








生物技术通报 ›› 2024, Vol. 40 ›› Issue (6): 126-133.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0129
王超敏1( ), 何美丹1, 王文治2, 袁潜华1, 张树珍2(
), 何美丹1, 王文治2, 袁潜华1, 张树珍2( ), 沈林波2(
), 沈林波2( )
)
收稿日期:2024-02-01
出版日期:2024-06-26
发布日期:2024-05-06
通讯作者:
张树珍,女,博士,研究员,研究方向:甘蔗生物技术 ;E-mail: zhangshuzhen@itbb.org.cn;作者简介:王超敏,女,硕士研究生,研究方向:作物病毒学;E-mail: 3492787588@qq.com
基金资助:
WANG Chao-min1( ), HE Mei-dan1, WANG Wen-zhi2, YUAN Qian-hua1, ZHANG Shu-zhen2(
), HE Mei-dan1, WANG Wen-zhi2, YUAN Qian-hua1, ZHANG Shu-zhen2( ), SHEN Lin-bo2(
), SHEN Lin-bo2( )
)
Received:2024-02-01
Published:2024-06-26
Online:2024-05-06
摘要:
【目的】 建立一种快速、灵敏、特异的检测甘蔗条点病毒(sugarcane striate virus,SStrV)的SYBR Green I 荧光定量PCR方法。【方法】 从SStrV基因组保守区域序列设计特异性扩增引物,构建含有SStrV基因组序列的重组质粒pMD19-T-SStrV-qN作为阳性质粒标准品,以其为模板建立SStrV荧光定量PCR检测方法,并对该方法的灵敏性、特异性、稳定性进行了测试,随后用该方法对甘蔗不同组织部位中SStrV载量进行检测。【结果】 将含有SStrV基因组序列的重组质粒按10倍比稀释成标准品,将其作为模板进行荧光定量PCR,获得标准曲线y = -3.337 x + 38.197,相关系数r2=0.999,说明Cq值与标准品浓度拷贝数的对数呈线性关系;建立的荧光定量PCR最低可以检测到13拷贝重组质粒/μL,是普通PCR灵敏度的100倍。该方法能特异的检测SStrV,特异性高,组内和组间的变异系数在0.13% - 0.94%之间,表明该方法重复性良好。SStrV的载量在甘蔗的不同组织部位中差异较大,+4叶中SStrV的载量最高,与其他组织部位达到了显著差异。【结论】 建立了能灵敏特异检测SStrV的SYBR Green I 荧光定量PCR方法,明确了+4叶是甘蔗中SStrV检测的最佳采样部位。
王超敏, 何美丹, 王文治, 袁潜华, 张树珍, 沈林波. 甘蔗条点病毒荧光定量PCR检测方法的建立及应用[J]. 生物技术通报, 2024, 40(6): 126-133.
WANG Chao-min, HE Mei-dan, WANG Wen-zhi, YUAN Qian-hua, ZHANG Shu-zhen, SHEN Lin-bo. Establishment and Application of Real-time PCR for Sugarcane Striate Virus[J]. Biotechnology Bulletin, 2024, 40(6): 126-133.
