








生物技术通报 ›› 2023, Vol. 39 ›› Issue (3): 163-175.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0753
收稿日期:2022-06-21
出版日期:2023-03-26
发布日期:2023-04-10
通讯作者:
郝林,男,博士,教授,研究方向:植物逆境生物学;E-mail:haolinwj2001@163.com作者简介:王琪,女,硕士研究生,研究方向:植物与微生物相互作用;E-mail:2239960188@qq.com
基金资助:
WANG Qi( ), HU Zhe, FU Wei, LI Guang-zhe, HAO Lin(
), HU Zhe, FU Wei, LI Guang-zhe, HAO Lin( )
)
Received:2022-06-21
Published:2023-03-26
Online:2023-04-10
摘要:
干旱是影响农业生产的主要因子之一。根际促生菌可有效减轻干旱对植物的伤害,但其中的作用机理仍需探究。以接种Burkholderia sp. GD17菌和未接种的黄瓜幼苗为材料,经干旱处理(撤水7 d)后,分析其生理生化和相关基因表达,以此探究GD17菌促生拮抗的作用机理。结果表明,接菌5 d的植株根中GD17菌数量达到6.2×106 CFU/g鲜重,直至第15天撤水处理时仍维持这一数量级。正常浇水的加菌植株地上部鲜重与干重分别较未加菌对照高39%和36%;在干旱胁迫下,分别高38%和32%,叶片相对含水量高8.5%。干旱胁迫下,加菌叶片丙二醛和电解质渗透率分别较未加菌的低45%和26%。正常浇水的加菌植株叶片超氧化物歧化酶、过氧化物酶和过氧化氢酶活性均显著低于未加菌植株的水平,而在干旱胁迫下,加菌植株的3种抗氧化酶活性明显高于未加菌的。干旱显著提高叶片的脯氨酸含量,其中,加菌植株的幅度更大。正常浇水的加菌植株净光合速率高于未加菌植株,但气孔导度、胞间二氧化碳浓度和蒸腾速率没有明显变化,但在干旱条件下,这些参数在加菌植株中的值显著高于未加菌植株。叶绿素荧光成像进一步显示,接种GD17可有效减轻干旱对光合机构的损伤和光合效率的抑制。抗氧化、脯氨酸合成和转录因子等相关基因的表达受干旱诱导,且多数在加菌植株中的幅度更大。GD17菌可有效促进黄瓜幼苗生长和对干旱的耐受性。其可能机理包括增强抗氧化防卫、减轻光合作用损伤、提高渗透物质合成和细胞保水力、上调转录因子基因表达等。GD17菌在黄瓜农业生产中具有潜在的应用价值。
王琪, 胡哲, 富薇, 李光哲, 郝林. 伯克霍尔德氏菌GD17对黄瓜幼苗耐干旱的调节[J]. 生物技术通报, 2023, 39(3): 163-175.
WANG Qi, HU Zhe, FU Wei, LI Guang-zhe, HAO Lin. Regulation of Burkholderia sp. GD17 on the Drought Tolerance of Cucumber Seedlings[J]. Biotechnology Bulletin, 2023, 39(3): 163-175.
