








生物技术通报 ›› 2024, Vol. 40 ›› Issue (2): 146-159.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0676
杨艳( ), 胡洋, 刘霓如, 殷璐, 杨锐, 王鹏飞, 穆霄鹏, 张帅, 程春振(
), 胡洋, 刘霓如, 殷璐, 杨锐, 王鹏飞, 穆霄鹏, 张帅, 程春振( ), 张建成(
), 张建成( )
)
收稿日期:2023-07-14
出版日期:2024-02-26
发布日期:2024-03-13
通讯作者:
程春振,男,博士,副教授,研究方向:果实品质形成及调控机制解析;E-mail: ld0532cheng@sxau.edu.cn;作者简介:杨艳,女,硕士研究生,研究方向:果树生物技术; E-mail: 18404968975@163.com
基金资助:
YANG Yan( ), HU Yang, LIU Ni-ru, YIN Lu, YANG Rui, WANG Peng-fei, MU Xiao-peng, ZHANG Shuai, CHENG Chun-zhen(
), HU Yang, LIU Ni-ru, YIN Lu, YANG Rui, WANG Peng-fei, MU Xiao-peng, ZHANG Shuai, CHENG Chun-zhen( ), ZHANG Jian-cheng(
), ZHANG Jian-cheng( )
)
Received:2023-07-14
Published:2024-02-26
Online:2024-03-13
摘要:
【目的】为探究碱性亮氨酸拉链(bZIP)转录因子在‘红满堂’苹果花青素代谢中的调控作用。【方法】从‘红满堂’苹果克隆得到一个bZIP基因,分析了其基因及编码蛋白序列特性,利用洋葱表皮细胞瞬时转化确定其编码蛋白亚细胞定位,利用实时荧光定量PCR(RT-qPCR)研究了该基因在不同成熟时期‘红满堂’果实中的表达情况,基于烟草叶片、苹果叶片以及苹果果实瞬时转化研究了其对花青素积累和花青素合成相关结构基因表达的调控作用。【结果】该基因不含内含子,与MdbZIP43(XP_008393381.1)相似度最高,故命名为MbbZIP43。其编码序列长为522 bp,可编码由173 aa组成、含有bZIP_plant_GBF1结构域、定位于细胞核的亲水蛋白。RT-qPCR分析结果显示,随着果实成熟,MbbZIP43在‘红满堂’果实中的表达水平呈“先升后降”的变化趋势,在花后11周的果实中表达量最高。相关性分析显示MbbZIP43基因表达量与总黄酮和花青素含量正相关。瞬时过表达该基因的烟草叶片、苹果叶片和果皮中花青素含量分别提高了17.42%、25.66%和48.99%,过表达MbbZIP43的苹果叶片中花青素合成结构基因CHI、F3'H、DFR和UFGT分别提高了2.27倍、1.84倍、2.39倍和2.89倍;过表达MbbZIP43的苹果果皮中CHI、F3'H、DFR和UFGT分别提高了1.79倍、1.70倍、1.35倍和1.51倍,说明MbbZIP43可以通过上调这些结构基因的表达正调控苹果花青素合成。【结论】MbbZIP43可通过激活花青素合成相关结构基因CHI和UFGT等的表达进而促进花青素的积累。
杨艳, 胡洋, 刘霓如, 殷璐, 杨锐, 王鹏飞, 穆霄鹏, 张帅, 程春振, 张建成. ‘红满堂’苹果MbbZIP43基因的克隆与功能研究[J]. 生物技术通报, 2024, 40(2): 146-159.
YANG Yan, HU Yang, LIU Ni-ru, YIN Lu, YANG Rui, WANG Peng-fei, MU Xiao-peng, ZHANG Shuai, CHENG Chun-zhen, ZHANG Jian-cheng. Cloning and Functional Analysis of MbbZIP43 Gene in ‘Hongmantang’ Red-flesh Apple[J]. Biotechnology Bulletin, 2024, 40(2): 146-159.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 退火温度 Annealing temperature/℃ | 引物用途 Application |
|---|---|---|---|
| MbbZIP43-F | ATGGAGCCAAATGAATCAAAGG | 53 | 基因克隆 Gene cloning |
| MbbZIP43-R | TGGCGTACTTGAGTCTTCG | ||
| MbbZIP43-OE-F | ACGGGGGACTCTAGAGGATCCATGGAGCCAAATGAATCAAAGG | 65 | 载体构建 Vector construction |
| MbbZIP43-OE-R | GCTCACCATCGCTGCACTAGTTGGCGTACTTGAGTCTTCG | ||
| ACTIN-F | TGACCGAATGAGCAAGGAAATTACT | 60 | RT-qPCR |
| ACTIN-R | TACTCAGCTTTGGCAATCCACATC | ||
| Q-MbbZIP43-F | GGAGCCAAATGAATCAAAGG | ||
| Q-MbbZIP43-R | CGCATTCTTCTTCGTCTAACT | ||
| CHS-F | GGAGACAACTGGAGAAGGACTGGAA | ||
| CHS-R | CGACATTGATACTGGTGTCTTC | ||
| CHI-F | GGGATAACCTCGCGGCCAAA | ||
| CHI-R | GCATCCATGCCGGAAGCTACAA | ||
| F3'H-F | TGGAAGCTTGTGAGGACTGGGGT | ||
| F3'H-R | CTCCTCCGATGGCAAATCAAAGA | ||
| DFR-F | CCAAGTGAAGCGGGTTGTGCT | ||
| DFR-R | CAAAGCAGGCGGACAGGAGTAGC | ||
| ANS-F | GATAGGGTTTGAGTTCAAGTA | ||
| ANS-R | TCTCCTCAGCAGCCTCAGTTTTCT | ||
| UFGT-F | CCACCGCCCTTCCAAACACTCT | ||
| UFGT-R | CACCCTTATGTTACGCGGCATGT |
表1 本研究所用引物信息
Table 1 Information for the primers used in this study
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 退火温度 Annealing temperature/℃ | 引物用途 Application |