图1 SStrV 片段PCR扩增 M:Marker DS2000; 1-2:扩增的SStrV目的片段;3:阴性对照
Fig. 1 PCR amplification of SStrV fragments M:Marker DS2000; 1-2: amplified SStrV target fragment; 3: negative control

图2 荧光定量PCR标准曲线(A)、扩增曲线(B)以及熔解曲线(C) A为标准曲线,分别由拷贝数为1.31 × 108 copies /μL SStrV质粒DNA标准品经10倍梯度稀释而构建;B为扩增曲线,从左到右分别是由拷贝数为1.31 × 108 copies /μL SStrV质粒DNA标准品经10倍梯度稀释而构建;C为熔解曲线,其峰值对应的温度大于80℃
Fig. 2 PCR standard curve(A), amplification curve(B)and melting curve(C) A is standard curve, built with a 10-fold serial dilution of SStrV plasmid DNA of 1.31 ×108 copies /μL constructed, respectively. B is amplification curve, from left to right were developed with a 10-fold serial dilution of SStrV plasmid DNA. C is melting curve, and its peak corresponds to a temperature greater than 80℃

图4 荧光定量PCR灵敏性实验扩增曲线(A)和普通PCR检测方法的敏感性分析(B) A:从左到右分别是由拷贝数为1.31×108 copies /μL SStrv质粒DNA标准品经10倍梯度稀释而构建;B:M:MarkerDS2000;1-8:1.31×108 copies /μL-1.31×10 copies /μL;9:空白对照
Fig. 4 Amplification curve for qPCR sensitivity experiments(A) and sensitivity analysis of conventional PCR(B) A: From left to right were developed with a 10-fold serial dilution of SStrv plasmid DNA; B: M: DSTM 2000(marker);1-8: 1.31×108 copies /μL-1.31×10 copies /μL. 9: Blank control
| 质粒浓度Plasmid concentration/(copies·µL-1) | 组内实验Within-group experiments | 组间实验Intergroup experiments | ||||
|---|---|---|---|---|---|---|
| Ct值(x±s) Ct value | 变异系数(CV) Coefficient variation/% | Ct值(x±s) Ct value | 变异系数(CV) Coefficient variation/% | |||
| 1.31×108 | 10.94±0.09 | 0.81 | 10.89±0.10 | 0.94 | ||