| 基因Gene | 基因ID Gene ID | 功能Function | 引物序列Primer sequence(5'-3') | 产物长度Product length/bp |
|---|---|---|---|---|
| ACT7 | Csa6G484600 | 肌动蛋白 Actin | F:TGAACTGAGATTGGTTGGCGT R:TTGCCCAAATCTGGAGGGTC | 178 |
| EF1α | Csa6G023009 | 延长因子 Elongation factor | F:CAGACAAGCCACTCCGTCTT R:GCCTCGGGTAGAGATTCGTG | 181 |
| TUA | Csa4G000580 | 微管蛋白 Tubulin | F:CTCCCTCCTTTTGGAGCGTT R:GAAGCACAGCAACGTCAGTG | 161 |
| Cu/Zn-SOD1 | Csa2G013250 | 铜/锌-超氧化物歧化酶 Cu/Zn-superoxide dismutase | F:GCCACATTTCAACCCTGCTG R:GTCCACCCTTGCCAAGATCA | 209 |
| Mn-SOD | Csa1G025980 | 锰-超氧化物歧化酶 Mn-superoxide dismutase | F:AGAAGCTCCCCTGGTTGAGA R:CTCTCGTGGTCTCACGCATT | 200 |
| POD25 | Csa1G019820 | 过氧化物酶 Peroxidase | F:CGAGCCCATCGATAACCACA R:TCTTTGTCCATGGCCACTCC | 243 |
| CAT1 | Csa4G658590 | 过氧化氢酶 Catalase | F:CCGAGAGGTATCCTCACCCA R:AAATGCTTGGCCTCACGTTG | 270 |
| APX | Csa1G479610 | 抗坏血酸过氧化物酶 Ascorbate peroxidase | F:TTGCCTGATGCTACCAAGGG R:TCGTTCCTTGTGTGCCCTAC | 123 |
| GR | Csa7G378460 | 谷胱甘肽还原酶 Glutathione reductase | F:GTGGCATTGTGGTTCGTTCC R:CACCTCCAGCACTATCGGAC | 189 |
| MDAR1 | Csa6G451470 | 单脱氢抗坏血酸还原酶 Dehydroascorbate reductase | F:TGGGCGATGTGGCTACTTTT R:TAAGACTGCGTCGCCAACAT | 221 |
| P5CS | Csa3G733920 | 脯氨酸合成关键酶 A key enzyme in proline synthesis | F:CCAAGAATGCAAGGCGTATCG R:CAACAGCTGCACATGCCTTT | 264 |
| P5CR | Csa4G354630 | 脯氨酸合成关键酶 A key enzyme in proline synthesis | F:GGTTGAGCCGTTACTGTGGA R:TCCAGCTCCGATGAACCCTA | 126 |
| NAC35 | Csa3G852470 | 转录因子 Transcription factor | F:GGTCATCGTCCACGTGTTCT R:GCCTGAGACTGAGCAAGAGG | 250 |
| NAC41 | Csa4G361820 | 转录因子 Transcription factor | F:AGGGGGCAATCGAGAAACAG R:TGAACTCCGATGACACCACG | 252 |
| NAC66 | Csa6G382950 | 转录因子 Transcription factor | F:GGCGATGTGTTAATGCCGTC R:TCCTTCCATTTTCGCTCGCT | 164 |
| WRKY18 | Csa3G116700 | 转录因子 Transcription factor | F:AGCGATGTTGATGTGCTGGA R:GTAAAGGGTTCGTGCTCCGA | 233 |
| WRKY51 | Csa6G486960 | 转录因子 Transcription factor | F:GCAAGCCAAAACCAACGAGT R:GGACAACGAAAACGTGGTGG | 236 |
| DREB2A | Csa4G023742 | 干旱应答因子 Dehydration-responsive factor | F:ATGGCTTGGCACTTTCTCCA R:ACTTTCACCTTCAGTTCCTCCA | 285 |
| DREB2C | Csa6G004870 | 干旱应答因子 Dehydration-responsive factor | F:GGAGTAGGCTTTGGCTTGGT R:GTGAACTCCTCAGGCACACA | 259 |