|---|---|---|---|
| MbbZIP43-F | ATGGAGCCAAATGAATCAAAGG | 53 | 基因克隆 Gene cloning |
| MbbZIP43-R | TGGCGTACTTGAGTCTTCG | ||
| MbbZIP43-OE-F | ACGGGGGACTCTAGAGGATCCATGGAGCCAAATGAATCAAAGG | 65 | 载体构建 Vector construction |
| MbbZIP43-OE-R | GCTCACCATCGCTGCACTAGTTGGCGTACTTGAGTCTTCG | ||
| ACTIN-F | TGACCGAATGAGCAAGGAAATTACT | 60 | RT-qPCR |
| ACTIN-R | TACTCAGCTTTGGCAATCCACATC | ||
| Q-MbbZIP43-F | GGAGCCAAATGAATCAAAGG | ||
| Q-MbbZIP43-R | CGCATTCTTCTTCGTCTAACT | ||
| CHS-F | GGAGACAACTGGAGAAGGACTGGAA | ||
| CHS-R | CGACATTGATACTGGTGTCTTC | ||
| CHI-F | GGGATAACCTCGCGGCCAAA | ||
| CHI-R | GCATCCATGCCGGAAGCTACAA | ||
| F3'H-F | TGGAAGCTTGTGAGGACTGGGGT | ||
| F3'H-R | CTCCTCCGATGGCAAATCAAAGA | ||
| DFR-F | CCAAGTGAAGCGGGTTGTGCT | ||
| DFR-R | CAAAGCAGGCGGACAGGAGTAGC | ||
| ANS-F | GATAGGGTTTGAGTTCAAGTA | ||
| ANS-R | TCTCCTCAGCAGCCTCAGTTTTCT | ||
| UFGT-F | CCACCGCCCTTCCAAACACTCT | ||
| UFGT-R | CACCCTTATGTTACGCGGCATGT |
| 软件 Software | 用途 Application | 网址 Website |
|---|---|---|
| GDR | 序列下载 | |
| CELLO v.2.5 | 亚细胞定位预测 | |
| TMHMM-2.0 | 跨膜结构预测 | |
| SignalP-5.0 | 信号肽预测 | |
| NetPhos 3.1 | 蛋白磷酸化位点预测 | |
| SOPMA | 蛋白二级结构预测 | |
| SWISS-MODEL | 蛋白三级结构预测 | |
| MEME | 蛋白保守基序预测 | |
| CDD | 结构域验证 | |
| NCBI BLASTP | 同源蛋白搜索 | |
| PlantCARE | 启动子顺式作用元件预测 | |
| TFBS | 启动子转录因子结合位点预测 | |
表2 本研究所用生物信息学分析网站信息
Table 2 Information for the software used for the bioinformatic analysis in this study
| 软件 Software | 用途 Application | 网址 Website |
|---|---|---|
| GDR | 序列下载 | |
| CELLO v.2.5 | 亚细胞定位预测 | |
| TMHMM-2.0 | 跨膜结构预测 | |
| SignalP-5.0 | 信号肽预测 | |
| NetPhos 3.1 | 蛋白磷酸化位点预测 | |
| SOPMA | 蛋白二级结构预测 | |
| SWISS-MODEL | 蛋白三级结构预测 | |
| MEME | 蛋白保守基序预测 | |
| CDD | 结构域验证 | |
| NCBI BLASTP | 同源蛋白搜索 | |
| PlantCARE | 启动子顺式作用元件预测 | |
| TFBS | 启动子转录因子结合位点预测 | |

图1 MbbZIP43基因PCR产物电泳检测(A)及序列比对(B)结果 M:DL2000 marker;1:cDNA为模板;2:gDNA为模板;MbbZIP43-1为参考基因组CDS序列;MbbZIP43-2为测序序列
Fig. 1 Electrophoresis detection(A)and sequence alignment(B)results of PCR products for the MbbZIP43 M: DL2000 marker; 1: using cDNA as template; 2: using gDNA as template; MbbZIP43-1: reference CDS sequence in the Malus baccata genome; MbbZIP43-2: amplified MbbZIP43 gene sequence
| 种类 Type | 功能 Function | 元件 Element | 数目 Number |
|---|---|---|---|
| 光响应元件 Light responsive elements | 光响应Light responsiveness | GATA-motif | 1 |
| 光响应Light responsiveness | MRE | 1 | |
| 光响应Light responsiveness | Box 4 | 2 | |
| 光响应Light responsiveness | ATCT-motif | 1 | |
| 光响应Light responsiveness | G-box | 1 | |
| 光响应Light responsiveness | Gap-box | 1 | |
| 光响应Light responsiveness | 3-AF1 binding site | 3 | |
| 光响应Light responsiveness | GT1-motif | 6 | |
| 生长发育相关元件 Plant growth and development related elements | 分生组织表达Meristem expression | CAT-box | 1 |
| 胚乳表达Endosperm expression | GCN4_motif | 1 | |
| 激素响应元件 Phytohormone responsive elements | 水杨酸响应Salicylic acid responsiveness | TCA-element | 1 |
| 脱落酸响应Abscisic acid responsiveness | ABRE | 1 | |
| 茉莉酸甲酯响应MeJA-responsiveness | CGTCA-motif | 2 | |
| 茉莉酸甲酯响应MeJA-responsiveness | TGACG-motif | 2 | |