| 1.31×107 | 14.51±0.02 | 0.13 | 14.44±0.13 | 0.92 | ||
| 1.31×106 | 17.90±0.06 | 0.31 | 17.80±0.15 | 0.87 | ||
| 1.31×105 | 21.35±0.08 | 0.37 | 21.23±0.16 | 0.74 | ||
| 1.31×104 | 24.47±0.11 | 0.43 | 24.53±0.11 | 0.45 | ||
| 1.31×103 | 27.49±0.13 | 0.48 | 27.51±0.17 | 0.63 | ||
表1 荧光定量PCR重复性实验结果
Table 1 Results of qPCR repeatability test
| 质粒浓度Plasmid concentration/(copies·µL-1) | 组内实验Within-group experiments | 组间实验Intergroup experiments | ||||
|---|---|---|---|---|---|---|
| Ct值(x±s) Ct value | 变异系数(CV) Coefficient variation/% | Ct值(x±s) Ct value | 变异系数(CV) Coefficient variation/% | |||
| 1.31×108 | 10.94±0.09 | 0.81 | 10.89±0.10 | 0.94 | ||
| 1.31×107 | 14.51±0.02 | 0.13 | 14.44±0.13 | 0.92 | ||
| 1.31×106 | 17.90±0.06 | 0.31 | 17.80±0.15 | 0.87 | ||
| 1.31×105 | 21.35±0.08 | 0.37 | 21.23±0.16 | 0.74 | ||
| 1.31×104 | 24.47±0.11 | 0.43 | 24.53±0.11 | 0.45 | ||
| 1.31×103 | 27.49±0.13 | 0.48 | 27.51±0.17 | 0.63 | ||
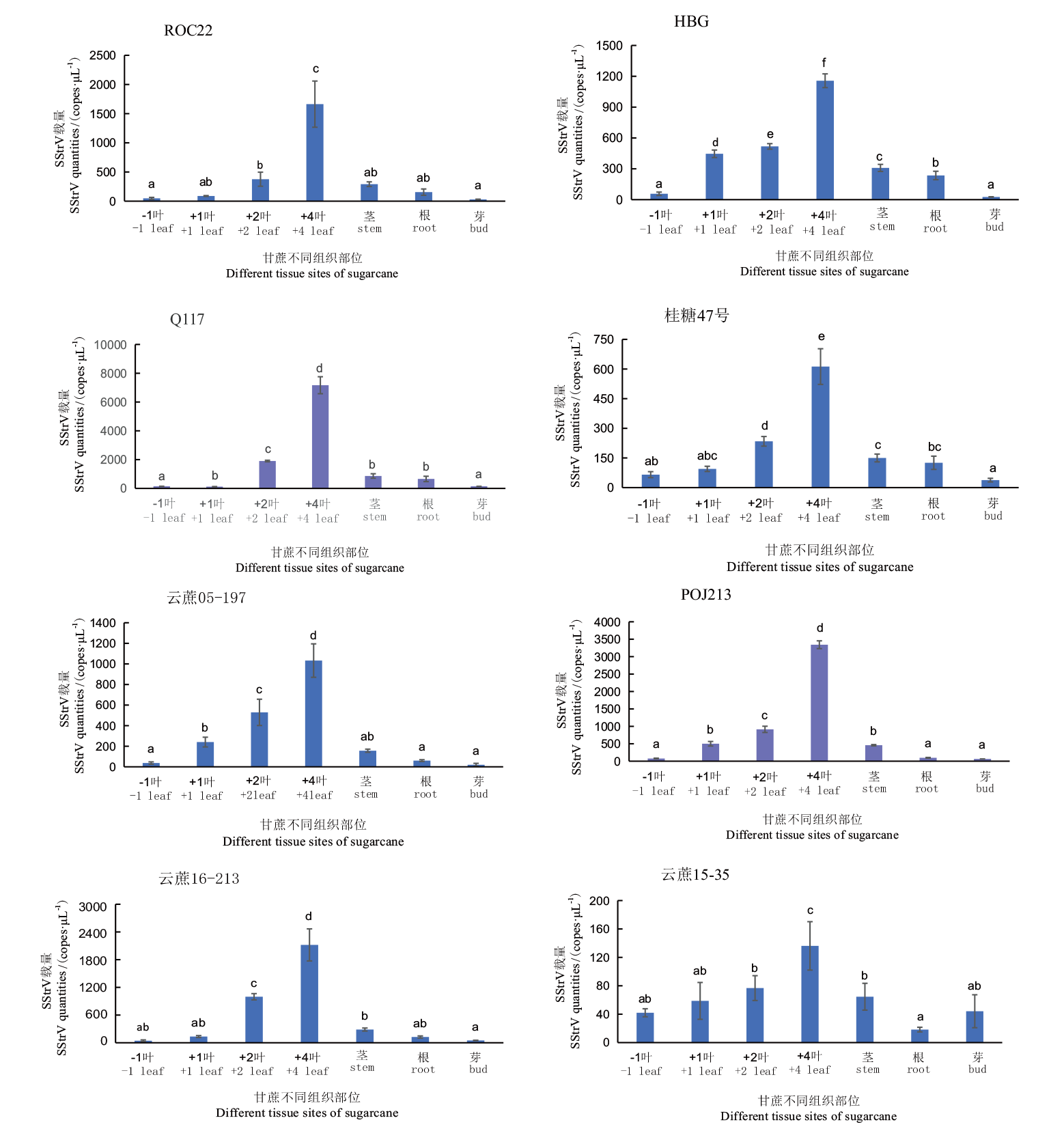
图5 八个株系不同组织部位SStrV检测 图中误差线表示标准偏差,小写字母表示不同组织部位中病毒SStrV载量差异达到(P <0.05)显著水平(使用Duncan法计算)
Fig. 5 Detection of SStrV in different tissue parts of eight lines The error line in the figure refers to the standard deviation. The lowercase letters indicates that the SStrV load of the virus in different tissue sites is significantly different(P <0.05)(Calculated using the Duncan method)
| [1] |
Kandel R, Yang XP, Song J, et al. Potentials, challenges, and genetic and genomic resources for sugarcane biomass improvement[J]. Front Plant Sci, 2018, 9: 151.