| DREB2D | Csa2G363010 | 干旱应答因子 Dehydration-responsive factor | F:GAACCAAATCGTGGTGCTCG R:AGCAACATCTTCCACCGTAGG | 258 |
表1 基因表达分析特异性引物
Table 1 Specific primers for analyzing gene expression
| 基因Gene | 基因ID Gene ID | 功能Function | 引物序列Primer sequence(5'-3') | 产物长度Product length/bp |
|---|---|---|---|---|
| ACT7 | Csa6G484600 | 肌动蛋白 Actin | F:TGAACTGAGATTGGTTGGCGT R:TTGCCCAAATCTGGAGGGTC | 178 |
| EF1α | Csa6G023009 | 延长因子 Elongation factor | F:CAGACAAGCCACTCCGTCTT R:GCCTCGGGTAGAGATTCGTG | 181 |
| TUA | Csa4G000580 | 微管蛋白 Tubulin | F:CTCCCTCCTTTTGGAGCGTT R:GAAGCACAGCAACGTCAGTG | 161 |
| Cu/Zn-SOD1 | Csa2G013250 | 铜/锌-超氧化物歧化酶 Cu/Zn-superoxide dismutase | F:GCCACATTTCAACCCTGCTG R:GTCCACCCTTGCCAAGATCA | 209 |
| Mn-SOD | Csa1G025980 | 锰-超氧化物歧化酶 Mn-superoxide dismutase | F:AGAAGCTCCCCTGGTTGAGA R:CTCTCGTGGTCTCACGCATT | 200 |
| POD25 | Csa1G019820 | 过氧化物酶 Peroxidase | F:CGAGCCCATCGATAACCACA R:TCTTTGTCCATGGCCACTCC | 243 |
| CAT1 | Csa4G658590 | 过氧化氢酶 Catalase | F:CCGAGAGGTATCCTCACCCA R:AAATGCTTGGCCTCACGTTG | 270 |
| APX | Csa1G479610 | 抗坏血酸过氧化物酶 Ascorbate peroxidase | F:TTGCCTGATGCTACCAAGGG R:TCGTTCCTTGTGTGCCCTAC | 123 |
| GR | Csa7G378460 | 谷胱甘肽还原酶 Glutathione reductase | F:GTGGCATTGTGGTTCGTTCC R:CACCTCCAGCACTATCGGAC | 189 |
| MDAR1 | Csa6G451470 | 单脱氢抗坏血酸还原酶 Dehydroascorbate reductase | F:TGGGCGATGTGGCTACTTTT R:TAAGACTGCGTCGCCAACAT | 221 |
| P5CS | Csa3G733920 | 脯氨酸合成关键酶 A key enzyme in proline synthesis | F:CCAAGAATGCAAGGCGTATCG R:CAACAGCTGCACATGCCTTT | 264 |
| P5CR | Csa4G354630 | 脯氨酸合成关键酶 A key enzyme in proline synthesis | F:GGTTGAGCCGTTACTGTGGA R:TCCAGCTCCGATGAACCCTA | 126 |
| NAC35 | Csa3G852470 | 转录因子 Transcription factor | F:GGTCATCGTCCACGTGTTCT R:GCCTGAGACTGAGCAAGAGG | 250 |
| NAC41 | Csa4G361820 | 转录因子 Transcription factor | F:AGGGGGCAATCGAGAAACAG R:TGAACTCCGATGACACCACG | 252 |
| NAC66 | Csa6G382950 | 转录因子 Transcription factor | F:GGCGATGTGTTAATGCCGTC R:TCCTTCCATTTTCGCTCGCT | 164 |
| WRKY18 | Csa3G116700 | 转录因子 Transcription factor | F:AGCGATGTTGATGTGCTGGA R:GTAAAGGGTTCGTGCTCCGA | 233 |
| WRKY51 | Csa6G486960 | 转录因子 Transcription factor | F:GCAAGCCAAAACCAACGAGT R:GGACAACGAAAACGTGGTGG | 236 |
