








生物技术通报 ›› 2024, Vol. 40 ›› Issue (9): 291-300.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0336
张曼玉1,2( ), 董嘉诚1,2, 苟福凡1,2, 弓朝晖1,2, 刘倩2, 孙文良2, 孔臻3, 郝捷4, 王敏1, 田朝光2(
), 董嘉诚1,2, 苟福凡1,2, 弓朝晖1,2, 刘倩2, 孙文良2, 孔臻3, 郝捷4, 王敏1, 田朝光2( )
)
收稿日期:2024-04-09
出版日期:2024-09-26
发布日期:2024-10-12
通讯作者:
田朝光,男,博士,研究员,研究方向:真菌合成生物学;E-mail: tian_cg@tib.cas.cn作者简介:张曼玉,女,研究方向:真菌合成生物学;E-mail: zhangmy@tib.cas.cn
基金资助:
ZHANG Man-yu1,2( ), DONG Jia-cheng1,2, GOU Fu-fan1,2, GONG Chao-hui1,2, LIU Qian2, SUN Wen-liang2, KONG zhen3, HAO Jie4, WANG Min1, TIAN Chao-guang2(
), DONG Jia-cheng1,2, GOU Fu-fan1,2, GONG Chao-hui1,2, LIU Qian2, SUN Wen-liang2, KONG zhen3, HAO Jie4, WANG Min1, TIAN Chao-guang2( )
)
Received:2024-04-09
Published:2024-09-26
Online:2024-10-12
摘要:
【目的】挖潜新型果胶酯酶的酶资源,在嗜热毁丝霉(Myceliophthora thermophila)中高水平表达烟草生物质诱导显著上调的同源果胶酯酶,对其进行酶学性质研究,并探究该果胶酯酶在协同降解烟草生物质过程中的作用。【方法】利用RT-qPCR的方法分析嗜热毁丝霉在烟草生物质中果胶酯酶MtCE12-1的表达水平,通过2A肽介导的表达筛选系统,在嗜热毁丝霉菌株ATCC 42462中高表达果胶酯酶基因Mtce12-1,对高活性的阳性转化子进行产酶培养和蛋白纯化,表征了果胶酯酶MtCE12-1的酶学性质;以两种烟草生物质烟草压棒和烟草梗丝为底物,通过检测纤维素的剩余含量,分析了与纤维素酶的协同作用效果。【结果】与葡萄糖培养条件相比较,烟草生物质条件下果胶酯酶基因Mtce12-1的转录水平显著上调109-110倍,SDS-PAGE电泳分析、拷贝数和Western检测显示,重组蛋白MtCE12-1成功进行了表达和分泌,表达水平达到464.08 U/mL。该酶在75℃、pH 8.0时表现出最佳酶活力,在50-85℃和pH 7.0-9.0的范围内表现出较好的酶活力,具有良好的热稳定性。将100-300 µg的MtCE12-1添加至降解体系后,烟草生物质中纤维素降解效率分别提高了18.5%-30.7%和14.6%-30.5%。【结论】用2A肽介导的表达系统在嗜热毁丝霉中可以高效表达和纯化制备目标蛋白,碱性果胶酯酶MtCE12-1不仅具有良好的温度稳定性,在烟草生物质降解过程中也具有突出的作用效果,为烟草工业生产应用提供了潜在的优质酶资源。
张曼玉, 董嘉诚, 苟福凡, 弓朝晖, 刘倩, 孙文良, 孔臻, 郝捷, 王敏, 田朝光. 嗜热毁丝霉果胶酯酶MtCE12-1的克隆表达及其酶学性质和应用研究[J]. 生物技术通报, 2024, 40(9): 291-300.
