGAGTCC | 添加酶切位点 Adding enzyme cutting site |
| 1302-OsRAI1-R | AAGTTCTTCTCCTTTACTAGTCAGACACCTTCCGCCATAGC | |
| pCA-F | TGAGACTTTTCAACAAAGGGTAATATCCGG | 过表达载体鉴定 Identification of overexpression vectors |
| pCA-R | GATCTAGTAACATAGATGACACCGC |
表1 PCR引物信息
Table 1 Information of PCR primers
| 名称Name | 序列 Sequences(5'-3') | 作用 Function |
|---|---|---|
| OsRAI1-F | ATGGAGCTTGACGAG | 基因克隆 Gene cloning |
| OsRAI1-R | CAGACACCTTCCGCC | |
| T7 | CCTATAGTGAGTCGTATTA | 原核表达载体鉴定 Identification of prokaryotic expression vectors |
| T7ter | CTAGCATAACCCCTTGGGGC | |
| pET28a-OsRAI1-F | CAGCAAATGGGTCGCGGATCCATGGAGCTTGACGAG | 添加酶切位点 Adding enzyme cutting site |
| pET28a-OsRAI1-R | ACTCGAGCACCACCAGCGGCCGCCAGACACCTTCCGCC | |
| 1302-OsRAI1-F | ACCATGGTAGATCTGACTAGTATGGAGCTTGACGAGGAGTCC | 添加酶切位点 Adding enzyme cutting site |
| 1302-OsRAI1-R | AAGTTCTTCTCCTTTACTAGTCAGACACCTTCCGCCATAGC | |
| pCA-F | TGAGACTTTTCAACAAAGGGTAATATCCGG | 过表达载体鉴定 Identification of overexpression vectors |
| pCA-R | GATCTAGTAACATAGATGACACCGC |
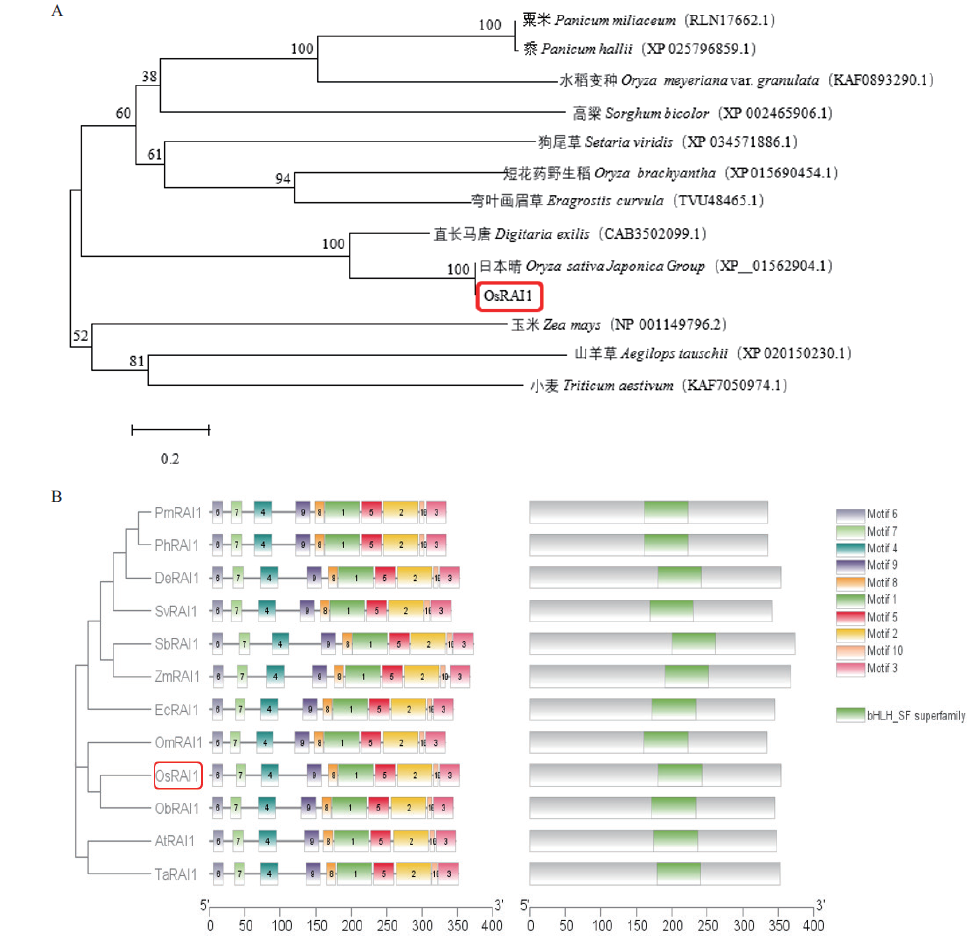
图5 OsRAI1蛋白系统的发育与进化 A:OsRAI1氨基酸序列的进化树分析;B:OsRAI1蛋白序列保守Motif元件预测
Fig. 5 Development and evolution of OsRAI1 protein system A: Phylogenetic tree of OsRAI1. B: Conserved motif element prediction of OsRAI1 protein sequences
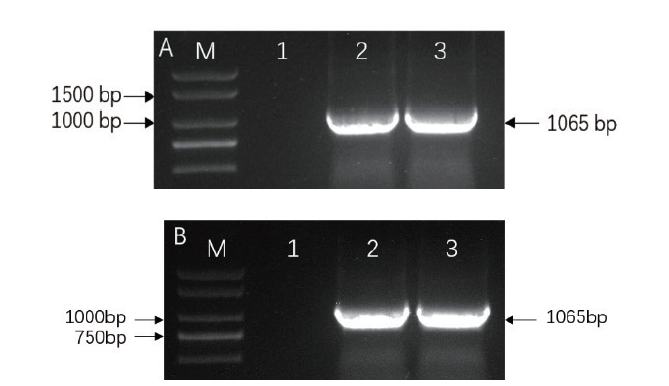
图6 OsRAI1基因PCR扩增图 A:基因OsRAI1原核表达扩增产物;B:基因OsRAI1过表达扩增产物。M为2 000 marker,1-3为扩增产物
Fig. 6 Electropherogram of PCR product for OsRAI1 gene A:Amplified product of gene OsRAI1 via prokaryotic expression; B:Amplified product of gene OsRAI1 via overexpression. M:2 000 marker; 1-3:Amplified product
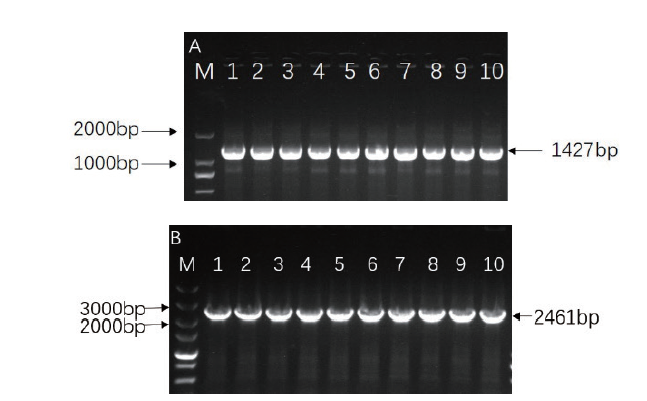
图7 基因OsRAI1表达载体阳性克隆鉴定 A:基因OsRAI1原核表达载体阳性克隆鉴定,M为2000 marker,1-10为单菌落PCR产物;B:基因OsRAI1过表达载体阳性克隆鉴定,M为5000 marker,1-10为单菌落PCR产物