| DREB2A | Csa4G023742 | 干旱应答因子 Dehydration-responsive factor | F:ATGGCTTGGCACTTTCTCCA R:ACTTTCACCTTCAGTTCCTCCA | 285 |
| DREB2C | Csa6G004870 | 干旱应答因子 Dehydration-responsive factor | F:GGAGTAGGCTTTGGCTTGGT R:GTGAACTCCTCAGGCACACA | 259 |
| DREB2D | Csa2G363010 | 干旱应答因子 Dehydration-responsive factor | F:GAACCAAATCGTGGTGCTCG R:AGCAACATCTTCCACCGTAGG | 258 |
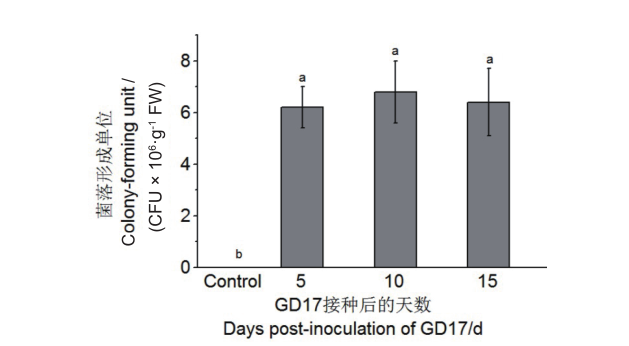
图1 GD17菌在根中的定殖效率 在种子催芽时加入GD17菌悬液,对照以蒸馏水代替。不同的小写字母表示在P<0.05具有显著性差异。下同
Fig. 1 Colonization efficiency of GD17 inside roots as indicated by colony-forming units GD17 inoculation was added during seed germination, control was replaced with distilled water. Bars with different lower-case letters indicate significant differences at P<0.05. The same below

图2 GD17和(或)干旱胁迫对植株生长和相对含水量的影响 A:地上部鲜重和干重;B:叶片相对含水量;C:叶片形态
Fig. 2 Effects of GD17 and(or)drought stress on plant growth and relative water content A: Fresh and dry weight of aerial part. B: Leaf relative water content. C: Representative pictures showing leaf morphology

图3 GD17和(或)干旱对植株氧化胁迫和抗氧化酶活性以及脯氨酸含量的影响
Fig. 3 Effects of GD17 and(or)drought on plant oxida-tion stress, activities of antioxidation enzymes and proline contents
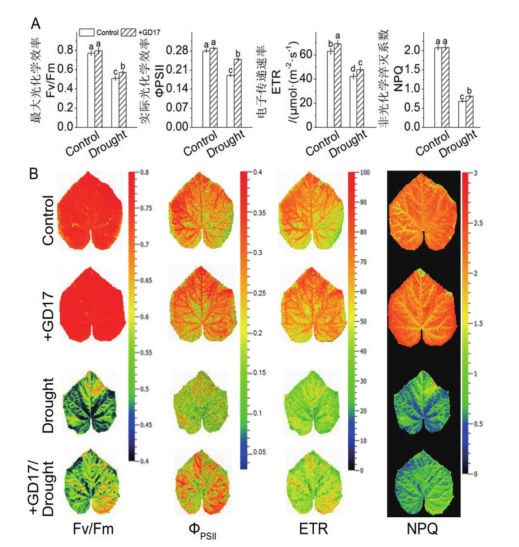
图5 GD17和(或)干旱胁迫对叶绿素荧光参数的影响 B:叶绿素荧光成像的代表图
Fig. 5 Effects of GD17 and(or)drought stress on chlor-ophyll fluorescence parameters B: Representative pictures of chlorophyll fluorescence imaging
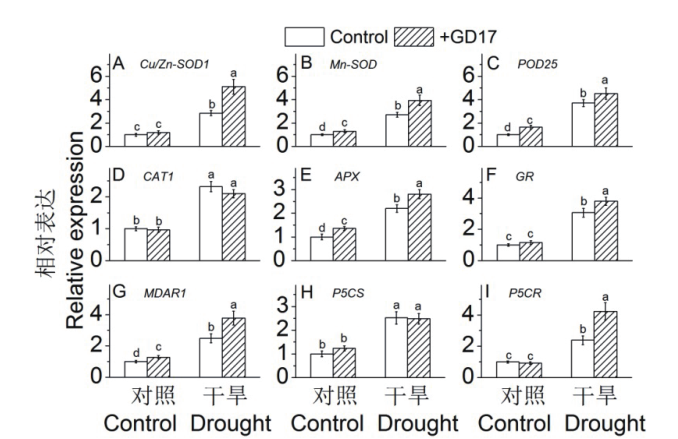
图6 GD17和(或)干旱胁迫对抗氧化酶和脯氨酸合成关键酶编码基因叶中表达的影响
Fig. 6 Effects of GD17 and(or)drought stress on the expressions of the genes related to antioxidation and proline synthesis in leaves
| [1] | Malepszy S. Cucumber(Cucumis Sativus L.)[M]// Bajaj YPS. Crops II. Berlin/Heidelberg: Springer, 1988. |
| [2] |
Vurukonda SSKP, Vardharajula S, Shrivastava M, et al. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria[J]. Microbiol Res, 2016, 184: 13-24.
doi: 10.1016/j.micres.2015.12.003 pmid: 26856449 |
| [3] |
Rodrigues J, Inzé D, Nelissen H, et al. Source-sink regulation in crops under water deficit[J]. Trends Plant Sci, 2019, 24(7): 652-663.
