








生物技术通报 ›› 2022, Vol. 38 ›› Issue (10): 18-28.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1572
赵玉雪1( ), 王芸2, 余璐瑶3, 刘京晶1, 斯金平1, 张新凤1(
), 王芸2, 余璐瑶3, 刘京晶1, 斯金平1, 张新凤1( ), 张磊1,2,3(
), 张磊1,2,3( )
)
收稿日期:2021-12-20
出版日期:2022-10-26
发布日期:2022-11-11
作者简介:赵玉雪,女,硕士研究生,研究方向:药用植物活性成分及质量评价;E-mail:基金资助:
ZHAO Yu-xue1( ), WANG Yun2, YU Lu-yao3, LIU Jing-jing1, SI Jin-ping1, ZHANG Xin-feng1(
), WANG Yun2, YU Lu-yao3, LIU Jing-jing1, SI Jin-ping1, ZHANG Xin-feng1( ), ZHANG Lei1,2,3(
), ZHANG Lei1,2,3( )
)
Received:2021-12-20
Published:2022-10-26
Online:2022-11-11
摘要:
糖基转移酶是一类专门催化糖基化反应的后修饰酶,根据与糖供体连接的受体分子不同,分为O-、C-、N-、S-四种糖基转移酶。近年来,伴随着结构生物学的不断发展,关于C-糖基转移酶的结构解析及定向改造的报道日益增多,为复杂天然产物修饰提供理论依据。本文综述了植物C-糖基转移酶的研究进展,从其底物杂泛性、糖供体结合的空间结构与生物技术应用的角度进行讨论,探究造成底物催化特征的结构基础,为后续通过合成生物学手段异源合成植物天然产物提供参考。
赵玉雪, 王芸, 余璐瑶, 刘京晶, 斯金平, 张新凤, 张磊. 植物中C-糖基转移酶的结构与应用[J]. 生物技术通报, 2022, 38(10): 18-28.
ZHAO Yu-xue, WANG Yun, YU Lu-yao, LIU Jing-jing, SI Jin-ping, ZHANG Xin-feng, ZHANG Lei. Structure and Application of C-glycosyltransferases in Plants[J]. Biotechnology Bulletin, 2022, 38(10): 18-28.
| 序号 Number | 基因名称 Gene name | 基因ID Gene ID | 植物来源 Species | 糖受体 Sugar receptor | 糖供体 Sugar donor | 参考文献 Reference |
|---|---|---|---|---|---|---|
| 1 | OsCGT | CAQ77160 | 水稻Oryza sativa | 黄酮 | UDP-Glc UDP-Ara | [ |
| 2 | UGT708A6 | NP_001132650.1 | 玉米Zea mays | 黄酮 二氢黄酮 | UDP-Glc | [ |
| 3 | FeCGTa(UGT708C1) | BAP90360 | 荞麦Fagopyrum esculentum | 二氢查耳酮 二氢黄酮 | UDP-Glc UDP-Xyl | [ |
| 4 | FeCGTb(UGT708C2) | BAP90361 | 荞麦F. esculentum | 二氢查耳酮 二氢黄酮 | UDP-Glc UDP-Xyl | [ |
| 5 | UGT708D1 | BAR73279 | 大豆Glycine max | 黄烷酮 黄酮 | UDP-Glc | [ |
| 6 | GtUF6CGT1 | BAQ19550 | 三花龙胆Gentiana triflora | 黄酮 | UDP-Glc | [ |
| 7 | MiCGT | ALD83754 | 芒果Mangifera indica | 苯丙素 | UDP-Glc | [ |
| 8 | MiCGTb | AMM73095 | 芒果M. indica | 苯丙素 | UDP-Glc | [ |
| 9 | PlUGT43 | A0A172J2G3.2 | 葛根Pueraria lobata | 异黄酮 | UDP-Glc | [ |
| 10 | FcCGT(UGT708G1) | BBA18062 | 金柑Fortunella crassifolia | 二氢查耳酮 二氢黄酮 | UDP-Glc UDP-Xyl UDP-Gal | [ |
| 11 | CuCGT(UGT708G2) | BBA18063 | 温州蜜柑Citrus unshiu | 二氢查耳酮 二氢黄酮 | UDP-Glc UDP-Xyl UDP-Gal | [ |
| 12 | TcCGT1 | MK644229 | 中国金莲花Trollius chinensis | 黄酮 黄酮醇 二氢查尔酮 | UDP-Xyl UDP-Gal UDP-Ara | [ |
| 13 | WjGT1 | LC465149 | 块茎山萮菜Eutrema japonicum | 黄酮 黄酮醇 二氢查耳酮 | UDP-Glc | [ |
| 14 | GgCGT | MH998596 | 光果甘草Glycyrrhiza glabra | 二氢查耳酮 异黄酮 | UDP-Glc UDP-Xyl UDP-Gal UDP-Ara | [ |
| 15 | OsUGT708A4 | Q5VMG8 | 水稻O. sativa | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 16 | OsUGT708A2 | Q5VME5 | 水稻O. sativa | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 17 | OsUGT708A1 | A2YBW7 | 水稻O. sativa | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 18 | OsUGT708A39 | A2YBW8 | 水稻O. sativa | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 19 | OsUGT708A40 | A2YBX1 | 水稻O. sativa | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 20 | ZmUGT708A5 | B6SWX3 | 玉米Z. mays | 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 21 | ZmUGT708A11 | A0A1D6LXE6 | 玉米Z. mays | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 22 | ZmUGT708A41 | B4FAN3 | 玉米Z. mays | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 23 | ZmUGT708A42 | B4FM13 | 玉米Z. mays | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 24 | SbUGT708A36 | C5Z8Y8 | 高粱Sorghum bicolor | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 25 | SbUGT708A35 | C5Z8Y5 | 高粱S. bicolor | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 26 | SbUGT708A34 | C5Z8Y2 | 高粱S. bicolor | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 27 | SbUGT708A38 | A0A194YGR9 | 高粱S. bicolor | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 28 | TaUGT708A14 | A0A3B6RGT4 | 小麦Triticum aestivum | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 29 | TaUGT708A52 | A0A3B6SEV6 | 小麦T. aestivum | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 30 | TaUGT708A53 | A0A3B6TPG6 | 小麦T. aestivum | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 31 | TaUGT708A15 | A0A3B6RGR9 | 小麦T. aestivum | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 32 | TaUGT708A54 | A0A3B6SB95 | 小麦T. aestivum | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 33 | TaUGT708A55 | A0A3B6TNL8 | 小麦T. aestivum | 二氢黄酮 | UDP-Glc | [ |
| 34 | BdUGT708A7 | I1GYZ6 | 二穗短柄草 Brachypodium distachyon | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 35 | BdUGT708A8 | I1GZD7 | 二穗短柄草B. distachyon | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 36 | SiUGT708A31 | K3XWN4 | 小米Setaria italica | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 37 | SiUGT708A32 | K3XWJ1 | 小米S. italica | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 38 | SiUGT708A33 | K3Y181 | 小米S. italica | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 39 | PhUGT708A43 | MK616588 | 毛竹Phyllostachys heterocycla | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 40 | PhUGT708A46 | MK616589 | 毛竹P. heterocycla | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 41 | PhUGT708A48 | MK616590 | 毛竹P. heterocycla | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 42 | PhUGT708A50 | MK616591 | 毛竹P. heterocycla | 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 43 | PgUGT708A44 | MK616592 | 淡竹P. heterocycla | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 44 | PpUGT708A45 | MK616593 | 高节竹Phyllostachys prominens | 二氢查耳酮 | UDP-Glc | [ |
| 45 | DcaCGT | MT452646 | 铁皮石斛Dendrobium catenatum | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 46 | NnCGT1(UGT708N1) | LOC104598527 | 莲Nelumbo nucifera | 黄酮 | UDP-Glc | [ |
| 47 | NnCGT2(UGT708N2) | LOC104603347 | 莲N. nucifera | 黄酮 | UDP-Ara UDP-Xyl | [ |
| 48 | SbCGT | MK894443 | 黄芩Scutellaria baicalensis | 黄酮 | UDP-Glc UDP-Ara | [ |
| 49 | LpCGT | MK894451 | 兰氏萍Landoltia punctata | 黄酮 | UDP-Glc UDP-Ara | [ |
| 50 | AbCGT | MN747045 | 芦荟Aloe barbadensis | 二氢查尔酮酚 | UDP-Glc | [ |
表1 已有报道的CGT基因研究汇总
Table 1 Summary of reported CGT gene studies
| 序号 Number | 基因名称 Gene name | 基因ID Gene ID | 植物来源 Species | 糖受体 Sugar receptor | 糖供体 Sugar donor | 参考文献 Reference |
|---|---|---|---|---|---|---|
| 1 | OsCGT | CAQ77160 | 水稻Oryza sativa | 黄酮 | UDP-Glc UDP-Ara | [ |
| 2 | UGT708A6 | NP_001132650.1 | 玉米Zea mays | 黄酮 二氢黄酮 | UDP-Glc | [ |
| 3 | FeCGTa(UGT708C1) | BAP90360 | 荞麦Fagopyrum esculentum | 二氢查耳酮 二氢黄酮 | UDP-Glc UDP-Xyl | [ |
| 4 | FeCGTb(UGT708C2) | BAP90361 | 荞麦F. esculentum | 二氢查耳酮 二氢黄酮 | UDP-Glc UDP-Xyl | [ |
| 5 | UGT708D1 | BAR73279 | 大豆Glycine max | 黄烷酮 黄酮 | UDP-Glc | [ |
| 6 | GtUF6CGT1 | BAQ19550 | 三花龙胆Gentiana triflora | 黄酮 | UDP-Glc | [ |