ZHANG Man-yu, DONG Jia-cheng, GOU Fu-fan, GONG Chao-hui, LIU Qian, SUN Wen-liang, KONG zhen, HAO Jie, WANG Min, TIAN Chao-guang. Cloning, Expression, Characterization and Application of the Pectin Esterase MtCE12-1 from Myceliophthora thermophila[J]. Biotechnology Bulletin, 2024, 40(9): 291-300.
| 名称 Primer | 序列 DNA sequence(5'-3') | 用途 Application |
|---|---|---|
| Mtce12-1-RT-F | GACGACATTGTAGTGATC | 基因表达分析 |
| Mtce12-1-RT-R | TAGTGGTTGAAGGTGTAG | 基因表达分析 |
| actin-RT-F | AACGCTCCTGCCTTCTAC | 基因表达分析 |
| actin-RT-R | GTAACACCATCACCAGAGTC | 基因表达分析 |
| Mtce12-1-F | GCCAGTTTCGTTCTTCAGAACTAGTATGCGACCGTGGTCGACTCT | 基因克隆 |
| Mtce12-1-R | GTGGTGGTGGTGGTGGTGGATATCGAACACCTGAGGCACCGGGG | 基因克隆 |
| Ptef1-F | CTTCGACCCCTCCTCAAATCTTCTT | 基因鉴定 |
| TtrpC-R | GAGCTATTAAATCACTAGAAGGCAC | 基因鉴定 |
| Mtalp1-ko-F | TTCTGGCCTGCCCTTTTCTTTCAAC | 基因鉴定 |
| Mtalp1-ko-R | GCCCCTTCTTCCGAAAGGGGAGGTA | 基因鉴定 |
表1 扩增靶序列引物
Table 1 Primers for amplifying the target sequence
| 名称 Primer | 序列 DNA sequence(5'-3') | 用途 Application |
|---|---|---|
| Mtce12-1-RT-F | GACGACATTGTAGTGATC | 基因表达分析 |