doi: S1360-1385(19)30101-3 pmid: 31109763 |
| [4] |
Bailey-Serres J, Parker JE, Ainsworth EA, et al. Genetic strategies for improving crop yields[J]. Nature, 2019, 575(7781): 109-118.
doi: 10.1038/s41586-019-1679-0 |
| [5] |
Zia R, Nawaz MS, Siddique MJ, et al. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation[J]. Microbiol Res, 2021, 242: 126626.
doi: 10.1016/j.micres.2020.126626 URL |
| [6] |
Bechtold U, Field B. Molecular mechanisms controlling plant growth during abiotic stress[J]. J Exp Bot, 2018, 69(11): 2753-2758.
doi: 10.1093/jxb/ery157 pmid: 29788471 |
| [7] |
Gupta A, Rico-Medina A, Caño-Delgado AI. The physiology of plant responses to drought[J]. Science, 2020, 368(6488): 266-269.
doi: 10.1126/science.aaz7614 pmid: 32299946 |
| [8] |
Wang CJ, Yang W, Wang C, et al. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains[J]. PLoS One, 2012, 7(12): e52565.
doi: 10.1371/journal.pone.0052565 URL |
| [9] |
Ullah A, Nisar M, Ali H, et al. Drought tolerance improvement in plants: an endophytic bacterial approach[J]. Appl Microbiol Biotechnol, 2019, 103(18): 7385-7397.
doi: 10.1007/s00253-019-10045-4 pmid: 31375881 |
| [10] |
郭英, 杨萍, 张丹雨, 等. 野大豆多功能根际促生菌的筛选鉴定和促生效果研究[J]. 生物技术通报, 2018, 34(10): 108-115.
doi: 10.13560/j.cnki.biotech.bull.1985.2018-0437 |
| Guo Y, Yang P, Zhang DY, et al. Screening, identification and growth-promoting effect of multifunction rhizosphere growth-promoting strain of wild soybean[J]. Biotechnol Bull, 2018, 34(10): 108-115. | |
| [11] |
Zhu RM, Cao YT, Li GZ, et al. Paraburkholderia sp. GD17 improves rice seedling tolerance to salinity[J]. Plant Soil, 2021, 467(1/2): 373-389.
doi: 10.1007/s11104-021-05108-3 |
| [12] |
祖国蔷, 胡哲, 王琪, 等. Burkholderia sp. GD17对水稻幼苗镉耐受的调节[J]. 生物技术通报, 2022, 38(4): 153-162.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0915 |
| Zu GQ, Hu Z, Wang Q, et al. Regulatory role of Burkholderia sp. GD17 in rice seedling’s responses to cadmium stress[J]. Biotechnol Bull, 2022, 38(4): 153-162. | |
| [13] | Yang AZ, Akhtar SS, Fu Q, et al. Burkholderia phytofirmans PsJN stimulate growth and yield of quinoa under salinity stress[J]. Plants(Basel), 2020, 9(6): 672. |
| [14] | 李合生. 植物生理生化实验原理和技术[M]. 北京: 高等教育出版社, 2000. |
| Li HS. Principles and techniques of plant physiological biochemical experiment[M]. Beijing: Higher Education Press, 2000. | |
| [15] | 王学奎, 黄见良. 植物生理生化实验原理与技术[M]. 3版. 北京: 高等教育出版社, 2015. |
| Wang XK, Huang JL. Principles and techniques of plant physiological biochemical experiment[M]. 3rd ed. Beijing: Higher Education Press, 2015. | |
| [16] |
Hemeda HM, Klein BP. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts[J]. J Food Sci, 1990, 55(1): 184-185.
doi: 10.1111/jfds.1990.55.issue-1 URL |
| [17] |
Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies[J]. Plant Soil, 1973, 39(1): 205-207.
doi: 10.1007/BF00018060 URL |
| [18] |
Wang YY, Wang Y, Li GZ, et al. Salicylic acid-altering Arabidopsis plant response to cadmium exposure: underlying mechanisms affecting antioxidation and photosynthesis-related processes[J]. Ecotoxicol Environ Saf, 2019, 169: 645-653.
doi: 10.1016/j.ecoenv.2018.11.062 URL |
| [19] |
Wan HJ, Zhao ZG, Qian CT, et al. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber[J]. Anal Biochem, 2010, 399(2): 257-261.