doi: 10.3389/fpls.2018.00151 pmid: 29503654 |
| [2] | Apon TA, Ahmde SF, Bony ZF, et al. Sett priming with salicylic acid improves salinity tolerance of sugarcane(Saccharum officinarum L.) during early stages of crop development[J]. Heliyon, 2023, 9(5): e16030. |
| [3] | 张跃彬, 吴才文. 国内外甘蔗产业技术进展及发展分析[J]. 中国糖料, 2017, 39(3): 47-50. |
| Zhang YB, Wu CW. Progress and development analysis of sugarcane industry in the world[J]. Sugar Crops China, 2017, 39(3): 47-50. | |
| [4] |
Swapna M, Kumar S. MicroRNAs and their regulatory role in sugarcane[J]. Front Plant Sci, 2017, 8: 997.
doi: 10.3389/fpls.2017.00997 pmid: 28659947 |
| [5] |
黄佳艳, 冯小艳, 沈林波, 等. 甘蔗ShPR10基因的克隆及其编码蛋白与甘蔗线条花叶病毒P1蛋白的互作研究[J]. 生物技术通报, 2023, 39(10): 163-174.
doi: 10.13560/j.cnki.biotech.bull.1985.2023-0403 |
| Huang JY, Feng XY, Shen LB, et al. Cloning of sugarcane ShPR10 gene and study on the interaction between ShPR10 protein and P1 protein encoded by sugarcane streak mosaic virus[J]. Biotechnol Bull, 2023, 39(10): 163-174. | |
| [6] | Calderan-Rodrigues MJ, de Barros Dantas LL, Cheavegatti Gianotto A, et al. Applying molecular phenotyping tools to explore sugarcane carbon potential[J]. Front Plant Sci, 2021, 12: 637166. |
| [7] | 李明, 田洪春, 黄智刚. 我国甘蔗产业发展现状研究[J]. 中国糖料, 2017, 39(1): 67-70. |
| Li M, Tian HC, Huang ZG. Research on the development status of sugarcane industry in China[J]. Sugar Crops China, 2017, 39(1): 67-70. | |
| [8] | 价格成本调查中心. 2022/23 年制糖期行业运行情况及新制糖期展望[EB/OL].[2023-12-26]. https://www.ndrc.gov.cn/wsdwhfz/202312/t20231226_1362936.html. |
| Price Cost Survey Center. Industry operation in 2022/23 sugar production period and outlook for new sugar production period[EB/OL].[2023-12-26]. https://www.ndrc.gov.cn/wsdwhfz/202312/t202312261362936.html. | |
| [9] | 许孚, 汪洲涛, 路贵龙, 等. 甘蔗遗传改良中的基因工程:适用、成就、局限和展望[J]. 农业生物技术学报, 2022, 30(3): 580-593. |
| Xu F, Wang ZT, Lu GL, et al. Genetic engineering in sugarcane improvement: adaptability, achievements, limitations and prospects[J]. J Agric Biotechnol, 2022, 30(3): 580-593. | |
| [10] | Gallan DZ, Penteriche AB, Henrique MO, et al. Sugarcane multitrophic interactions: integrating belowground and aboveground organisms[J]. Genet Mol Biol, 2022, 46(Suppl 1): e20220163. |
| [11] | 许小洁, 于伟鹏, 杨广玲, 等. 甘蔗花叶病毒辅助成分-蛋白酶基因原核表达及抗血清制备[J]. 山东农业科学, 2019, 51(3): 87-91. |
| Xu XJ, Yu WP, Yang GL, et al. Prokaryotic expression and antiserum preparation of helper component-proteinase of sugarcane mosaic virus[J]. Shandong Agric Sci, 2019, 51(3): 87-91. | |
| [12] | 陈海, 申亚南, 吕文竹, 等. 甘蔗高粱花叶病毒RT-LAMP快速检测方法的建立及评价[J]. 分子植物育种, 2020, 18(10): 3282-3287. |