| 赤霉素响应Gibberellin-responsiveness | P-box | 2 | |
| 赤霉素响应Gibberellin-responsiveness | TATC-box | 1 | |
| 逆境响应元件 Stress responsive elements | 厌氧诱导Anaerobic induction | ARE | 1 |
| 低温Low-temperature | LTR | 1 | |
| 伤害反应元件Wound-responsive element | WUN-motif | 2 | |
| 高温High-temperature | STRE | 4 | |
| 干旱响应Drought-inducibility | MYC | 4 | |
| 防御和胁迫Defense and stress | DRE core | 1 | |
| 防御和胁迫Defense and stress | TC-rich repeats | 1 | |
| 防御和胁迫Defense and stress | W box | 2 | |
| 防御和胁迫Defense and stress | MYB | 4 |
表3 MbbZIP43启动子区顺式作用元件预测结果
Table 3 Predicted cis-regulatory elements in the promoter region of MbbZIP43
| 种类 Type | 功能 Function | 元件 Element | 数目 Number |
|---|---|---|---|
| 光响应元件 Light responsive elements | 光响应Light responsiveness | GATA-motif | 1 |
| 光响应Light responsiveness | MRE | 1 | |
| 光响应Light responsiveness | Box 4 | 2 | |
| 光响应Light responsiveness | ATCT-motif | 1 | |
| 光响应Light responsiveness | G-box | 1 | |
| 光响应Light responsiveness | Gap-box | 1 | |
| 光响应Light responsiveness | 3-AF1 binding site | 3 | |
| 光响应Light responsiveness | GT1-motif | 6 | |
| 生长发育相关元件 Plant growth and development related elements | 分生组织表达Meristem expression | CAT-box | 1 |
| 胚乳表达Endosperm expression | GCN4_motif | 1 | |
| 激素响应元件 Phytohormone responsive elements | 水杨酸响应Salicylic acid responsiveness | TCA-element | 1 |
| 脱落酸响应Abscisic acid responsiveness | ABRE | 1 | |
| 茉莉酸甲酯响应MeJA-responsiveness | CGTCA-motif | 2 | |
| 茉莉酸甲酯响应MeJA-responsiveness | TGACG-motif | 2 | |
| 赤霉素响应Gibberellin-responsiveness | P-box | 2 | |
| 赤霉素响应Gibberellin-responsiveness | TATC-box | 1 | |
| 逆境响应元件 Stress responsive elements | 厌氧诱导Anaerobic induction | ARE | 1 |
| 低温Low-temperature | LTR | 1 | |
| 伤害反应元件Wound-responsive element | WUN-motif | 2 | |
| 高温High-temperature | STRE | 4 | |
| 干旱响应Drought-inducibility | MYC | 4 | |
| 防御和胁迫Defense and stress | DRE core | 1 | |
| 防御和胁迫Defense and stress | TC-rich repeats | 1 | |
| 防御和胁迫Defense and stress | W box | 2 | |
| 防御和胁迫Defense and stress | MYB | 4 |

图2 不同植物bZIP43以及已知花青素调控相关bZIP蛋白多序列比对结果 MdbZIP43:苹果bZIP43(XP_008393381.1);PbbZIP43:白梨bZIP43(XP_009339530.2);PybZIP43:沙梨bZIP43(UXW88004.1);PabZIP43:甜樱桃bZIP43(XP_021809905.1);PsbZIP43:李子bZIP43(XM_008241838.1);PdbZIP43:扁桃bZIP43(XP_034216145.1);PpbZIP43:桃bZIP43(XP_007209676.1);RcbZIP43:月季bZIP43(XP_024190316.2);MdbZIP44:苹果bZIP44(XP_008377201.2);AtHY5:拟南芥HY5(BAA21327.1);VvHY5:葡萄HY5(AGX85877.1);MdHY5:苹果HY5(NP_001280752.1);PaHY5:甜樱桃HY5(XP_021827650.1);PpHY5:桃HY5(XP_020411091.1);GbHY5:银杏HY5(Gb_12012)。图中红色框标出的是bZIP保守结构,红色线条表示α螺旋,绿色线条代表β折叠