| Mtce12-1-RT-R | TAGTGGTTGAAGGTGTAG | 基因表达分析 |
| actin-RT-F | AACGCTCCTGCCTTCTAC | 基因表达分析 |
| actin-RT-R | GTAACACCATCACCAGAGTC | 基因表达分析 |
| Mtce12-1-F | GCCAGTTTCGTTCTTCAGAACTAGTATGCGACCGTGGTCGACTCT | 基因克隆 |
| Mtce12-1-R | GTGGTGGTGGTGGTGGTGGATATCGAACACCTGAGGCACCGGGG | 基因克隆 |
| Ptef1-F | CTTCGACCCCTCCTCAAATCTTCTT | 基因鉴定 |
| TtrpC-R | GAGCTATTAAATCACTAGAAGGCAC | 基因鉴定 |
| Mtalp1-ko-F | TTCTGGCCTGCCCTTTTCTTTCAAC | 基因鉴定 |
| Mtalp1-ko-R | GCCCCTTCTTCCGAAAGGGGAGGTA | 基因鉴定 |
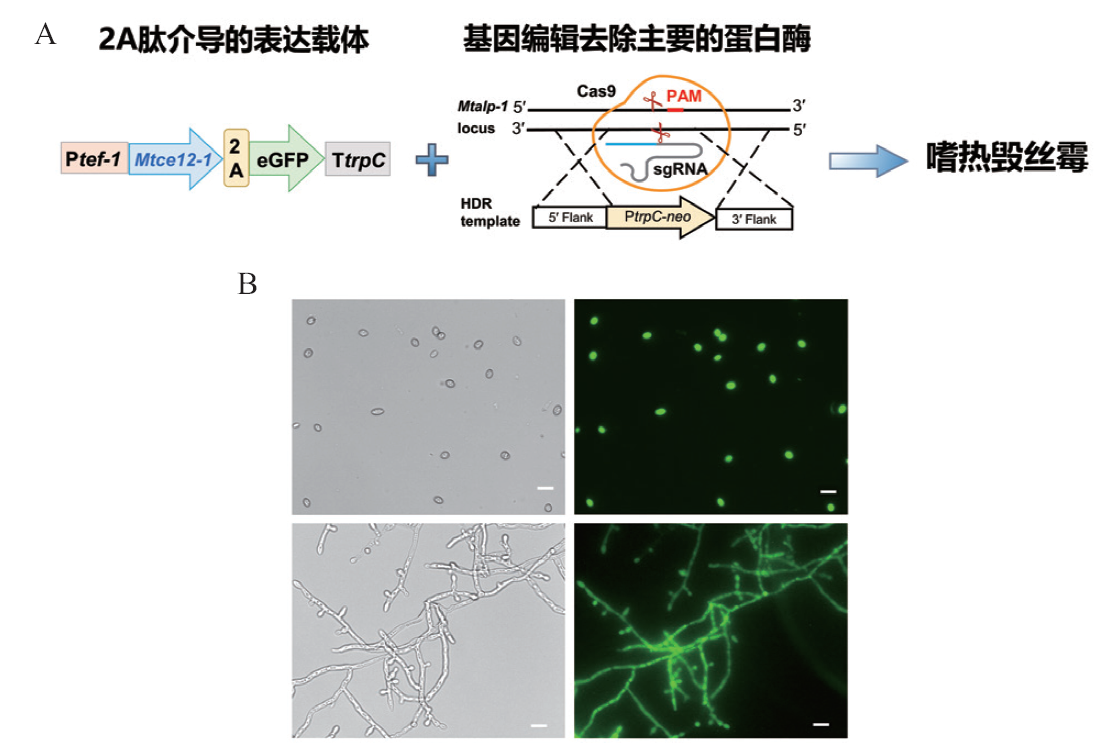
图2 嗜热毁丝霉重组表达果胶酯酶MtCE12-1 A:表达载体构建以及重组表达MtCE12-1的示意图;B:荧光显微镜观察重组菌株OE-MtCE12-1-GFP的孢子和菌丝荧光,B中的标尺为 10 µm
Fig. 2 Recombinant expressed pectin esterase MtCE12-1 in M. thermophile A: Schematic illustration of the expressed vector and recombinant expression of MtCE12-1. B: Microscopic fluorescence imaging of conidia and mycelia of the overexpressing strain OE-MtCE12-1-GFP. Scale bar 10 μm
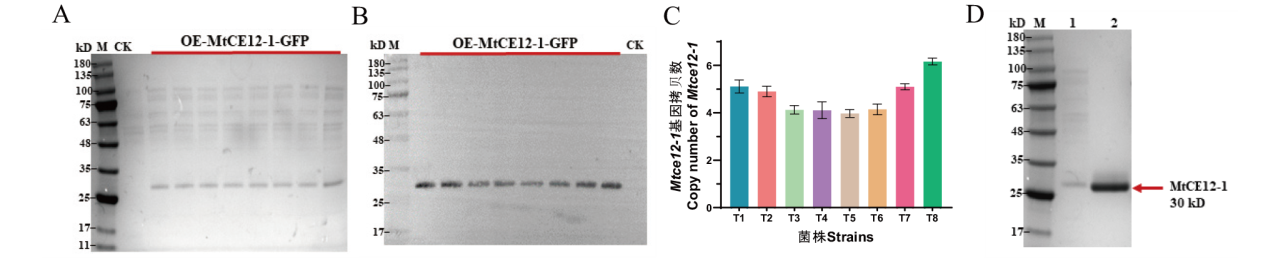
图3 SDS-PAGE电泳、Western-blotting和拷贝数检测重组蛋白MtCE12-1的表达 A:上清液SDS-PAGE电泳分析 M: 蛋白Marker,泳道CK为空载体pAN52-1N转入宿主菌株的阴性对照,其它泳道为重组菌株OE-MtCE12-1-GFP;B:Western-blotting检测分析,泳道CK为阴性对照,其它泳道为重组菌株OE-MtCE12-1-GFP;C:Mtce12-1在重组菌株中的基因拷贝数分析;D:纯化后的蛋白SDS-PAGE电泳分析, M: 蛋白Marker,泳道1为重组菌株OE-MtCE12-1-GFP蛋白上清液,泳道2为MtCE12-1纯化样品