| Chen H, Shen YN, Lü WZ, et al. Establishment and evaluation of RT-LAMP for the rapid detection of Sorghum mosaic virus[J]. Mol Plant Breed, 2020, 18(10): 3282-3287. | |
| [13] | 徐小伟. 甘蔗线条花叶病毒P1蛋白诱导的广谱抗病蛋白的鉴定及功能研究[D]. 扬州: 扬州大学, 2023. |
| Xu XW. Identification and functional analysis of broad-spectrum resistance protein induced by P1 protein of sugarcane streak mosaic virus[D]. Yangzhou: Yangzhou University, 2023. | |
| [14] | 许跃语. 甘蔗响应黄叶病毒侵染的转录组差异表达研究[D]. 福州: 福建农林大学, 2019. |
| Xu YY. Transcriptomic analysis of differentially expressed genes in sugarcane in response to yellow leaf virus infection[D]. Fuzhou: Fujian Agriculture and Forestry University, 2019. | |
| [15] | 孙生仁, 张慧丽, 吴小斌, 等. 甘蔗杆状病毒及其启动子功能的研究进展[J]. 植物生理学报, 2018, 54(6): 943-950. |
| Sun SR, Zhang HL, Wu XB, et al. Research advances in sugarcane bacilliform virus and its encoded promoter[J]. Plant Physiol J, 2018, 54(6): 943-950. | |
| [16] | 周雪平, 崔晓峰, 陶小荣. 双生病毒—— 一类值得重视的植物病毒[J]. 植物病理学报, 2003, 33(6): 487-492. |
| Zhou XP, Cui XF, Tao XR. Geminiviruses—an emerging threat for crop production[J]. Acta Phytopathol Sin, 2003, 33(6): 487-492. | |
| [17] | 李静. 我国六种双生病毒的分子鉴定及两种病毒的致病性研究[D]. 杭州: 浙江大学, 2010. |
| Li J. Molecular identification of six begomoviruses and pathogenicity of two begomoviruses in china[D]. Hangzhou: Zhejiang University, 2010. | |
| [18] |
Kraberger S, Saumtally S, Pande D, et al. Molecular diversity, geographic distribution and host range of monocot-infecting mastreviruses in Africa and surrounding islands[J]. Virus Res, 2017, 238: 171-178.
doi: S0168-1702(17)30377-5 pmid: 28687345 |
| [19] | Lin YF, Ali N, Hajimorad MR, et al. Incidence, geographical distribution, and genetic diversity of sugarcane striate virus in Saccharum species in China[J]. Plant Dis, 2021, 105(11): 3531-3537. |
| [20] |
Filloux D, Fernandez E, Comstock JC, et al. Viral metagenomic-based screening of sugarcane from Florida reveals occurrence of six sugarcane-Infecting viruses and high prevalence of sugarcane yellow leaf virus[J]. Plant Dis, 2018, 102(11): 2317-2323.
doi: 10.1094/PDIS-04-18-0581-RE pmid: 30207899 |
| [21] | Boukari W, Alcalá-Briseño RI, Kraberger S, et al. Occurrence of a novel mastrevirus in sugarcane germplasm collections in Florida, Guadeloupe and Réunion[J]. Virol J, 2017, 14(1): 146. |
| [22] | 于志亚, 杨鸣发, 马云云, 等. SFTSV实时荧光定量RT-PCR检测方法的建立及应用[J]. 中国兽医科学, 2022, 52(3): 292-297. |
| Yu ZY, Yang MF, Ma YY, et al. Establishment and application of real-time fluorescent quantitative RT-PCR for the detection of SFTSV[J]. Chin Vet Sci, 2022, 52(3): 292-297. | |
| [23] |
张记宇, 韩郁茹, 时洪艳, 等. 猪急性腹泻综合征冠状病毒SYBR Green荧光定量PCR检测方法的建立及应用[J]. 畜牧兽医学报, 2021, 52(10): 2887-2894.