| 7 | MiCGT | ALD83754 | 芒果Mangifera indica | 苯丙素 | UDP-Glc | [ |
| 8 | MiCGTb | AMM73095 | 芒果M. indica | 苯丙素 | UDP-Glc | [ |
| 9 | PlUGT43 | A0A172J2G3.2 | 葛根Pueraria lobata | 异黄酮 | UDP-Glc | [ |
| 10 | FcCGT(UGT708G1) | BBA18062 | 金柑Fortunella crassifolia | 二氢查耳酮 二氢黄酮 | UDP-Glc UDP-Xyl UDP-Gal | [ |
| 11 | CuCGT(UGT708G2) | BBA18063 | 温州蜜柑Citrus unshiu | 二氢查耳酮 二氢黄酮 | UDP-Glc UDP-Xyl UDP-Gal | [ |
| 12 | TcCGT1 | MK644229 | 中国金莲花Trollius chinensis | 黄酮 黄酮醇 二氢查尔酮 | UDP-Xyl UDP-Gal UDP-Ara | [ |
| 13 | WjGT1 | LC465149 | 块茎山萮菜Eutrema japonicum | 黄酮 黄酮醇 二氢查耳酮 | UDP-Glc | [ |
| 14 | GgCGT | MH998596 | 光果甘草Glycyrrhiza glabra | 二氢查耳酮 异黄酮 | UDP-Glc UDP-Xyl UDP-Gal UDP-Ara | [ |
| 15 | OsUGT708A4 | Q5VMG8 | 水稻O. sativa | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 16 | OsUGT708A2 | Q5VME5 | 水稻O. sativa | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 17 | OsUGT708A1 | A2YBW7 | 水稻O. sativa | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 18 | OsUGT708A39 | A2YBW8 | 水稻O. sativa | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 19 | OsUGT708A40 | A2YBX1 | 水稻O. sativa | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 20 | ZmUGT708A5 | B6SWX3 | 玉米Z. mays | 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 21 | ZmUGT708A11 | A0A1D6LXE6 | 玉米Z. mays | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 22 | ZmUGT708A41 | B4FAN3 | 玉米Z. mays | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 23 | ZmUGT708A42 | B4FM13 | 玉米Z. mays | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 24 | SbUGT708A36 | C5Z8Y8 | 高粱Sorghum bicolor | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 25 | SbUGT708A35 | C5Z8Y5 | 高粱S. bicolor | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 26 | SbUGT708A34 | C5Z8Y2 | 高粱S. bicolor | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 27 | SbUGT708A38 | A0A194YGR9 | 高粱S. bicolor | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 28 | TaUGT708A14 | A0A3B6RGT4 | 小麦Triticum aestivum | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 29 | TaUGT708A52 | A0A3B6SEV6 | 小麦T. aestivum | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 30 | TaUGT708A53 | A0A3B6TPG6 | 小麦T. aestivum | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 31 | TaUGT708A15 | A0A3B6RGR9 | 小麦T. aestivum | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 32 | TaUGT708A54 | A0A3B6SB95 | 小麦T. aestivum | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 33 | TaUGT708A55 | A0A3B6TNL8 | 小麦T. aestivum | 二氢黄酮 | UDP-Glc | [ |
| 34 | BdUGT708A7 | I1GYZ6 | 二穗短柄草 Brachypodium distachyon | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 35 | BdUGT708A8 | I1GZD7 | 二穗短柄草B. distachyon | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 36 | SiUGT708A31 | K3XWN4 | 小米Setaria italica | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 37 | SiUGT708A32 | K3XWJ1 | 小米S. italica | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 38 | SiUGT708A33 | K3Y181 | 小米S. italica | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 39 | PhUGT708A43 | MK616588 | 毛竹Phyllostachys heterocycla | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 40 | PhUGT708A46 | MK616589 | 毛竹P. heterocycla | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 41 | PhUGT708A48 | MK616590 | 毛竹P. heterocycla | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 42 | PhUGT708A50 | MK616591 | 毛竹P. heterocycla | 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 43 | PgUGT708A44 | MK616592 | 淡竹P. heterocycla | 二氢黄酮 二氢查耳酮 | UDP-Glc UDP-Ara | [ |