Fig. 3 Detecting the expression of recombinant protein MtCE12-1 by using the SDS-PAGE electrophoresis, Western blotting, and copy number A: SDS-PAGE analysis of secreted proteins from the CK and OE-MtCE12-1-GFP strains after 4-day of growth. M: the protein molecular weight markers. CK is used as the negative control for the transformation of the empty vector pAN52-1N into the host wild-type strain. B: Western blotting of culture supernatants from the strains and probed with anti-His antibody. C: Assay of Mtce12-1 copy number in overexpressing strains by RT-qPCR. D: SDS-PAGE analysis of the purified MtCE12-1. M: the protein molecular weight markers; Lane 1: the culture supernatants of the OE-MtCE12-1-GFP strain T8; Lane 2: the purified enzyme of MtCE12-1
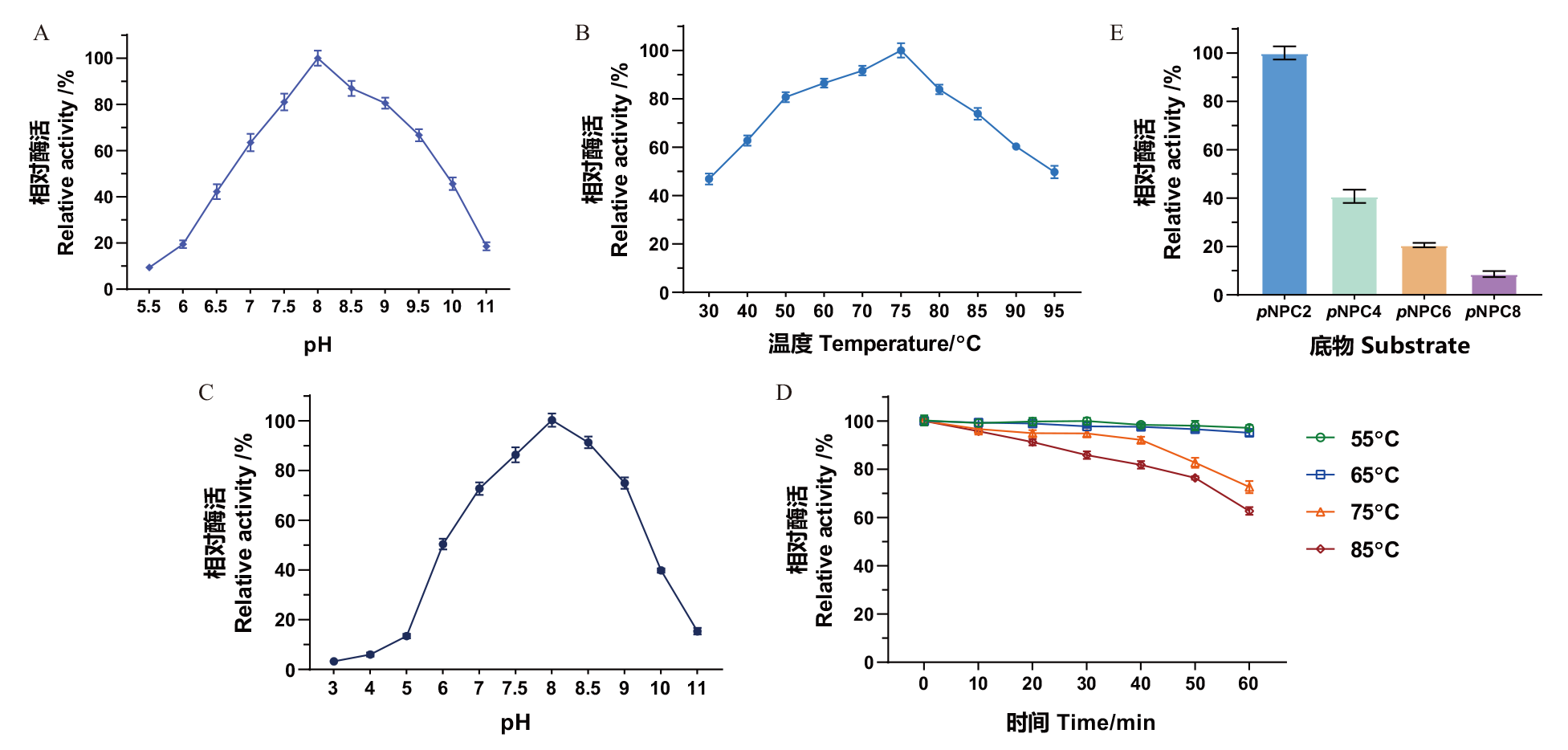
图4 果胶酯酶MtCE12-1的酶学性质 A:pH对MtCE12-1酶活力的影响;B:温度对MtCE12-1酶活力的影响;C:pH对MtCE12-1酶活力稳定性的影响;D:温度对MtCE12-1酶活力稳定性的影响;E:底物特异性