doi: 10.11843/j.issn.0366-6964.2021.010.019 |
| Zhang JY, Han YR, Shi HY, et al. Establishment and application of SYBR green real-time PCR for swine acute diarrhea syndrome coronavirus[J]. Acta Vet Zootechnica Sin, 2021, 52(10): 2887-2894. | |
| [24] | 王坤, 陈开闯, 胡筱璇, 等. 甘蔗杆状病毒荧光定量PCR检测方法的建立及初步应用[J]. 基因组学与应用生物学, 2020, 39(4): 1779-1784. |
| Wang K, Chen KC, Hu XX, et al. Establishment and preliminary application of fluorescence quantitative PCR for detection of sugarcane bacilliform virus[J]. Genom Appl Biol, 2020, 39(4): 1779-1784. | |
| [25] | 王洪星, 龚殿, 张雨良, 等. 甘蔗黄叶病毒实时荧光RT-PCR检测体系研究[J]. 中国糖料, 2013, 35(1): 1-4. |
| Wang HX, Gong D, Zhang YL, et al. Research of real-time RT-PCR detection system for sugarcane yellow leaf virus[J]. Sugar Crops China, 2013, 35(1): 1-4. | |
| [26] | 王会敏. 甘蔗抗甘蔗条纹花叶病毒精准评价体系的建立与应用[D]. 南京: 南京农业大学, 2022. |
| Wang HM. Establishment and application of an accurate evaluation system for sugarcane resistance to Sugarcane streak mosaic virus[D]. Nanjing: Nanjing Agricultural University, 2022. | |
| [27] | 王洪星. 侵染海南甘蔗的高粱花叶病毒鉴定及检测[D]. 海口: 海南大学, 2012. |
| Wang HX. Characterization and detection of sorghum mosaic virus of sugarcane in Hainan[D]. Haikou: Hainan University, 2012. | |
| [28] | 淡明, 李松, 余坤兴, 等. 甘蔗健康种苗宿根矮化病的荧光定量PCR检测[J]. 中国农学通报, 2011, 27(5): 372-376. |
|
Dan M, Li S, Yu KX, et al. Detection of ratoon stunting disease in virus-free seedcane of sugarcane by real-time fluorescence quantitative PCR[J]. Chin Agric Sci Bull, 2011, 27(5): 372-376.
doi: 10.11924/j.issn.1000-6850.2010-2614 |
|
| [29] |
Garces FF, Gutierrez A, Hoy JW. Detection and quantification of Xanthomonas albilineans by qPCR and potential characterization of sugarcane resistance to leaf scald[J]. Plant Dis, 2014, 98(1): 121-126.
doi: 10.1094/PDIS-04-13-0431-RE pmid: 30708616 |
| [30] | 夏宝山. 我国蔗区甘蔗杆状病毒的发生及其qPCR和RCA检测方法的建立与应用[D]. 海口: 海南大学, 2023. |
| Xia BS. The occurrence of Sugarcane bacilliform virus in sugarcane planting areas of China and the establishment and application of qPCR and RCA detection methods[D]. Haikou: Hainan University, 2023. | |
| [31] | Wang D, Zhang X, Chen DX, et al. First report of Sida leaf curl virus and associated Betasatellite from tobacco[J]. Plant Dis, 2022, 106(3): 1078. |
| [32] | Wang D, Zhang X, Yao XM, et al. A 7-amino-acid motif of rep protein essential for virulence is critical for triggering host defense against Sri Lankan cassava mosaic virus[J]. Mol Plant Microbe Interact, 2020, 33(1): 78-86. |
| [33] | Wang D, Zhang X, Yin YT, et al. First report of Ludwigia yellow vein vietnam virus causing leaf curling on tobacco plants in Hainan province, China[J]. Plant Dis, 2023,107:2559. |
| [34] | Wang N, Zhao PZ, Wang D, et al. Diverse begomoviruses evolutionarily hijack plant terpenoid-based defense to promote whitefly performance[J]. Cells, 2022, 12(1): 149. |
| [35] |
Ye J, Zhang LL, Zhang X, et al. Plant defense networks against insect-borne pathogens[J]. Trends Plant Sci, 2021, 26(3): 272-287.