| 44 | PpUGT708A45 | MK616593 | 高节竹Phyllostachys prominens | 二氢查耳酮 | UDP-Glc | [ |
| 45 | DcaCGT | MT452646 | 铁皮石斛Dendrobium catenatum | 二氢黄酮 二氢查耳酮 | UDP-Glc | [ |
| 46 | NnCGT1(UGT708N1) | LOC104598527 | 莲Nelumbo nucifera | 黄酮 | UDP-Glc | [ |
| 47 | NnCGT2(UGT708N2) | LOC104603347 | 莲N. nucifera | 黄酮 | UDP-Ara UDP-Xyl | [ |
| 48 | SbCGT | MK894443 | 黄芩Scutellaria baicalensis | 黄酮 | UDP-Glc UDP-Ara | [ |
| 49 | LpCGT | MK894451 | 兰氏萍Landoltia punctata | 黄酮 | UDP-Glc UDP-Ara | [ |
| 50 | AbCGT | MN747045 | 芦荟Aloe barbadensis | 二氢查尔酮酚 | UDP-Glc | [ |
| 蛋白质ID PDB ID | 描述Description | 植物来源Species | 参考文献Reference |
|---|---|---|---|
| 6JTD | TcCGT1与UDP复合的晶体结构 | 中国金莲花T. chinensis | [ |
| 6L5P | GgCGT与UDP-Glu复合物的晶体结构 | 光果甘草G. glabra | [ |
| 6LLW | UGT708C1与UDP复合的晶体结构 | 荞麦F. esculentum | [ |
| 6LF6 | ZmCGTa与UDP复合的晶体结构 | 玉米Z. mays | [ |
| 6LG0 | SbCGTa与UDP复合的晶体结构 | 黄芩S. baicalensis | [ |
| 6LG1 | LpCGTa与UDP复合物的晶体结构 | 兰氏萍L. punctata | [ |
表2 已有解析晶体结构的CGT蛋白
Table 2 Resolved crystal structures of available CGT proteins
| 蛋白质ID PDB ID | 描述Description | 植物来源Species | 参考文献Reference |
|---|---|---|---|
| 6JTD | TcCGT1与UDP复合的晶体结构 | 中国金莲花T. chinensis | [ |
| 6L5P | GgCGT与UDP-Glu复合物的晶体结构 | 光果甘草G. glabra | [ |
| 6LLW | UGT708C1与UDP复合的晶体结构 | 荞麦F. esculentum | [ |
| 6LF6 | ZmCGTa与UDP复合的晶体结构 | 玉米Z. mays | [ |
| 6LG0 | SbCGTa与UDP复合的晶体结构 | 黄芩S. baicalensis | [ |
| 6LG1 | LpCGTa与UDP复合物的晶体结构 | 兰氏萍L. punctata | [ |
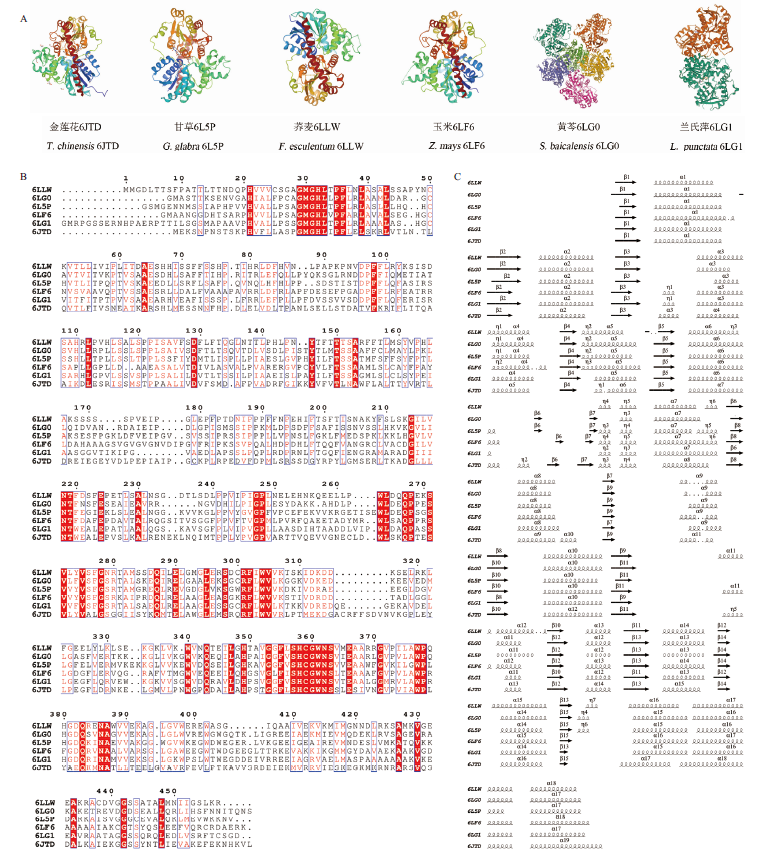
图3 已有结构解析的植物CGT的蛋白结构 A为已有结构解析的植物CGT的晶体结构;B为蛋白序列比对结果;C为蛋白二级结构的结构域比对
Fig. 3 Protein crystal structure of structurally resolved plant CGT A shows the crystal structure of plant CGT with structure resolution. B shows the results of protein sequence comparison. C shows the structural domain comparison of protein secondary structure
| [1] |
Jones P, Vogt T. Glycosyltransferases in secondary plant metabolism:tranquilizers and stimulant controllers[J]. Planta, 2001, 213(2):164-174.
pmid: 11469580 |
| [2] | 姬向楠, 何非, 段长青, 等. 植物UDP-糖基转移酶生化特性和功能研究进展[J]. 食品科学, 2013, 34(9):316-323. |
| Ji XN, He F, Duan CQ, et al. Recent progress in biochemical properties and functions of UDP-glycosyl transferase during plant secondary metabolism[J]. Food Sci, 2013, 34(9):316-323. | |
| [3] |
Lim EK, Baldauf S, Li Y, et al. Evolution of substrate recognition across a multigene family of glycosyltransferases in Arabidopsis[J]. Glycobiology, 2003, 13(3):139-145.
doi: 10.1093/glycob/cwg017 URL |
| [4] | Lim EK. Plant glycosyltransferases:their potential as novel biocatalysts[J]. Chemistry, 2005, 11(19):5486-5494. |
| [5] | 秦晶晶, 孙春玉, 张美萍, 等. 植物UDP-糖基转移酶分类、功能以及进化[J]. 基因组学与应用生物学, 2018, 37(1):440-450. |
| Qin JJ, Sun CY, Zhang MP, et al. Classification, function and evolution of plant UDP-glycosyltransferase[J]. Genom Appl Biol, 2018, 37(1):440-450. | |
| [6] |
Cobucci-Ponzano B, Moracci M. Glycosynthases as tools for the production of glycan analogs of natural products[J]. Nat Prod Rep, 2012, 29(6):697-709.