Fig. 2 Multiple sequence alignment results for bZIP43 proteins and bZIP proteins related to known anthocyanin biosynthesis from different plant species MdbZIP43: Malus domestica bZIP43(XP_008393381.1); PbbZIP43: Pyrus bretschneideri bZIP43(XP_009339530.2); PybZIP43: Pyrus pyrifolia bZIP43(UXW88004.1); PabZIP43: Prunus avium bZIP43(XP_021809905.1); PsbZIP43: P. salicina bZIP43(XM_008241838.1); PdbZIP43: P. dulcis bZIP43(XP_034216145.1); PpbZIP43: P. persica bZIP43(XP_007209676.1); RcbZIP43: Rosa chinensis bZIP43(XP_024190316.2); MdbZIP44: M. domestica bZIP44(XP_008377201.2); AtHY5: Arabidopsis thaliana HY5(BAA21327.1); VvHY5: Vitis vinifera HY5(AGX85877.1); MdHY5: M. domestica HY5(NP_001280752.1); PaHY5: P. avium HY5(XP_021827650.1); PpHY5: P. persica HY5(XP_020411091.1); GbHY5: Ginkgo biloba HY5(Gb_12012). Red box, red line and green line indicates the conserved bZIP domain, α-helix and β folding, respectively

图5 不同发育时期‘红满堂’苹果MbbZIP43相对表达量、总酚含量、总黄酮含量、总黄酮醇含量和花青素含量 PS1-PS5:分别指花后7周、11周、15周、19周和23周的果实;FW:鲜重。不同小写字母表示差异显著(P<0.05)。下同
Fig. 5 Relative expressions of MbbZIP43, total phenol content, total flavonoid content, total flavonol content and anthocyanin content in ‘Hongmantang’ apple fruits at different ripening stages PS1-PS5 represent fruits at 7, 11, 15, 19 and 23 weeks post flowering, respectively. FW: Fresh weight. Different lowercase letters indicate significant differences(P<0.05). The same below
| 测定指标 Index | MbbZIP43表达水平 MbbZIP43 expression level | 总黄酮含量 Total flavonoids content | 总酚含量 Total phenolic content | 总黄酮醇含量 Total flavonol content | 花青素含量 Anthocyanin content |
|---|---|---|---|---|---|
| MbbZIP43表达水平 MbbZIP43 expression level | 1.0 | ||||
| 总黄酮含量 Total flavonoids content | 0.60 | 1.0 | |||
| 总酚含量 Total phenolic content | 0.08 | 0.69 | 1.0 | ||
| 总黄酮醇含量 Total flavonol content | -0.60 | -0.97* | -0.81 | 1.0 | |
| 花青素含量 Anthocyanin content | 0.34 | -0.02 | -0.70 | 0.21 | 1.0 |
表4 MbbZIP43基因表达水平与总黄酮、总酚、总黄酮醇和花青素含量相关性分析结果
Table 4 Correlation analysis results among the MbbZIP43 expressions, total flavonoids content, total phenolic content, total flavonol content and anthocyanin content
| 测定指标 Index | MbbZIP43表达水平 MbbZIP43 expression level | 总黄酮含量 Total flavonoids content | 总酚含量 Total phenolic content | 总黄酮醇含量 Total flavonol content | 花青素含量 Anthocyanin content |
|---|---|---|---|---|---|
| MbbZIP43表达水平 MbbZIP43 expression level | 1.0 | ||||
| 总黄酮含量 Total flavonoids content | 0.60 | 1.0 | |||
| 总酚含量 Total phenolic content | 0.08 | 0.69 | 1.0 | ||
| 总黄酮醇含量 Total flavonol content | -0.60 | -0.97* | -0.81 | 1.0 | |
| 花青素含量 Anthocyanin content | 0.34 | -0.02 | -0.70 | 0.21 | 1.0 |
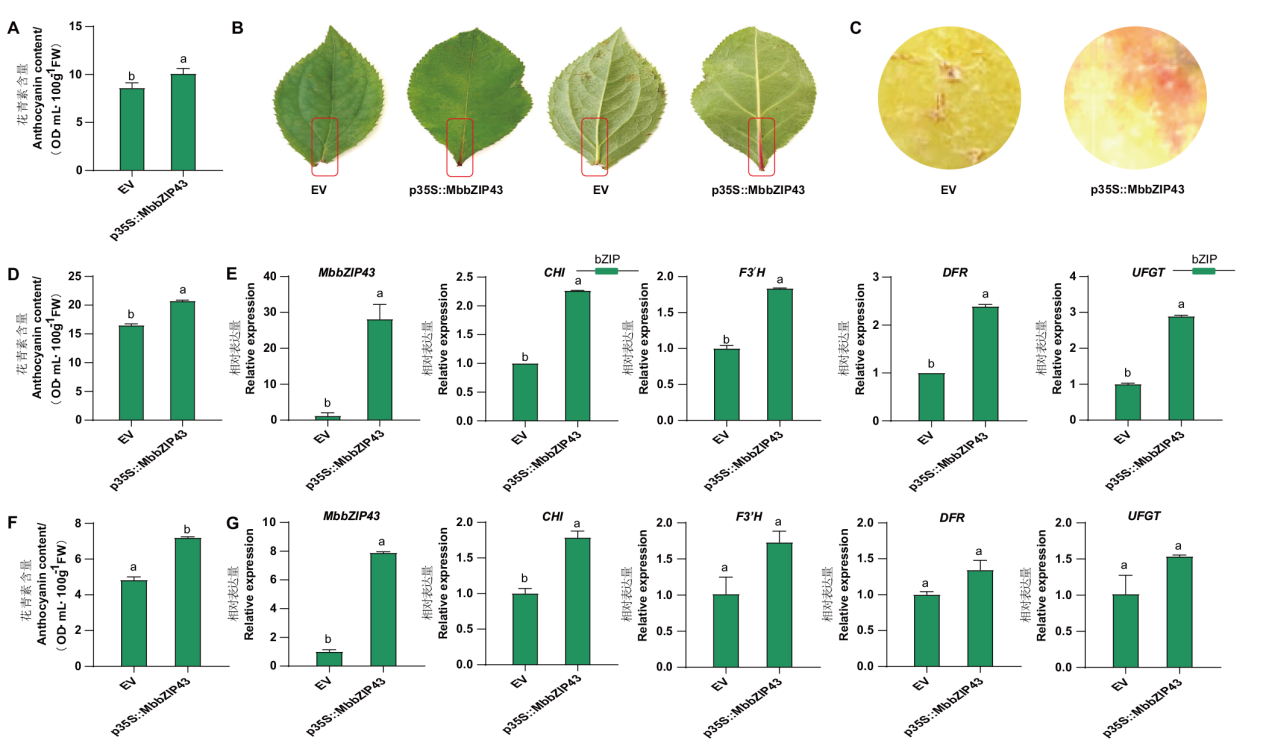
图6 MbbZIP43瞬时过表达对花青素积累的影响 A:瞬时过表达MbbZIP43对烟草叶片花青素积累的影响;B:瞬时过表达MbbZIP43的苹果叶片;C:瞬时过表达MbbZIP43的苹果果皮;D:瞬时过表达MbbZIP43对苹果叶片花青素积累的影响;E:瞬时过表达MbbZIP43对苹果叶片中MbbZIP43和花青素合成相关结构基因表达的影响;F:瞬时过表达MbbZIP43对苹果果皮花青素积累的影响;G:瞬时过表达MbbZIP43对苹果果皮中MbbZIP43和花青素合成相关结构基因表达的影响
Fig. 6 Influences of transient overexpression of MbbZIP43 gene on anthocyanin accumulations A: Influences of transient overexpression of MbbZIP43 gene on the anthocyanin accumulation in tobacco. B: Apple leaves overexpressing MbbZIP43. C: Apple peel overexpressing MbbZIP43. D: Influences of transient overexpression of MbbZIP43 gene on anthocyanin accumulation in apple leaves. E: Influences of transient overexpression of MbbZIP43 gene on the expressions of MbbZIP43 and structural genes related to anthocyanin biosynthesis in apple leaves. F: Influences of transient overexpression of MbbZIP43 gene on anthocyanin accumulation in apple. G: Influences of transient overexpression of MbbZIP43 gene on the expressions of MbbZIP43 and structural genes related to anthocyanin biosynthesis in apple peel