Fig. 7 Identification of positive clones of OsRAI1 gene via vectors A:Identification of positive clones of gene OsRAI1 via prokaryotic expression vector; M:2000 marker. 1-10:PCR product of single colony. B:Identification of positive clones of gene OsRAI1 via overexpression vector; M:5000 marker. 1-10:PCR product of single colony

图8 基因OsRAI1在烟草叶片中亚细胞定位 A:目标蛋白荧光通道;B:叶绿体荧光通道;C:明场;D:叠加图
Fig. 8 Subcellular localization of gene OsRAI1 in the leaves of Nicotiana benthamiana A:Target protein fluorescence channel. B:Chloroplast fluorescence channel. C:Brightfield. D:Overlay chart

图9 不同浓度IPTG诱导重组蛋白表达产物的WB分析 M:Marker;1:1 mmol/L诱导全菌;2:0.8 mmol/L诱导上清;3:0.8 mmol/L诱导全菌;4:0.6 mmol/L诱导上清;5:0.6 mmol/L诱导全菌;6:0.2 mmol/L诱导上清;7:0.2 mmol/L诱导全菌
Fig. 9 WB analysis of recombinant protein expression products induced by different concentrations of IPTG M:Protein marker. 1:Whole bacteria induced by 1 mmol/L. 2:Supernatant induced by 0.8 mmol/L. 3:Whole bacteria induced by 0.8 mmol/L. 4:Supernatant induced by 0.6 mmol/L. 5:Whole bacteria induced by 0.6 mmol/L. 6:Supernatant induced by 0.2 mmol/L. 7:Whole bacteria induced by 0.2 mmol/L

图10 不同诱导时间和温度诱导重组蛋白表达产物的SDS-PAGE分析 M:Protein marker;1:37℃ 3 h诱导全菌;2:37℃ 6 h诱导全菌;3:37℃ 18 h诱导全菌;4:28℃ 3 h诱导全菌;5:28℃ 6 h诱导全菌;6:28℃ 18 h诱导全菌;7:16℃ 3 h诱导全菌;8:16℃ 6 h诱导全菌;9:16℃ 18 h诱导全菌;10:37℃ 3 h诱导上清;11:37℃ 6 h诱导上清;12:37℃ 18 h诱导上清;13:28℃ 3 h诱导上清;14:28℃ 6 h诱导上清;15:28℃ 18 h诱导上清;16:16℃ 3 h诱导上清;17:16℃ 6 h诱导上清;18:16℃ 18 h诱导上清
Fig. 10 SDS-PAGE analysis of recombinant protein expression products induced by different inducing time and temperature M:Protein Marker. 1:Whole bacteria induced by 37℃ for 3 h. 2:Whole bacteria induced by 37℃ for 6 h. 3:Whole bacteria induced by 37℃ for 18 h. 4:Whole bacteria induced by 28℃ for 3 h. 5:Whole bacteria induced by 28℃ for 6 h. 6:Whole bacteria induced by 28℃ for 18 h. 7:Whole bacteria induced by 16℃ for 3 h. 8:Whole bacteria induced by 16℃ for 6 h. 9:Whole bacteria induced by 16℃ for 18 h. 10:Supernatant induced by 37℃ for 3 h. 11:Supernatant induced by 37℃ for 6 h. 12:Supernatant induced by 37℃ for 18 h. 13:Supernatant induced by 28℃ for 3 h. 14:Supernatant induced by 28℃ for 6 h. 15:Supernatant induced by 28℃ for 18 h. 16:Supernatant induced by 16℃ for 3 h. 17:Supernatant induced by 16℃ for 6 h. 18:Supernatant induced by 16℃ for 18 h