doi: 10.1039/c2np20032e pmid: 22504390 |
| [7] |
Gantt RW, Peltier-Pain P, Thorson JS. Enzymatic methods for glyco(diversification/randomization)of drugs and small molecules[J]. Nat Prod Rep, 2011, 28(11):1811-1853.
doi: 10.1039/c1np00045d URL |
| [8] | 冉军舰, 梁新红, 陈晓静, 等. 苹果根皮苷-2-O-糖基转移酶基因克隆与表达模式分析[J]. 食品科学, 2019, 40(6):202-207. |
| Ran JJ, Liang XH, Chen XJ, et al. Cloning and expression pro? le analysis of the phloridzin 2’-O-glycosyltransferase gene in apple[J]. Food Sci, 2019, 40(6):202-207. | |
| [9] |
Cui LL, Yao SB, Dai XL, et al. Identification of UDP-glycosyltransferases involved in the biosynthesis of astringent taste compounds in tea(Camellia sinensis)[J]. J Exp Bot, 2016, 67(8):2285-2297.
doi: 10.1093/jxb/erw053 URL |
| [10] |
Zhao MY, Zhang N, Gao T, et al. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants[J]. New Phytol, 2020, 226(2):362-372.
doi: 10.1111/nph.16364 pmid: 31828806 |
| [11] |
Martin RC, Mok MC, Mok DW. A gene encoding the cytokinin enzyme Zeatin O-xylosyltransferase of Phaseolus vulgaris[J]. Plant Physiol, 1999, 120(2):553-558.
pmid: 10364407 |
| [12] |
Vogt T, Jones P. Glycosyltransferases in plant natural product synthesis:characterization of a supergene family[J]. Trends Plant Sci, 2000, 5(9):380-386.
pmid: 10973093 |
| [13] |
Jones P, Messner B, Nakajima JI, et al. UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana[J]. J Biol Chem, 2003, 278(45):43910-43918.
doi: 10.1074/jbc.M303523200 URL |
| [14] |
Sawada S, Suzuki H, Ichimaida F, et al. Udp-glucuronic acid:anthocyanin glucuronosyltransferase from red daisy(Bellis perennis)flowers:enzymology and phylogenetics of a novel glucuronosyltransferase involved in flower pigment biosynthesis[J]. J Biol Chem, 2005, 280(2):899-906.
doi: 10.1074/jbc.M410537200 URL |
| [15] |
Bowles D, Isayenkova J, Lim EK, et al. Glycosyltransferases:managers of small molecules[J]. Curr Opin Plant Biol, 2005, 8(3):254-263.
doi: 10.1016/j.pbi.2005.03.007 URL |
| [16] |
Campbell JA, Davies GJ, et al. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities[J]. Biochem J, 1998, 329(Pt 3)(Pt 3):719.
doi: 10.1042/bj3290719 URL |
| [17] |
Breton C, Snajdrová L, Jeanneau C, et al. Structures and mechanisms of glycosyltransferases[J]. Glycobiology, 2006, 16(2):29R-37R.
pmid: 16037492 |
| [18] |
Tam HK, Härle J, Gerhardt S, et al. Structural characterization of O- and C-glycosylating variants of the landomycin glycosyltransferase LanGT2[J]. Angew Chem Int Ed Engl, 2015, 54(9):2811-2815.
doi: 10.1002/anie.201409792 URL |
| [19] |
Song CK, Gu L, Liu JY, et al. Functional characterization and substrate promiscuity of UGT71 glycosyltransferases from strawberry(Fragaria×ananassa)[J]. Plant Cell Physiol, 2015, 56(12):2478-2493.
doi: 10.1093/pcp/pcv151 URL |
| [20] |
Rahimi S, Kim J, Mijakovic I, et al. Triterpenoid-biosynthetic UDP-glycosyltransferases from plants[J]. Biotechnol Adv, 2019, 37(7):107394.
doi: 10.1016/j.biotechadv.2019.04.016 URL |
| [21] | Zhao MY, Cai BB Jin JY, et al. Cold stress-induced glucosyltransferase CsUGT78A15 is involved in the formation of eugenol glucoside in Camellia sinensis[J]. Hortic Plant J, 2020, 6(6):11. |
| [22] |
Martin RC, Mok DWS, Smets R, et al. Development of transgenic tobacco harboring a Zeatin O-glucosyltransferase gene from Phaseolus[J]. Vitro Cell Dev Biol Plant, 2001, 37(3):354-360.
doi: 10.1007/s11627-001-0063-5 URL |
| [23] |
Xu C, Liberatore KL, MacAlister CA, et al. A cascade of Arabinosyltransferases controls shoot meristem size in tomato[J]. Nat Genet, 2015, 47(7):784-792.
doi: 10.1038/ng.3309 pmid: 26005869 |
| [24] |
Li Y, Lin HX, Wang J, et al. Glucosyltransferase capable of catalyzing the last step in neoandrographolide biosynthesis[J]. Org Lett, 2018, 20(19):5999-6002.
doi: 10.1021/acs.orglett.8b02146 pmid: 30234309 |
| [25] |
Hou BK, Lim EK, Higgins GS, et al. N-glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana[J]. J Biol Chem, 2004, 279(46):47822-47832.