| [1] |
Hurst HC. Transcription factors 1: bZIP proteins[J]. Protein Profile, 1995, 2(2): 101-168.
pmid: 7780801 |
| [2] |
Ellenberger T. Getting a grip on DNA recognition: structures of the basic region leucine zipper, and the basic region helix-loop-helix DNA-binding domains[J]. Curr Opin Struct Biol, 1994, 4(1): 12-21.
doi: 10.1016/S0959-440X(94)90054-X URL |
| [3] |
Wei KF, Chen J, et al. Genome-wide analysis of bZIP-encoding genes in maize[J]. DNA Res, 2012, 19(6): 463-476.
doi: 10.1093/dnares/dss026 pmid: 23103471 |
| [4] |
Silveira AB, Gauer L, Tomaz JP, et al. The Arabidopsis AtbZIP9 protein fused to the VP16 transcriptional activation domain alters leaf and vascular development[J]. Plant Sci, 2007, 172(6): 1148-1156.
doi: 10.1016/j.plantsci.2007.03.003 URL |
| [5] |
Yamamoto MP, Onodera Y, Touno SM, et al. Synergism between RPBF Dof and RISBZ1 bZIP activators in the regulation of rice seed expression genes[J]. Plant Physiol, 2006, 141(4): 1694-1707.
doi: 10.1104/pp.106.082826 pmid: 16798940 |
| [6] |
Burman N, Bhatnagar A, Khurana JP. OsbZIP48, a HY5 transcription factor ortholog, exerts pleiotropic effects in light-regulated development[J]. Plant Physiol, 2018, 176(2): 1262-1285.
doi: 10.1104/pp.17.00478 pmid: 28775143 |
| [7] |
Babu Rajendra Prasad V, Gupta N, Nandi A, et al. HY1 genetically interacts with GBF1 and regulates the activity of the Z-box containing promoters in light signaling pathways in Arabidopsis thaliana[J]. Mech Dev, 2012, 129(9/10/11/12): 298-307.
doi: 10.1016/j.mod.2012.06.004 URL |
| [8] |
Yang SS, Zhang XX, Zhang XM, et al. A bZIP transcription factor, PqbZIP1, is involved in the plant defense response of American ginseng[J]. PeerJ, 2022, 10: e12939.
doi: 10.7717/peerj.12939 URL |
| [9] |
Collin A, Daszkowska-Golec A, Szarejko I. Updates on the role of abscisic acid insensitive 5(abi5)and abscisic acid-responsive element binding factors(abfs)in ABA signaling in different developmental stages in plants[J]. Cells, 2021, 10(8): 1996.
doi: 10.3390/cells10081996 URL |
| [10] |
Alves M, Dadalto S, Gonçalves A, et al. Plant bZIP transcription factors responsive to pathogens: a review[J]. Int J Mol Sci, 2013, 14(4): 7815-7828.
doi: 10.3390/ijms14047815 pmid: 23574941 |
| [11] |
刘恺媛, 王茂良, 辛海波, 等. 植物花青素合成与调控研究进展[J]. 中国农学通报, 2021, 37(14): 41-51.
doi: 10.11924/j.issn.1000-6850.casb2020-0390 |
|
Liu KY, Wang ML, Xin HB, et al. Anthocyanin biosynthesis and regulate mechanisms in plants: a review[J]. Chin Agric Sci Bull, 2021, 37(14): 41-51.
doi: 10.11924/j.issn.1000-6850.casb2020-0390 |
|
| [12] |
Dubos C, Stracke R, Grotewold E, et al. MYB transcription factors in Arabidopsis[J]. Trends Plant Sci, 2010, 15(10): 573-581.