| 基因名称 Gene name | 蛋白描述 Protein description | 功能结构域 Functional domain | 氨基酸数/个 Number of amino acids | 互作系数 Interaction coefficient |
|---|---|---|---|---|
| Os05T0586300-00 | Expressed protein;Os03g0137400 protein | HLH | 387 | 0.644 |
| Os04T0486400-01 | OJ000223_09.13 protein;Os04g0486400 protein | SANT | 385 | 0.612 |
| Os03T0137400-01 | Expressed protein;Os03g0137400 protein | transmembrane helix region | 792 | 0.578 |
| Os01T0752500-00 | Putative ethylene responsive protein Os01g0752500 protein | AP2 | 212 | 0.570 |
| Os06T0164400-01 | annotation not available;Os06g0164400 protein | coiled coil region;HLH | 197 | 0.503 |
表2 OsRAI1功能互作蛋白预测
Table 2 Functional interaction protein prediction of OsRAI1
| 基因名称 Gene name | 蛋白描述 Protein description | 功能结构域 Functional domain | 氨基酸数/个 Number of amino acids | 互作系数 Interaction coefficient |
|---|---|---|---|---|
| Os05T0586300-00 | Expressed protein;Os03g0137400 protein | HLH | 387 | 0.644 |
| Os04T0486400-01 | OJ000223_09.13 protein;Os04g0486400 protein | SANT | 385 | 0.612 |
| Os03T0137400-01 | Expressed protein;Os03g0137400 protein | transmembrane helix region | 792 | 0.578 |
| Os01T0752500-00 | Putative ethylene responsive protein Os01g0752500 protein | AP2 | 212 | 0.570 |
| Os06T0164400-01 | annotation not available;Os06g0164400 protein | coiled coil region;HLH | 197 | 0.503 |
| [1] |
de Silva WSI, Perera MMN, Perera KLNS, et al. In silico analysis of osr40c1 promoter sequence isolated from indica variety pokkali[J]. Rice Sci, 2017, 24(4):228-234.
doi: 10.1016/j.rsci.2016.11.002 URL |
| [2] |
Wani SH, Kumar Sah S. Biotechnology and abiotic stress tolerance in rice[J]. J Rice Res, 2014, 2(2):e105. DOI: 10.4172/jrr.1000e105.