doi: 10.1074/jbc.M409569200 URL |
| [26] |
Li W, Zhang FX, Chang YW, et al. Nicotinate O-glucosylation is an evolutionarily metabolic trait important for seed germination under stress conditions in Arabidopsis thaliana[J]. Plant Cell, 2015, 27(7):1907-1924.
doi: 10.1105/tpc.15.00223 URL |
| [27] |
Yin QG, Zhang J, Wang SH, et al. N-glucosyltransferase GbNGT1 from Ginkgo complement the auxin metabolic pathway[J]. Hortic Res, 2021, 8(1):229.
doi: 10.1038/s41438-021-00658-0 URL |
| [28] |
Grubb CD, Zipp BJ, Ludwig-Müller J, et al. Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis[J]. Plant J, 2004, 40(6):893-908.
doi: 10.1111/j.1365-313X.2004.02261.x URL |
| [29] | 李玉, 路福平, 王正祥. 功能性低聚糖合成中糖基转移酶研究进展[J]. 食品科学, 2013, 34(9):358-363. |
| Li Y, Lu FP, Wang ZX. Recent advance in research and application of several glycosyltransferases in the synthesis of functional oligosaccharides[J]. Food Sci, 2013, 34(9):358-363. | |
| [30] |
Kitamura K, Ando Y, Matsumoto T, et al. Total synthesis of aryl C-glycoside natural products:strategies and tactics[J]. Chem Rev, 2018, 118(4):1495-1598.
doi: 10.1021/acs.chemrev.7b00380 pmid: 29281269 |
| [31] |
Sato S, Koide T. Synthesis of vicenin-1 and 3, 6, 8- and 8, 6-di-C-β-d-(glucopyranosyl-xylopyranosyl)-4’, 5, 7-trihydroxyflavones using two direct C-glycosylations of naringenin and phloroacetophenone with unprotected d-glucose and d-xylose in aqueous solution as the key reactions[J]. Carbohydr Res, 2010, 345(13):1825-1830.
doi: 10.1016/j.carres.2010.04.001 URL |
| [32] |
Kitamura K, Maezawa Y, Ando Y, et al. Synthesis of the pluramycins 2:total synthesis and structure assignment of saptomycin B[J]. Angew Chem Int Ed Engl, 2014, 53(5):1262-1265.
doi: 10.1002/anie.201308017 URL |
| [33] |
Kitamura K, Ando Y, Matsumoto T, et al. Synthesis of the pluramycins 1:two designed anthrones as enabling platforms for flexible bis-C-glycosylation[J]. Angew Chem Int Ed Engl, 2014, 53(5):1258-1261.
doi: 10.1002/anie.201308016 URL |
| [34] |
Ho TC, Kamimura H, Ohmori K, et al. Total synthesis of(+)-vicenin-2[J]. Org Lett, 2016, 18(18):4488-4490.
doi: 10.1021/acs.orglett.6b02203 URL |
| [35] |
Brazier-Hicks M, Evans KM, Gershater MC, et al. The C-glycosylation of flavonoids in cereals[J]. J Biol Chem, 2009, 284(27):17926-17934.
doi: 10.1074/jbc.M109.009258 pmid: 19411659 |
| [36] |
Falcone Ferreyra ML, Rodriguez E, Casas MI, et al. Identification of a bifunctional maize C- and O-glucosyltransferase[J]. J Biol Chem, 2013, 288(44):31678-31688.
doi: 10.1074/jbc.M113.510040 pmid: 24045947 |
| [37] |
Nagatomo Y, Usui S, Ito T, et al. Purification, molecular cloning and functional characterization of flavonoid C-glucosyltransferases from Fagopyrum esculentum M. (buckwheat)Cotyledon[J]. Plant J, 2014, 80(3):437-448.
doi: 10.1111/tpj.12645 URL |
| [38] |
Hirade Y, Kotoku N, Terasaka K, et al. Identification and functional analysis of 2-hydroxyflavanone C-glucosyltransferase in soybean(Glycine max)[J]. FEBS Lett, 2015, 589(15):1778-1786.
doi: 10.1016/j.febslet.2015.05.010 URL |
| [39] |
Sasaki N, Nishizaki Y, Yamada E, et al. Identification of the glucosyltransferase that mediates direct flavone C-glucosylation in Gentiana triflora[J]. FEBS Lett, 2015, 589(1):182-187.
doi: 10.1016/j.febslet.2014.11.045 URL |
| [40] |
Chen DW, Fan S, Chen RD, et al. Probing and engineering key residues for bis-C-glycosylation and promiscuity of a C-glycosyltransferase[J]. ACS Catal, 2018, 8(6):4917-4927.
doi: 10.1021/acscatal.8b00376 URL |
| [41] |
Wang X, Li CF, Zhou C, et al. Molecular characterization of the C-glucosylation for puerarin biosynthesis in Pueraria Lobata[J]. Plant J, 2017, 90(3):535-546.
doi: 10.1111/tpj.13510 URL |
| [42] | Ito T, Fujimoto S, Suito F, et al. C-Glycosyltransferases catalyzing the formation of di-C-glucosyl flavonoids in Citrus plants[J]. PlantJ, 2017, 91(2):187-198. |
| [43] |
He JB, Zhao P, Hu ZM, et al. Molecular and structural characterization of a promiscuous C-glycosyltransferase from Trollius chinensis[J]. Angew Chem Int Ed Engl, 2019, 58(33):11513-11520.