doi: 10.1016/j.tplants.2020.10.009 pmid: 33277186 |
| [1] | 桑森骅. 基于实时动态成像系统对NK细胞毒性的检测方法[J]. 生物技术通报, 2024, 40(4): 77-84. |
| [2] | 杨伟杰, 杨周林, 朱浩东, 魏煜, 刘君, 刘训. 地衣素合成酶关键模块 LchAD 蛋白的性质和功能研究[J]. 生物技术通报, 2024, 40(3): 322-332. |
| [3] | 杨文莉, 朱梨梨, 陈健, 陈燕欣, 姚涓, 姜大刚. 我国作物病菌标准品的研究进展[J]. 生物技术通报, 2024, 40(2): 31-37. |
| [4] | 刘星雨, 李洁, 朱龙佼, 李相阳, 许文涛. 铜绿假单胞菌适配体的获得及应用[J]. 生物技术通报, 2024, 40(1): 186-193. |
| [5] | 谢宏, 周丽莹, 李舒文, 王梦迪, 艾晔, 晁跃辉. 蒺藜苜蓿MtCIM基因结构和功能分析[J]. 生物技术通报, 2024, 40(1): 262-269. |
| [6] | 余慧, 王静, 梁昕昕, 辛亚平, 周军, 赵会君. 宁夏枸杞铁镉响应基因的筛选及其功能验证[J]. 生物技术通报, 2023, 39(7): 195-205. |
| [7] | 张雪萍, 鲁雨晴, 张月倩, 李晓娟. 植物细胞外囊泡及其分析技术的进展[J]. 生物技术通报, 2023, 39(5): 32-43. |
| [8] | 姚姿婷, 曹雪颖, 肖雪, 李瑞芳, 韦小妹, 邹承武, 朱桂宁. 火龙果溃疡病菌实时荧光定量PCR内参基因的筛选[J]. 生物技术通报, 2023, 39(5): 92-102. |
| [9] | 宋海娜, 吴心桐, 杨鲁豫, 耿喜宁, 张华敏, 宋小龙. 葱鳞葡萄孢菌诱导下韭菜RT-qPCR内参基因的筛选和验证[J]. 生物技术通报, 2023, 39(3): 101-115. |
| [10] | 穆德添, 万凌云, 章瑶, 韦树根, 陆英, 付金娥, 田艺, 潘丽梅, 唐其. 钩藤管家基因筛选及生物碱合成相关基因的表达分析[J]. 生物技术通报, 2023, 39(2): 126-138. |
| [11] | 高凯月, 郭雨婷, 杜奕谋, 郑小梅, 马欣荣, 赵伟, 郑平, 孙际宾. 黑曲霉葡萄糖吸收定量检测的方法建立及其在MstC功能研究中的应用[J]. 生物技术通报, 2023, 39(12): 71-80. |
| [12] | 陈晓琳, 刘洋儿, 许文涛, 郭明璋, 刘慧琳. 合成生物学细胞传感技术在食品安全快速检测中的应用[J]. 生物技术通报, 2023, 39(1): 137-149. |
| [13] | 胡海洋, 应婉琴, 何军, 吕芷贤, 谢小平, 邓仲良. 酶促重组等温扩增实时荧光法快速检测肺炎支原体方法的建立及应用[J]. 生物技术通报, 2022, 38(9): 264-270. |
| [14] | 刘娜, 焦京琳, 饶正华. 短链脂肪酸在动物样本中的检测方法研究进展[J]. 生物技术通报, 2022, 38(8): 84-91. |
| [15] | 曹英芳, 赵新, 刘双, 李瑞环, 刘娜, 徐石勇, 高芳瑞, 马卉, 兰青阔, 檀建新, 王永. 抗除草剂大豆GE-J12实时荧光定量PCR检测方法的建立[J]. 生物技术通报, 2022, 38(7): 146-152. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||