Fig. 4 Enzymatic properties of pectin esterase MtCE12-1 A: Effect of pH on the enzyme activity of MtCE12-1. B: Effect of temperature on the enzyme activity of MtCE12-1. C: Effect of pH on the enzyme activity of MtCE12-1. D: Effect of temperature on the stability of MtCE12-1. E: Substrate specificity of MtCE12-1
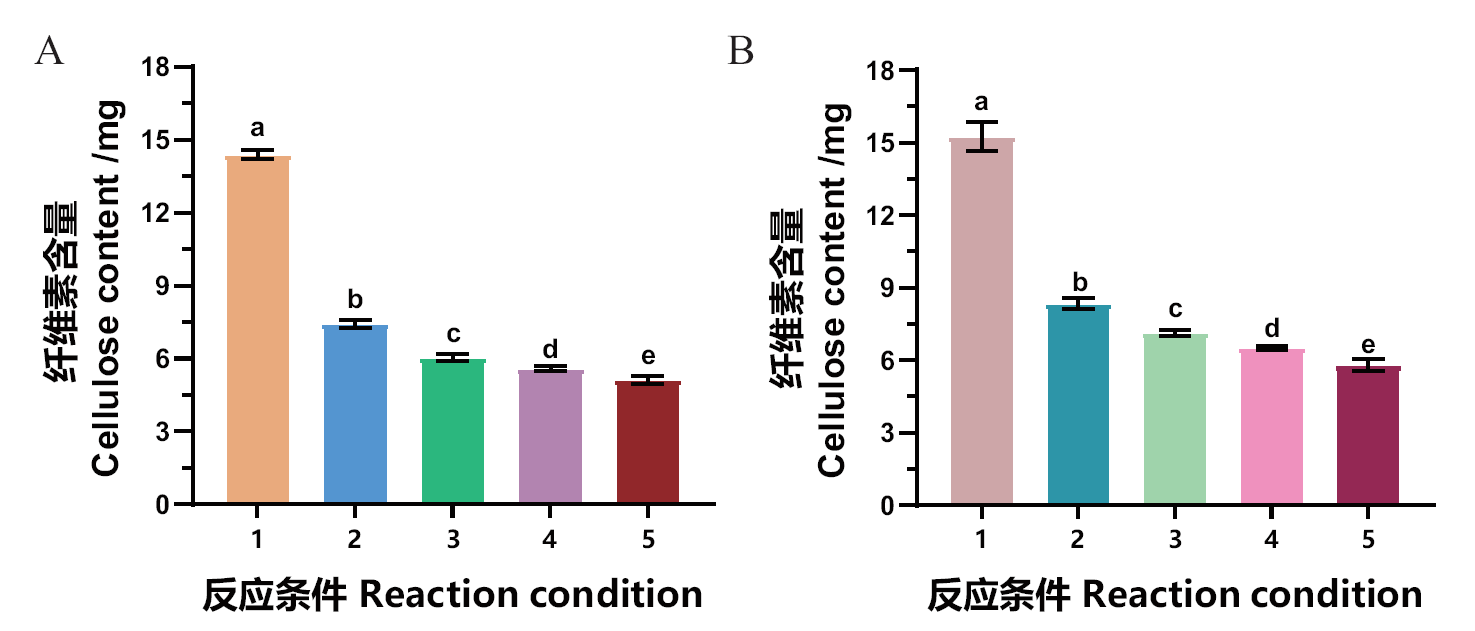
图5 果胶酯酶MtCE12-1与纤维素酶协同降解烟草废弃生物质 A:MtCE12-1与纤维素酶协同降解烟草压棒;B:MtCE12-1与纤维素酶协同降解烟草梗丝。其中条件1为不添加酶液的对照组,条件2为只添加纤维素酶,条件3为添加100 μg MtCE12-1和纤维素酶,条件4为添加200 μg MtCE12-1和纤维素酶,条件5为添加300 μg MtCE12-1和纤维素酶。不同字母表示具有统计学意义(Tukey’s HSD, P < 0.05)
Fig. 5 Synergistic degradation of tobacco waste biomass by pectin esterase MtCE12-1 and cellulase A: Synergistic degradation of tobacco bar by MtCE12-1 and cellulase. B: Synergistic degradation of tobacco stem by MtCE12-1 and cellulase. 1: Control(no enzyme); 2: only cellulase; 3: 100 μg MtCE12-1 and cellulase; 4: 200 μg MtCE12-1 and cellulase; 5: 300 μg MtCE12-1 and cellulase. Different letters are statistically significant(Tukey’s HSD, P < 0.05)
| [1] | Zhang GT, Zhan JJ, Fu HQ. Trends in smoking prevalence and intensity between 2010 and 2018: implications for tobacco control in China[J]. Int J Environ Res Public Health, 2022, 19(2): 670. |