doi: 10.1016/j.ab.2009.12.008 pmid: 20005862 |
| [20] |
Sun YL, Cheng ZY, Glick BR. The presence of a 1-aminocyclopropane-1-carboxylate(ACC)deaminase deletion mutation alters the physiology of the endophytic plant growth-promoting bacterium Burkholderia phytofirmans PsJN[J]. FEMS Microbiol Lett, 2009, 296(1): 131-136.
doi: 10.1111/fml.2009.296.issue-1 URL |
| [21] |
Miller G, Suzuki N, Ciftci-Yilmaz S, et al. Reactive oxygen species homeostasis and signalling during drought and salinity stresses[J]. Plant Cell Environ, 2010, 33(4): 453-467.
doi: 10.1111/pce.2010.33.issue-4 URL |
| [22] |
Yogendra SG, S Singh U, K Sharma A. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice(Oryza sativa L.)[J]. Afr J Biotechnol, 2015, 14(9): 764-773.
doi: 10.5897/AJB URL |
| [23] |
Begum N, Wang L, Ahmad H, et al. Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism[J]. Microb Ecol, 2022, 83(4): 971-988.
doi: 10.1007/s00248-021-01815-7 |
| [24] |
Noctor G, Mhamdi A, Foyer CH. The roles of reactive oxygen metabolism in drought: not so cut and dried[J]. Plant Physiol, 2014, 164(4): 1636-1648.
doi: 10.1104/pp.113.233478 pmid: 24715539 |
| [25] |
Ozturk M, Turkyilmaz Unal B, García-Caparrós P, et al. Osmoregulation and its actions during the drought stress in plants[J]. Physiol Plant, 2021, 172(2): 1321-1335.
doi: 10.1111/ppl.13297 pmid: 33280137 |
| [26] |
Blum A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production[J]. Plant Cell Environ, 2017, 40(1): 4-10.
doi: 10.1111/pce.12800 URL |
| [27] |
Vendruscolo ECG, Schuster I, Pileggi M, et al. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat[J]. J Plant Physiol, 2007, 164(10): 1367-1376.
doi: 10.1016/j.jplph.2007.05.001 URL |
| [28] |
Szabados L, Savouré A. Proline: a multifunctional amino acid[J]. Trends Plant Sci, 2010, 15(2): 89-97.
doi: 10.1016/j.tplants.2009.11.009 pmid: 20036181 |
| [29] |
Sheteiwy MS, Ali DFI, Xiong YC, et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress[J]. BMC Plant Biol, 2021, 21(1): 195.
doi: 10.1186/s12870-021-02949-z pmid: 33888066 |
| [30] |
Nadeem SM, Imran M, Naveed M, et al. Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions[J]. J Sci Food Agric, 2017, 97(15): 5139-5145.
doi: 10.1002/jsfa.2017.97.issue-15 URL |
| [31] |
Malinowski DP, Belesky DP. Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance[J]. Crop Sci, 2000, 40(4): 923-940.
doi: 10.2135/cropsci2000.404923x URL |
| [32] |
Pinheiro C, Chaves MM. Photosynthesis and drought: can we make metabolic connections from available data?[J]. J Exp Bot, 2011, 62(3): 869-882.
doi: 10.1093/jxb/erq340 pmid: 21172816 |
| [33] | 徐雪东, 张超, 秦成, 等. 干旱下接种根际促生细菌对苹果实生苗光合和生理生态特性的影响[J]. 应用生态学报, 2019, 30(10): 3501-3508. |
| Xu XD, Zhang C, Qin C, et al. Effects of PGPR inoculation on photosynthesis and physiological-ecological characteristics of apple seedlings under drought stress[J]. Chin J Appl Ecol, 2019, 30(10): 3501-3508. | |
| [34] |
Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo[J]. Annu Rev Plant Biol, 2008, 59: 89-113.
doi: 10.1146/annurev.arplant.59.032607.092759 pmid: 18444897 |
| [35] |
Lu CM, Qiu NW, Wang BS, et al. Salinity treatment shows no effects on photosystem II photochemistry, but increases the resistance of photosystem II to heat stress in halophyte Suaeda salsa[J]. J Exp Bot, 2003, 54(383): 851-860.