doi: 10.4172/jrr.1000e105 |
| [3] | 曾红根. 江西省高产优质直播水稻栽培技术及病虫草害防治探析[J]. 种子科技, 2020, 38(20):98-99. |
| Zeng HG. Analysis on cultivation technology of high yield and high quality direct seeding rice and prevention and control of pests and weeds in jiangxi province[J]. Seed Science & Technology, 2020, 38(20):98-99. | |
| [4] | 汪家旭, 潘沧桑. 潜根线虫的种类[J]. 厦门大学学报:自然科学版, 1999, 38(2):145-152. |
| Wang JX, Pan CS. Studies of rice root Nematodes(Hirschmanniella species)[J]. J Xiamen Univ:Nat Sci, 1999, 38(2):145-152. | |
| [5] |
Elling AA. Major emerging problems with minor Meloidogyne species[J]. Phytopathology, 2013, 103(11):1092-1102.
doi: 10.1094/PHYTO-01-13-0019-RVW pmid: 23777404 |
| [6] | 张绍升, 李茂胜, 严叔平. 水稻潜根线虫的致病性和综合防治技术[J]. 中国水稻科学, 1998, 12(1):31-34. |
| Zhang SS, Li MS, Yan SP. The pathogenicity and integrated control measures of rice root Nematodes[J]. Chin J Rice Sci, 1998, 12(1):31-34. | |
| [7] |
Sun XT, Zhang L, Tang ZQ, et al. Transcriptome analysis of roots from resistant and susceptible rice varieties infected with Hirschmanniella mucronata[J]. FEBS Open Bio, 2019, 9(11):1968-1982.
doi: 10.1002/feb4.v9.11 URL |
| [8] | 于冰, 田烨, 李海英, 等. 植物bHLH转录因子的研究进展[J]. 中国农学通报, 2019, 35(9):75-80. |
| Yu B, Tian Y, Li HY, et al. Research progress of plant bHLH transc-ription factor[J]. Chin Agric Sci Bull, 2019, 35(9):75-80. | |
| [9] |
Li X, Duan X, Jiang H, et al. Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis[J]. Plant Physiol, 2006, 141(4):1167-1184.
doi: 10.1104/pp.106.080580 URL |
| [10] | 刘晓月, 王文生, 傅彬英. 植物bHLH转录因子家族的功能研究进展[J]. 生物技术进展, 2011, 1(6):391-397. |
| Liu XY, Wang WS, Fu BY. Research progress of plant bHLH transcription factor family[J]. Curr Biotechnol, 2011, 1(6):391-397. | |
| [11] |
Thorstensen T, Grini PE, Mercy IS, et al. The Arabidopsis SET-domain protein ASHR3 is involved in stamen development and interacts with the bHLH transcription factor ABORTED MICROSPORES(AMS)[J]. Plant Mol Biol, 2008, 66(1/2):47-59.
doi: 10.1007/s11103-007-9251-y URL |
| [12] | 王艳敏, 白卉, 曹焱. bHLH转录因子研究进展及其在植物抗逆中的应用[J]. 安徽农业科学, 2015, 43(21):34-35, 50. |
| Wang YM, Bai H, Cao Y. Research progress of bHLH transcription factor and application in plant abiotic stress tolerance[J]. J Anhui Agric Sci, 2015, 43(21):34-35, 50. | |
| [13] |
Wang YJ, Zhang ZG, He XJ, et al. A rice transcription factor OsbHLH1 is involved in cold stress response[J]. Theor Appl Genet, 2003, 107(8):1402-1409.
doi: 10.1007/s00122-003-1378-x URL |
| [14] |
Ortolan F, Fonini LS, Pastori T, et al. Tightly controlled expression of OsbHLH35 is critical for anther development in rice[J]. Plant Sci, 2021, 302:110716.
doi: 10.1016/j.plantsci.2020.110716 URL |
| [15] |
Wang L, Ying Y, Narsai R, et al. Identification of OsbHLH133 as a regulator of iron distribution between roots and shoots in Oryza sativa[J]. Plant Cell Environ, 2013, 36(1):224-236.
doi: 10.1111/pce.2013.36.issue-1 URL |
| [16] |
Lee J, Moon S, Jang S, et al. OsbHLH073 negatively regulates internode elongation and plant height by modulating GA homeostasis in rice[J]. Plants, 2020, 9(4):547.