doi: 10.1016/j.tplants.2010.06.005 URL |
| [13] |
Shin DH, Choi M, Kim K, et al. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis[J]. FEBS Lett, 2013, 587(10): 1543-1547.
doi: 10.1016/j.febslet.2013.03.037 URL |
| [14] |
Zhao Y, Min T, et al. The photomorphogenic transcription factor PpHY5 regulates anthocyanin accumulation in response to UVA and UVB irradiation[J]. Front Plant Sci, 2021, 11: 603178.
doi: 10.3389/fpls.2020.603178 URL |
| [15] |
Wang YY, Zhang XD, Zhao YR, et al. Transcription factor PyHY5 binds to the promoters of PyWD40 and PyMYB10 and regulates its expression in red pear ‘Yunhongli No. 1’[J]. Plant Physiol Biochem, 2020, 154: 665-674.
doi: 10.1016/j.plaphy.2020.07.008 URL |
| [16] |
Liu YQ, Ye YT, et al. B-box transcription factor FaBBX22 promotes light-induced anthocyanin accumulation in strawberry(Fragar-ia×ananassa)[J]. Int J Mol Sci, 2022, 23(14): 7757.
doi: 10.3390/ijms23147757 URL |
| [17] |
Liu YQ, Tang L, Wang YP, et al. The blue light signal transduction module FaCRY1-FaCOP1-FaHY5 regulates anthocyanin accumulation in cultivated strawberry[J]. Front Plant Sci, 2023, 14: 1144273.
doi: 10.3389/fpls.2023.1144273 URL |
| [18] |
Liu HN, Su J, Zhu YF, et al. The involvement of PybZIPa in light-induced anthocyanin accumulation via the activation of PyUFGT through binding to tandem G-boxes in its promoter[J]. Hortic Res, 2019, 6: 134.
doi: 10.1038/s41438-019-0217-4 |
| [19] |
Xu ZJ, Wang JC, Ma YB, et al. The bZIP transcription factor SlAREB1 regulates anthocyanin biosynthesis in response to low temperature in tomato[J]. Plant J, 2023, 115(1): 205-219.
doi: 10.1111/tpj.v115.1 URL |
| [20] |
An JP, Yao JF, et al. Apple bZIP transcription factor MdbZIP44 regulates abscisic acid-promoted anthocyanin accumulation[J]. Plant Cell Environ, 2018, 41(11): 2678-2692.
doi: 10.1111/pce.v41.11 URL |
| [21] |
An JP, Zhang XW, Liu YJ, et al. ABI5 regulates ABA-induced anthocyanin biosynthesis by modulating the MYB1-bHLH3 complex in apple[J]. J Exp Bot, 2021, 72(4): 1460-1472.
doi: 10.1093/jxb/eraa525 URL |
| [22] |
Liu WJ, Mei ZX, Yu L, et al. The ABA-induced NAC transcription factor MdNAC1 interacts with a bZIP-type transcription factor to promote anthocyanin synthesis in red-fleshed apples[J]. Hortic Res, 2023, 10(5): uhad049.
doi: 10.1093/hr/uhad049 URL |
| [23] | 杨廷桢, 高敬东, 王骞, 等. 苹果属观赏新品种——‘红满堂’的选育[J]. 果树学报, 2015, 32(4): 727-729, 520. |
| Yang TZ, Gao JD, Wang Q, et al. A new Malus ornamental variety ‘Hongmantang’[J]. J Fruit Sci, 2015, 32(4): 727-729, 520. | |
| [24] | 郭子微, 侯文赫, 付鸿博, 等. 不同苹果果实发育过程中酚类物质含量及抗氧化能力变化研究[J]. 山东农业科学, 2021, 53(11): 35-44. |
| Guo ZW, Hou WH, Fu HB, et al. Changes of phenolic substances and antioxidant capacity during fruit development of different apple varieties[J]. Shandong Agric Sci, 2021, 53(11): 35-44. | |
| [25] |
Cheng CZ, Guo ZW, Li HA, et al. Integrated metabolic, transcriptomic and chromatin accessibility analyses provide novel insights into the competition for anthocyanins and flavonols biosynthesis during fruit ripening in red apple[J]. Front Plant Sci, 2022, 13: 975356.
doi: 10.3389/fpls.2022.975356 URL |
| [26] |
Chen CJ, Chen H, Zhang Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data[J]. Mol Plant, 2020, 13(8): 1194-1202.
doi: S1674-2052(20)30187-8 pmid: 32585190 |
| [27] | 刘嘉鹏, 武欢, 王斌, 等. 香蕉MaSNAT2基因的克隆与表达分析[J]. 西北植物学报, 2022, 42(4): 569-577. |
| Liu JP, Wu H, Wang B, et al. Cloning and expression analysis of a MaSNAT2 gene in banana[J]. Acta Bot Boreali Occidentalia Sin, 2022, 42(4): 569-577. | |
| [28] |
Cheng CZ, Zhong Y, et al. The upregulated expression of the Citrus RIN4 gene in HLB diseased Citrus aids Candidatus Liberibacter asiaticus infection[J]. Int J Mol Sci, 2022, 23(13): 6971.
doi: 10.3390/ijms23136971 URL |
| [29] |
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method[J]. Nat Protoc, 2008, 3(6): 1101-1108.
doi: 10.1038/nprot.2008.73 pmid: 18546601 |
| [30] |
Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods[J]. Am J Enol Vitic, 1977, 28(1): 49-55.
doi: 10.5344/ajev.1977.28.1.49 URL |
| [31] |
Zheng HZ, Kim YI, Chung SK. A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples[J]. Food Chem, 2012, 131(1): 106-110.