doi: 10.1002/anie.201905505 URL |
| [44] |
Mashima K, Hatano M, Suzuki H, et al. Identification and characterization of apigenin 6-C-glucosyltransferase involved in biosynthesis of isosaponarin in wasabi(Eutrema japonicum)[J]. Plant Cell Physiol, 2019, 60(12):2733-2743.
doi: 10.1093/pcp/pcz164 pmid: 31418788 |
| [45] |
Zhang M, Li FD, Li K, et al. Functional characterization and structural basis of an efficient di- C-glycosyltransferase from Glycyrrhiza glabra[J]. J Am Chem Soc, 2020, 142(7):3506-3512.
doi: 10.1021/jacs.9b12211 pmid: 31986016 |
| [46] |
Sun YW, Chen Z, Yang JY, et al. Pathway-specific enzymes from bamboo and crop leaves biosynthesize anti-nociceptive C-glycosylated flavones[J]. Commun Biol, 2020, 3(1):110.
doi: 10.1038/s42003-020-0834-3 pmid: 32144397 |
| [47] |
Ren ZY, Ji XY, Jiao ZB, et al. Functional analysis of a novel C-glycosyltransferase in the orchid Dendrobium catenatum[J]. Hortic Res, 2020, 7:111.
doi: 10.1038/s41438-020-0330-4 URL |
| [48] |
Feng CY, Li SS, Taguchi G, et al. Enzymatic basis for stepwise C-glycosylation in the formation of flavonoid di-C-glycosides in sacred Lotus(Nelumbo nucifera Gaertn.)[J]. Plant J, 2021, 106(2):351-365.
doi: 10.1111/tpj.15168 URL |
| [49] |
Wang ZL, Gao HM, Wang S, et al. Dissection of the general two-step di- C-glycosylation pathway for the biosynthesis of(Iso)schaftosides in higher plants[J]. Proc Natl Acad Sci USA, 2020, 117(48):30816-30823.
doi: 10.1073/pnas.2012745117 URL |
| [50] |
Xie KB, Zhang XL, Sui SY, et al. Exploring and applying the substrate promiscuity of a C-glycosyltransferase in the chemo-enzymatic synthesis of bioactive C-glycosides[J]. Nat Commun, 2020, 11(1):5162.
doi: 10.1038/s41467-020-18990-9 pmid: 33056984 |
| [51] |
Li J, Yang JG, Mu SC, et al. Efficient O-glycosylation of triterpenes enabled by protein engineering of plant glycosyltransferase UGT74AC1[J]. ACS Catal, 2020, 10(6):3629-3639.
doi: 10.1021/acscatal.9b05232 URL |
| [52] |
Liu MZ, Wang DD, Li Y, et al. Crystal structures of the C-glycosyltransferase UGT708C1 from buckwheat provide insights into the mechanism of C-glycosylation[J]. Plant Cell, 2020, 32(9):2917-2931.
doi: 10.1105/tpc.20.00002 URL |
| [53] | 刘美子, 王丹丹, 秦超, 等. 植物糖基转移酶的结构与机理及糖基化工程的研究进展[J]. 中国科学:生命科学, 2019, 49(9):1133-1142. |
|
Liu MZ, Wang DD, Qin C, et al. Research progress in understanding the structure, mechanism, and engineering of plant glycosyltransferases[J]. Sci Sin Vitae, 2019, 49(9):1133-1142.
doi: 10.1360/SSV-2019-0164 URL |
|
| [54] |
Coutinho PM, Deleury E, Davies GJ, et al. An evolving hierarchical family classification for glycosyltransferases[J]. J Mol Biol, 2003, 328(2):307-317.
pmid: 12691742 |
| [55] |
Wilson AE, Tian L. Phylogenomic analysis of UDP-dependent glycosyltransferases provides insights into the evolutionary landscape of glycosylation in plant metabolism[J]. Plant J, 2019, 100(6):1273-1288.
doi: 10.1111/tpj.14514 URL |
| [56] |
Jadhav SKR, Patel KA, Dholakia BB, et al. Structural characterization of a flavonoid glycosyltransferase from withania somnifera[J]. Bioinformation, 2012, 8(19):943-949.
doi: 10.6026/97320630008943 pmid: 23144555 |
| [57] | Yin QG, Shen GA, Chang ZZ, et al. Involvement of three putative glucosyltransferases from the UGT72 family in flavonol glucoside/rhamnoside biosynthesis in Lotus japonicus seeds[J]. J Exp Bot, 2017, 68(3):597-612. |
| [58] |
Wang XQ. Structure, mechanism and engineering of plant natural product glycosyltransferases[J]. FEBS Lett, 2009, 583(20):3303-3309.
doi: 10.1016/j.febslet.2009.09.042 pmid: 19796637 |
| [59] |
Xiao JB, Capanoglu E, Jassbi AR, et al. Advance on the flavonoid C-glycosides and health benefits[J]. Crit Rev Food Sci Nutr, 2016, 56(Suppl 1):S29-S45.