doi: 10.3390/plants9040547 URL |
| [17] | 邵雅芳, 徐非非, 唐富福, 等. 水稻花青素合成相关基因的时空表达研究[J]. 核农学报, 2013, 27(1):9-14. |
| Shao YF, Xu FF, Tang FF, et al. The temporal and spatial expression pattern of anthocyanin related genes in RICE(Oryza sativa L.)[J]. J Nucl Agric Sci, 2013, 27(1):9-14. | |
| [18] |
Carretero-Paulet L, Galstyan A, Roig-Villanova I, et al. Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae[J]. Plant Physiol, 2010, 153(3):1398-1412.
doi: 10.1104/pp.110.153593 pmid: 20472752 |
| [19] |
Chen ZH, Nimmo GA, et al. BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis[J]. Biochem J, 2007, 405(1):191-198.
doi: 10.1042/BJ20070102 URL |
| [20] |
Sun XH, Copeland NG, Jenkins NA, et al. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins[J]. Mol Cell Biol, 1991, 11(11):5603-5611.
pmid: 1922066 |
| [21] | Qin Y, L i X, Guo M, et al. Regulation of salt and ABA responses by CIPK14, a calcium sensor interacting protein kinase in Arabidopsis[J]. Sci China C Life Sci, 2008, 51(5):391-401. |
| [1] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [2] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [3] | 梅欢, 李玥, 刘可蒙, 刘吉华. 小檗碱桥酶高效原核表达及生物合成l-SLR的研究[J]. 生物技术通报, 2023, 39(7): 277-287. |
| [4] | 杨旭妍, 赵爽, 马天意, 白玉, 王玉书. 三个甘蓝WRKY基因的克隆及其对非生物胁迫的表达[J]. 生物技术通报, 2023, 39(11): 261-269. |
| [5] | 郭志浩, 金泽鑫, 刘琦, 高利. 小麦矮腥黑粉菌效应蛋白g11335的生物信息学分析、亚细胞定位及毒性验证[J]. 生物技术通报, 2022, 38(8): 110-117. |
| [6] | 索青青, 吴楠, 杨慧, 李莉, 王锡锋. 水稻咖啡酰辅酶A-O-甲基转移酶基因的原核表达、抗体制备和应用[J]. 生物技术通报, 2022, 38(8): 135-141. |
| [7] | 覃雪晶, 王雨涵, 曹一博, 张凌云. 青杄PwHAP5基因原核表达及多克隆抗体制备[J]. 生物技术通报, 2022, 38(8): 142-149. |
| [8] | 王光丽, 范婵, 王辉, 卢惠芳, 夏灵尹, 黄健, 闵迅. 霍乱弧菌溶血素HlyA的原核表达、纯化及多克隆抗体制备与鉴定[J]. 生物技术通报, 2022, 38(7): 269-277. |
| [9] | 杨佳宝, 周至铭, 张展, 冯丽, 孙黎. 向日葵HaLACS1的克隆、表达及酵母功能互补鉴定[J]. 生物技术通报, 2022, 38(6): 147-156. |
| [10] | 镐青青, 姚圣, 刘佳禾, 陈佩珍, 张梦洋, 季孔庶. 马尾松NAC转录因子基因PmNAC8的克隆及表达分析[J]. 生物技术通报, 2022, 38(4): 202-216. |
| [11] | 赵婷婷, 王俊刚, 王文治, 冯翠莲, 冯小艳, 张树珍. 甘蔗单糖转运蛋白基因ShSTP7序列分析及组织表达特征测定[J]. 生物技术通报, 2022, 38(4): 72-78. |
| [12] | 汪巧菊, 胡雨萌, 温亚亚, 宋丽, 孟闯, 潘志明, 焦新安. 新型冠状病毒S1蛋白的表达及活性鉴定[J]. 生物技术通报, 2022, 38(3): 157-163. |
| [13] | 冯建英, 李立芹, 鲁黎明. 马铃薯bHLH转录因子家族全基因组鉴定与表达分析[J]. 生物技术通报, 2022, 38(2): 21-33. |
| [14] | 郑向, 段左平, 张杰, 潘素君, 戴良英, 刘世名, 李魏. 大豆疫霉菌效应子研究进展[J]. 生物技术通报, 2022, 38(11): 10-20. |
| [15] | 党瑗, 李维, 苗向, 修宇, 林善枝. 山杏油体蛋白基因PsOLE4克隆及其调控油脂累积功能分析[J]. 生物技术通报, 2022, 38(11): 151-161. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||