| [2] | Zhang Y, Li RD, Shang GL, et al. Effects of multiscale-mechanical fragmentation on techno-functional properties of industrial tobacco waste[J]. Powder Technol, 2022, 402: 117327. |
| [3] | Tao JM, Chen QS, Chen SY, et al. Metagenomic insight into the microbial degradation of organic compounds in fermented plant leaves[J]. Environ Res, 2022, 214(Pt 1): 113902. |
| [4] | Lin YN, Wang C, Yu GF, et al. Transformation of tobacco biomass into value-added carbohydrate, aromatics, and biochar[J]. Biomass Convers Biorefin, 2024, 14(10): 11697-11705. |
| [5] | 黄申, 芦尧, 刘强, 等. 生物酶在烟草工业中的应用研究进展[J]. 轻工学报, 2023, 38(5): 112-118. |
| Huang S, Lu Y, Liu Q, et al. Review on application of biological enzymes in tobacco industry[J]. J Light Ind, 2023, 38(5): 112-118. | |
| [6] |
郝捷, 李选文, 张宝, 等. 纤维素酶在烟草中的应用进展[J]. 生物技术进展, 2023, 13(2): 166-173.
doi: 10.19586/j.2095-2341.2022.0127 |
|
Hao J, Li XW, Zhang B, et al. Application progress of cellulase in tobacco[J]. Curr Biotechnol, 2023, 13(2): 166-173.