doi: 10.1080/10408398.2015.1067595 URL |
| [60] |
Gorzalczany S, Marrassini C, Miño J, et al. Antinociceptive activity of ethanolic extract and isolated compounds of Urtica circularis[J]. J Ethnopharmacol, 2011, 134(3):733-738.
doi: 10.1016/j.jep.2011.01.025 pmid: 21277970 |
| [61] |
Negri G, Mattei R, Mendes FR. Antinociceptive activity of the HPLC- and MS-standardized hydroethanolic extract of Pterodon emarginatus Vogel leaves[J]. Phytomedicine, 2014, 21(8/9):1062-1069.
doi: 10.1016/j.phymed.2014.04.009 URL |
| [62] |
Borghi SM, Carvalho TT, Staurengo-Ferrari L, et al. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines[J]. J Nat Prod, 2013, 76(6):1141-1149.
doi: 10.1021/np400222v pmid: 23742617 |
| [63] |
Zhu Q, Mao LN, Liu CP, et al. Antinociceptive effects of vitexin in a mouse model of postoperative pain[J]. Sci Rep, 2016, 6:19266.
doi: 10.1038/srep19266 pmid: 26763934 |
| [64] |
Liu B, Wu ZY, Li YP, et al. Puerarin prevents cardiac hypertrophy induced by pressure overload through activation of autophagy[J]. Biochem Biophys Res Commun, 2015, 464(3):908-915.
doi: 10.1016/j.bbrc.2015.07.065 URL |
| [65] | 吴佳茜, 王旻, 陈代杰, 等. 糖基转移酶定向进化研究进展[J]. 生命科学, 2009, 21(3):388-393. |
| Wu JQ, Wang M, Chen DJ, et al. Research advances in directed evolution of glycosyltransferase[J]. Chin Bull Life Sci, 2009, 21(3):388-393. | |
| [66] |
Wen ZX, Zhang ZM, Zhong L, et al. Directed evolution of a plant glycosyltransferase for chemo- and regioselective glycosylation of pharmaceutically significant flavonoids[J]. ACS Catal, 2021, 11(24):14781-14790.
doi: 10.1021/acscatal.1c04191 URL |
| [1] | 王佳蕊, 孙培媛, 柯瑾, 冉彬, 李洪有. 苦荞糖基转移酶基因FtUGT143的克隆及表达分析[J]. 生物技术通报, 2023, 39(8): 204-212. |
| [2] | 王玲, 卓燊, 付学森, 刘紫璇, 刘笑蓉, 王志辉, 周日宝, 刘湘丹. 莲生物碱生物合成途径及相关基因研究进展[J]. 生物技术通报, 2023, 39(7): 56-66. |
| [3] | 成婷, 苑帅, 张晓元, 林良才, 李欣, 张翠英. 酿酒酵母异丁醇合成途径调控的研究进展[J]. 生物技术通报, 2023, 39(7): 80-90. |
| [4] | 王晓梅, 杨小薇, 李辉尚, 何微, 辛竹琳. 全球合成生物学发展现状及对我国的启示[J]. 生物技术通报, 2023, 39(2): 292-302. |
| [5] | 陈晓琳, 刘洋儿, 许文涛, 郭明璋, 刘慧琳. 合成生物学细胞传感技术在食品安全快速检测中的应用[J]. 生物技术通报, 2023, 39(1): 137-149. |
| [6] | 周琳, 梁轩铭, 赵磊. 天然类胡萝卜素的生物合成研究进展[J]. 生物技术通报, 2022, 38(7): 119-127. |
| [7] | 郭晓真, 张学福. 植物合成生物学领域发展态势的文献计量分析[J]. 生物技术通报, 2022, 38(2): 289-296. |
| [8] | 叶敏, 高教琪, 周雍进. 非常规酵母细胞工厂合成天然产物[J]. 生物技术通报, 2021, 37(8): 12-24. |
| [9] | 周正, 李卿, 陈万生, 张磊. 药用植物天然产物生物合成途径及关键催化酶的研究策略[J]. 生物技术通报, 2021, 37(8): 25-34. |
| [10] | 叶健文, 陈江楠, 张旭, 吴赴清, 陈国强. 动态调控:一种高效的细胞工厂工程化代谢改造策略[J]. 生物技术通报, 2020, 36(6): 1-12. |
| [11] | 常瀚文, 郑鑫铃, 骆健美, 王敏, 申雁冰. 抗逆元件及其在高效微生物细胞工厂构建中的应用进展[J]. 生物技术通报, 2020, 36(6): 13-34. |
| [12] | 张慧, 田方方, 吴毅. 合成型酵母基因组重排技术[J]. 生物技术通报, 2020, 36(4): 13-18. |
| [13] | 曹燕亭, 刘延峰, 李江华, 刘龙, 堵国成. 基于细胞亚群调控提升生物合成效率的研究进展[J]. 生物技术通报, 2020, 36(4): 19-25. |
| [14] | 李佳秀, 蔡倩茹, 吴杰群. 萜类化合物在酿酒酵母中的合成生物学研究进展[J]. 生物技术通报, 2020, 36(12): 199-207. |
| [15] | 贺越, 赵圣国, 张晓音, 郑楠, 王加启. 细菌脲酶蛋白结构与催化机制[J]. 生物技术通报, 2020, 36(12): 208-217. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||