doi: 10.1016/j.foodchem.2011.08.038 URL |
| [32] | 仝月澳, 周厚基. 果树营养诊断法[M]. 北京: 农业出版社, 1982. |
| Tong YA, Zhou HJ. Nutritional diagnosis of fruit trees[M]. Beijing: Agricultural Publishing House, 1982. | |
| [33] |
Wang B, Xu YB, Xu SY, et al. Characterization of banana SNARE genes and their expression analysis under temperature stress and mutualistic and pathogenic fungal colonization[J]. Plants, 2023, 12(8): 1599.
doi: 10.3390/plants12081599 URL |
| [34] |
Alabd A, Ahmad M, Zhang X, et al. Light-responsive transcription factor PpWRKY44 induces anthocyanin accumulation by regulating PpMYB10 expression in pear[J]. Hortic Res, 2022, 9: uhac199.
doi: 10.1093/hr/uhac199 URL |
| [35] |
An JP, Wang XF, Li YY, et al. EIN3-LIKE1, MYB1, and ETHYLENE RESPONSE FACTOR3 act in a regulatory loop that synergistically modulates ethylene biosynthesis and anthocyanin accumulation[J]. Plant Physiol, 2018, 178(2): 808-823.
doi: 10.1104/pp.18.00068 URL |
| [36] |
Yu LJ, Sun YY, et al. ROS1 promotes low temperature-induced anthocyanin accumulation in apple by demethylating the promoter of anthocyanin-associated genes[J]. Hortic Res, 2022, 9: uhac007.
doi: 10.1093/hr/uhac007 URL |
| [37] | Zhang YY, Huang DQ, Wang B, et al. Characterization of highbush blueberry(Vaccinium corymbosum L.)anthocyanin biosynthesis related MYBs and functional analysis of VcMYB gene[J]. Curr News Mol Biol, 2023, 45(1): 379-399. |
| [38] |
Zhang YY, Liu F, Wang B, et al. Identification, characterization and expression analysis of anthocyanin biosynthesis-related bHLH genes in blueberry(Vaccinium corymbosum L.)[J]. Int J Mol Sci, 2021, 22(24): 13274.
doi: 10.3390/ijms222413274 URL |
| [39] |
Takos AM, Jaffé FW, Jacob SR, et al. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples[J]. Plant Physiol, 2006, 142(3): 1216-1232.
doi: 10.1104/pp.106.088104 pmid: 17012405 |
| [40] |
Telias A, Kui LW, Stevenson DE, et al. Apple skin patterning is associated with differential expression of MYB10[J]. BMC Plant Biol, 2011, 11: 93.
doi: 10.1186/1471-2229-11-93 pmid: 21599973 |
| [41] |
Vimolmangkang S, Han YP, Wei GC, et al. An apple MYB transcription factor, MdMYB3, is involved in regulation of anthocyanin biosynthesis and flower development[J]. BMC Plant Biol, 2013, 13: 176.
doi: 10.1186/1471-2229-13-176 pmid: 24199943 |
| [42] |
An JP, Li HH, Song LQ, et al. The molecular cloning and functional characterization of MdMYC2, a bHLH transcription factor in apple[J]. Plant Physiol Biochem, 2016, 108: 24-31.
doi: 10.1016/j.plaphy.2016.06.032 URL |
| [43] |
An XH, Tian Y, Chen KQ, et al. The apple WD40 protein MdTTG1 interacts with bHLH but not MYB proteins to regulate anthocyanin accumulation[J]. J Plant Physiol, 2012, 169(7): 710-717.
doi: 10.1016/j.jplph.2012.01.015 URL |
| [44] |
An JP, Qu FJ, Yao JF, et al. The bZIP transcription factor MdHY5 regulates anthocyanin accumulation and nitrate assimilation in apple[J]. Hortic Res, 2017, 4: 17023.
doi: 10.1038/hortres.2017.23 URL |
| [45] |
Li XL, Cheng YD, et al. Weighted gene coexpression correlation network analysis reveals a potential molecular regulatory mechanism of anthocyanin accumulation under different storage temperatures in ‘Friar’ plum[J]. BMC Plant Biol, 2021, 21(1): 576.
doi: 10.1186/s12870-021-03354-2 |
| [46] |
Hu JF, Fang HC, Wang J, et al. Ultraviolet B-induced MdWRKY72 expression promotes anthocyanin synthesis in apple[J]. Plant Sci, 2020, 292: 110377.
doi: 10.1016/j.plantsci.2019.110377 URL |
| [47] |
An JP, Wang XF, et al. MdBBX22 regulates UV-B-induced anthocyanin biosynthesis through regulating the function of MdHY5 and is targeted by MdBT2 for 26S proteasome-mediated degradation[J]. Plant Biotechnol J, 2019, 17(12): 2231-2233.
doi: 10.1111/pbi.v17.12 URL |
| [48] |
Rudell DR, Mattheis JP. Synergism exists between ethylene and methyl jasmonate in artificial light-induced pigment enhancement of ‘Fuji’ apple fruit peel[J]. Postharvest Biol Technol, 2008, 47(1): 136-140.
doi: 10.1016/j.postharvbio.2007.05.021 URL |
| [49] |
Sun JJ, Wang YC, Chen XS, et al. Effects of methyl jasmonate and abscisic acid on anthocyanin biosynthesis in callus cultures of red-fleshed apple(Malus sieversii f. niedzwetzkyana)[J]. Plant Cell Tiss Organ Cult, 2017, 130(2): 227-237.