doi: 10.19586/j.2095-2341.2022.0127 |
|
| [7] | Zou G, Shi SH, Jiang YP, et al. Construction of a cellulase hyper-expression system in Trichoderma reesei by promoter and enzyme engineering[J]. Microb Cell Fact, 2012, 11: 21. |
| [8] | Daly P, Cai F, Kubicek CP, et al. From lignocellulose to plastics: knowledge transfer on the degradation approaches by fungi[J]. Biotechnol Adv, 2021, 50: 107770. |
| [9] |
Meng JL, Mäkelä MR, de Vries RP. Molecular engineering to improve lignocellulosic biomass based applications using filamentous fungi[J]. Adv Appl Microbiol, 2021, 114: 73-109.
doi: 10.1016/bs.aambs.2020.09.001 pmid: 33934853 |
| [10] | Kun RS, Gomes ACS, Hildén KS, et al. Developments and opportunities in fungal strain engineering for the production of novel enzymes and enzyme cocktails for plant biomass degradation[J]. Biotechnol Adv, 2019, 37(6): 107361. |
| [11] | Berka RM, Grigoriev IV, Otillar R, et al. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris[J]. Nat Biotechnol, 2011, 29: 922-927. |
| [12] | Kolbusz MA, Di Falco M, Ishmael N, et al. Transcriptome and exoproteome analysis of utilization of plant-derived biomass by Myceliophthora thermophila[J]. Fungal Genet Biol, 2014, 72: 10-20. |
| [13] |
Liu Q, Li JG, Gao RR, et al. CLR-4, a novel conserved transcription factor for cellulase gene expression in ascomycete fungi[J]. Mol Microbiol, 2019, 111(2): 373-394.
doi: 10.1111/mmi.14160 pmid: 30474279 |
| [14] | Li N, Liu Y, Liu DF, et al. MtTRC-1, a novel transcription factor, regulates cellulase production via directly modulating the genes expression of the Mthac-1 and Mtcbh-1 in Myceliophthora thermophila[J]. Appl Environ Microbiol, 2022, 88(19): e0126322. |
| [15] | Zhu ZJ, Zhang MY, Liu DD, et al. Development of the thermophilic fungus Myceliophthora thermophila into glucoamylase hyperproduction system via the metabolic engineering using improved AsCas12a variants[J]. Microb Cell Fact, 2023, 22(1): 150. |
| [16] | Li JG, Lin LC, Sun T, et al. Direct production of commodity chemicals from lignocellulose using Myceliophthora thermophila[J]. Metab Eng, 2020, 61: 416-426. |
| [17] | Liu J, Chen MX, Gu SY, et al. Independent metabolism of oligosaccharides is the keystone of synchronous utilization of cellulose and hemicellulose in Myceliophthora[J]. PNAS Nexus, 2024, 3(2): pgae053. |
| [18] | Gu SY, Wu TJ, Zhao JQ, et al. Rewiring metabolic flux to simultaneously improve malate production and eliminate by-product succinate accumulation by Myceliophthora thermophila[J]. Microb Biotechnol, 2024, 17(2): e14410. |
| [19] | Kashyap DR, Vohra PK, Chopra S, et al. Applications of pectinases in the commercial sector: a review[J]. Bioresour Technol, 2001, 77(3): 215-227. |
| [20] | Hoondal G, Tiwari R, Tewari R, et al. Microbial alkaline pectinases and their industrial applications: a review[J]. Appl Microbiol Biotechnol, 2002, 59(4): 409-418. |
| [21] |
Lara-Márquez A, Zavala-Páramo MG, López-Romero E, et al. Biotechnological potential of pectinolytic complexes of fungi[J]. Biotechnol Lett, 2011, 33(5): 859-868.
doi: 10.1007/s10529-011-0520-0 pmid: 21246254 |
| [22] | Sista Kameshwar AK, Qin WS. Structural and functional properties of pectin and lignin-carbohydrate complexes de-esterases: a review[J]. Bioresour Bioprocess, 2018, 5(1): 43. |
| [23] |
Schmitz K, Protzko R, Zhang LS, et al. Spotlight on fungal pectin utilization-from phytopathogenicity to molecular recognition and industrial applications[J]. Appl Microbiol Biotechnol, 2019, 103(6): 2507-2524.