doi: 10.1093/jxb/erg080 URL |
| [36] |
Kang CQ, Zhang YQ, Cheng RF, et al. Acclimating cucumber plants to blue supplemental light promotes growth in full sunlight[J]. Front Plant Sci, 2021, 12: 782465.
doi: 10.3389/fpls.2021.782465 URL |
| [37] |
Shu S, Chen LF, Lu W, et al. Effects of exogenous spermidine on photosynthetic capacity and expression of Calvin cycle genes in salt-stressed cucumber seedlings[J]. J Plant Res, 2014, 127(6): 763-773.
doi: 10.1007/s10265-014-0653-z pmid: 25069716 |
| [38] |
Martins SJ, Rocha GA, de Melo HC, et al. Plant-associated bacteria mitigate drought stress in soybean[J]. Environ Sci Pollut Res Int, 2018, 25(14): 13676-13686.
doi: 10.1007/s11356-018-1610-5 |
| [39] |
Huang W, Zhang SB, Liu T. Moderate photoinhibition of photosystem II significantly affects linear electron flow in the shade-demanding plant Panax notoginseng[J]. Front Plant Sci, 2018, 9: 637.
doi: 10.3389/fpls.2018.00637 pmid: 29868090 |
| [40] |
Nordstedt NP, Jones ML. Isolation of rhizosphere bacteria that improve quality and water stress tolerance in greenhouse ornamentals[J]. Front Plant Sci, 2020, 11: 826.
doi: 10.3389/fpls.2020.00826 pmid: 32612623 |
| [41] |
Li QM, Liu BB, Wu Y, et al. Interactive effects of drought stresses and elevated CO2 concentration on photochemistry efficiency of cucumber seedlings[J]. J Integr Plant Biol, 2008, 50(10): 1307-1317.
doi: 10.1111/jipb.2008.50.issue-10 URL |
| [42] |
Porcel R, Redondo-Gómez S, Mateos-Naranjo E, et al. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress[J]. J Plant Physiol, 2015, 185: 75-83.
doi: 10.1016/j.jplph.2015.07.006 URL |
| [43] |
Diao PF, Chen C, Zhang YZ, et al. The role of NAC transcription factor in plant cold response[J]. Plant Signal Behav, 2020, 15(9): 1785668.
doi: 10.1080/15592324.2020.1785668 URL |
| [44] |
Manna M, Thakur T, Chirom O, et al. Transcription factors as key molecular target to strengthen the drought stress tolerance in plants[J]. Physiol Plant, 2021, 172(2): 847-868.
doi: 10.1111/ppl.13268 pmid: 33180329 |
| [45] |
Zhang XM, Yu HJ, Sun C, et al. Genome-wide characterization and expression profiling of the NAC genes under abiotic stresses in Cucumis sativus[J]. Plant Physiol Biochem, 2017, 113: 98-109.
doi: 10.1016/j.plaphy.2017.01.023 URL |
| [46] |
Wang JF, Zhang L, Cao YY, et al. CsATAF1 positively regulates drought stress tolerance by an ABA-dependent pathway and by promoting ROS scavenging in cucumber[J]. Plant Cell Physiol, 2018, 59(5): 930-945.
doi: 10.1093/pcp/pcy030 pmid: 29415202 |
| [47] | Li WX, Pang SY, Lu ZG, et al. Function and mechanism of WRKY transcription factors in abiotic stress responses of plants[J]. Plants(Basel), 2020, 9(11): 1515. |
| [48] |
Sun YD, Yu DQ. Activated expression of AtWRKY53 negatively regulates drought tolerance by mediating stomatal movement[J]. Plant Cell Rep, 2015, 34(8): 1295-1306.
doi: 10.1007/s00299-015-1787-8 pmid: 25861729 |
| [49] |
Jiang YJ, Liang G, Yu DQ. Activated expression of WRKY57 confers drought tolerance in Arabidopsis[J]. Mol Plant, 2012, 5(6): 1375-1388.
doi: 10.1093/mp/sss080 URL |
| [50] |
Chen CH, Chen XQ, Han J, et al. Genome-wide analysis of the WRKY gene family in the cucumber genome and transcriptome-wide identification of WRKY transcription factors that respond to biotic and abiotic stresses[J]. BMC Plant Biol, 2020, 20(1): 443.