doi: 10.1007/s11240-017-1217-4 URL |
| [50] |
Chen ZJ, Yu L, Liu WJ, et al. Research progress of fruit color development in apple(Malus domestica Borkh.)[J]. Plant Physiol Biochem, 2021, 162: 267-279.
doi: 10.1016/j.plaphy.2021.02.033 URL |
| [51] |
Ubi BE, Honda C, Bessho H, et al. Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature[J]. Plant Sci, 2006, 170(3): 571-578.
doi: 10.1016/j.plantsci.2005.10.009 URL |
| [52] |
Kui LW, Micheletti D, Palmer J, et al. High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex[J]. Plant Cell Environ, 2011, 34(7): 1176-1190.
doi: 10.1111/pce.2011.34.issue-7 URL |
| [53] |
An JP, Zhang XW, Bi SQ, et al. The ERF transcription factor MdERF38 promotes drought stress-induced anthocyanin biosynthesis in apple[J]. Plant J, 2020, 101(3): 573-589.
doi: 10.1111/tpj.v101.3 URL |
| [54] |
An JP, Zhang XW, You CX, et al. MdWRKY40 promotes wounding-induced anthocyanin biosynthesis in association with MdMYB1 and undergoes MdBT2-mediated degradation[J]. New Phytol, 2019, 224(1): 380-395.
doi: 10.1111/nph.v224.1 URL |
| [55] | 杜婷婷, 宋治华, 董碧莹, 等. 木豆类黄酮代谢通路关键基因家族的鉴定与表达分析[J]. 农业生物技术学报, 2021, 29(12): 2289-2303. |
| Du TT, Song ZH, Dong BY, et al. Identification and expression analysis of key gene families in flavonoid metabolism pathway in pigeon pea(Cajanus cajan)[J]. J Agric Biotechnol, 2021, 29(12): 2289-2303. | |
| [56] |
Meng JX, Yin J, Wang H, et al. A TCP transcription factor in Malus halliana, MhTCP4, positively regulates anthocyanins biosynthesis[J]. Int J Mol Sci, 2022, 23(16): 9051.
doi: 10.3390/ijms23169051 URL |
| [1] | 任延靖, 张鲁刚, 赵孟良, 李江, 邵登魁. 白菜种子cDNA酵母文库的构建及BrTTG1互作蛋白的筛选及分析[J]. 生物技术通报, 2024, 40(2): 223-232. |
| [2] | 朱毅, 柳唐镜, 宫国义, 张洁, 王晋芳, 张海英. 西瓜ClPP2C3克隆及表达分析[J]. 生物技术通报, 2024, 40(1): 243-249. |
| [3] | 谢宏, 周丽莹, 李舒文, 王梦迪, 艾晔, 晁跃辉. 蒺藜苜蓿MtCIM基因结构和功能分析[J]. 生物技术通报, 2024, 40(1): 262-269. |
| [4] | 唐伟林, 康琴, 汪霞, 谌明洋, 孙欣江, 王棵, 侯凯, 吴卫, 徐东北. 薄荷茉莉酸受体McCOI1a基因的克隆与表达模式分析[J]. 生物技术通报, 2024, 40(1): 270-280. |
| [5] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [6] | 王佳蕊, 孙培媛, 柯瑾, 冉彬, 李洪有. 苦荞糖基转移酶基因FtUGT143的克隆及表达分析[J]. 生物技术通报, 2023, 39(8): 204-212. |
| [7] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [8] | 孙明慧, 吴琼, 刘丹丹, 焦小雨, 王文杰. 茶树CsTMFs的克隆与表达分析[J]. 生物技术通报, 2023, 39(7): 151-159. |
| [9] | 赵雪婷, 高利燕, 王俊刚, 沈庆庆, 张树珍, 李富生. 甘蔗AP2/ERF转录因子基因ShERF3的克隆、表达及其编码蛋白的定位[J]. 生物技术通报, 2023, 39(6): 208-216. |
| [10] | 姜晴春, 杜洁, 王嘉诚, 余知和, 王允, 柳忠玉. 虎杖转录因子PcMYB2的表达特性和功能分析[J]. 生物技术通报, 2023, 39(5): 217-223. |
| [11] | 姚姿婷, 曹雪颖, 肖雪, 李瑞芳, 韦小妹, 邹承武, 朱桂宁. 火龙果溃疡病菌实时荧光定量PCR内参基因的筛选[J]. 生物技术通报, 2023, 39(5): 92-102. |
| [12] | 王艺清, 王涛, 韦朝领, 戴浩民, 曹士先, 孙威江, 曾雯. 茶树SMAS基因家族的鉴定及互作分析[J]. 生物技术通报, 2023, 39(4): 246-258. |
| [13] | 刘思佳, 王浩楠, 付宇辰, 闫文欣, 胡增辉, 冷平生. ‘西伯利亚’百合LiCMK基因克隆及功能分析[J]. 生物技术通报, 2023, 39(3): 196-205. |
| [14] | 王涛, 漆思雨, 韦朝领, 王艺清, 戴浩民, 周喆, 曹士先, 曾雯, 孙威江. CsPPR和CsCPN60-like在茶树白化叶片中的表达分析及互作蛋白验证[J]. 生物技术通报, 2023, 39(3): 218-231. |
| [15] | 庞强强, 孙晓东, 周曼, 蔡兴来, 张文, 王亚强. 菜心BrHsfA3基因克隆及其对高温胁迫的响应[J]. 生物技术通报, 2023, 39(2): 107-115. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||