doi: 10.1186/s12870-020-02625-8 pmid: 32977756 |
| [51] |
Ling J, Jiang WJ, Zhang Y, et al. Genome-wide analysis of WRKY gene family in Cucumis sativus[J]. BMC Genomics, 2011, 12: 471.
doi: 10.1186/1471-2164-12-471 pmid: 21955985 |
| [52] |
Yamaguchi-Shinozaki K, Shinozaki K. A novel Cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress[J]. Plant Cell, 1994, 6(2): 251-264.
doi: 10.1105/tpc.6.2.251 pmid: 8148648 |
| [53] | 张梅, 刘炜, 毕玉平. 植物中DREBs类转录因子及其在非生物胁迫中的作用[J]. 遗传, 2009, 31(3): 236-244. |
| Zhang M, Liu W, Bi YP. Dehydration-responsive element-binding(DREB)transcription factor in plants and its role during abiotic stresses[J]. Hereditas, 2009, 31(3): 236-244. |
| [1] | 王子颖, 龙晨洁, 范兆宇, 张蕾. 利用酵母双杂交系统筛选水稻中与OsCRK5互作蛋白[J]. 生物技术通报, 2023, 39(9): 117-125. |
| [2] | 刘雯锦, 马瑞, 刘升燕, 杨江伟, 张宁, 司怀军. 马铃薯StCIPK11的克隆及响应干旱胁迫分析[J]. 生物技术通报, 2023, 39(9): 147-155. |
| [3] | 杨志晓, 侯骞, 刘国权, 卢志刚, 曹毅, 芶剑渝, 王轶, 林英超. 不同抗性烟草品系Rubisco及其活化酶对赤星病胁迫的响应[J]. 生物技术通报, 2023, 39(9): 202-212. |
| [4] | 褚睿, 李昭轩, 张学青, 杨东亚, 曹行行, 张雪艳. 黄瓜枯萎病拮抗芽孢杆菌的筛选、鉴定及其生防潜力[J]. 生物技术通报, 2023, 39(8): 262-271. |
| [5] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [6] | 丁凯鑫, 王立春, 田国奎, 王海艳, 李凤云, 潘阳, 庞泽, 单莹. 烯效唑缓解植物干旱损伤的研究进展[J]. 生物技术通报, 2023, 39(6): 1-11. |
| [7] | 王春语, 李政君, 王平, 张丽霞. 高粱表皮蜡质缺失突变体sb1抗旱生理生化分析[J]. 生物技术通报, 2023, 39(5): 160-167. |
| [8] | 王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132. |
| [9] | 谢洋, 邢雨蒙, 周国彦, 刘美妍, 银珊珊, 闫立英. 黄瓜二倍体及其同源四倍体果实转录组分析[J]. 生物技术通报, 2023, 39(3): 152-162. |
| [10] | 杨东亚, 祁瑞雪, 李昭轩, 林薇, 马慧, 张雪艳. 黄瓜茄病镰刀菌拮抗芽孢杆菌的筛选、鉴定及促生效果[J]. 生物技术通报, 2023, 39(2): 211-220. |
| [11] | 周恒, 谢彦杰. 植物氧化胁迫信号应答的研究进展[J]. 生物技术通报, 2023, 39(11): 36-43. |
| [12] | 于波, 秦晓惠, 赵杨. 植物感应干旱信号的机制[J]. 生物技术通报, 2023, 39(11): 6-17. |
| [13] | 陈楚怡, 杨小梅, 陈胜艳, 陈斌, 岳莉然. ABA和干旱胁迫下菊花脑ZF-HD基因家族的表达分析[J]. 生物技术通报, 2023, 39(11): 270-282. |
| [14] | 冯策婷, 江律, 刘鑫颖, 罗乐, 潘会堂, 张启翔, 于超. 单叶蔷薇NAC基因家族鉴定及干旱胁迫响应分析[J]. 生物技术通报, 2023, 39(11): 283-296. |
| [15] | 鄢梦雨, 韦晓薇, 曹婧, 兰海燕. 异子蓬SabHLH169基因的克隆及抗旱功能分析[J]. 生物技术通报, 2023, 39(11): 328-339. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||