doi: 10.1007/s00253-019-09622-4 pmid: 30694345 |
| [24] | Benz JP, Chau BH, Zheng DA, et al. A comparative systems analysis of polysaccharide-elicited responses in Neurospora crassa reveals carbon source-specific cellular adaptations[J]. Mol Microbiol, 2014, 91(2): 275-299. |
| [25] | Liu Q, Zhang YL, Li FY, et al. Upgrading of efficient and scalable CRISPR-Cas-mediated technology for genetic engineering in thermophilic fungus Myceliophthora thermophila[J]. Biotechnol Biofuels, 2019, 12: 293. |
| [26] | Yang YJ, Liu Y, Liu DD, et al. Development of a flow cytometry-based plating-free system for strain engineering in industrial fungi[J]. Appl Microbiol Biotechnol, 2022, 106(2): 713-727. |
| [27] | Liu Q, Gao RR, Li JG, et al. Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering[J]. Biotechnol Biofuels, 2017, 10: 1. |
| [28] | Gao LW, Liu GD, Zhao QQ, et al. Customized optimization of lignocellulolytic enzyme cocktails for efficient conversion of pectin-rich biomass residues[J]. Carbohydr Polym, 2022, 297: 120025. |
| [29] |
Sakai T, Sakamoto T, Hallaert J, et al. Pectin, pectinase and protopectinase: production, properties, and applications[J]. Adv Appl Microbiol, 1993, 39: 213-294.
pmid: 8213306 |
| [30] | 华婷, 李雅楠, 王凯凯, 等. 蓝状菌(Talaromyces leycettanus JCM12802)高温果胶甲酯酶PmeT在毕赤酵母中的高效表达及酶学性质[J]. 微生物学报, 2018, 58(1): 122-130. |
| Hua T, Li YN, Wang KK, et al. High-level expression and characterization of pectin methylesterase Pme T from Talaromyces leycettanus JCM12802 in Pichia pastoris[J]. Acta Microbiol Sin, 2018, 58(1): 122-130. | |
| [31] | Kumar R, Meghwanshi GK, Marcianò D, et al. Sequence, structure and functionality of pectin methylesterases and their use in sustainable carbohydrate bioproducts: a review[J]. Int J Biol Macromol, 2023, 244: 125385. |
| [1] | 王茜, 周家燕, 王倩, 邓玉萍, 张敏慧, 陈静, 杨军, 邹建. 向日葵YABBY基因家族鉴定及表达分析[J]. 生物技术通报, 2024, 40(8): 199-211. |
| [2] | 杨巍, 赵丽芬, 唐兵, 周麟笔, 杨娟, 莫传园, 张宝会, 李飞, 阮松林, 邓英. 芥菜SRO基因家族全基因组鉴定与表达分析[J]. 生物技术通报, 2024, 40(8): 129-141. |
| [3] | 胡雅丹, 伍国强, 刘晨, 魏明. MYB转录因子在调控植物响应逆境胁迫中的作用[J]. 生物技术通报, 2024, 40(6): 5-22. |
| [4] | 秦健, 李振月, 何浪, 李俊玲, 张昊, 杜荣. 肌源性细胞分化的单细胞转录谱变化及细胞间通讯分析[J]. 生物技术通报, 2024, 40(6): 330-342. |
| [5] | 陈春林, 李白雪, 李金玲, 杜清洁, 李猛, 肖怀娟. 甜瓜CmEPF基因家族的鉴定及表达分析[J]. 生物技术通报, 2024, 40(4): 130-138. |
| [6] | 陈强, 黄馨慧, 张峥, 张冲, 柳叶飞. 褪黑素对薄皮甜瓜采后软化和乙烯合成的影响[J]. 生物技术通报, 2024, 40(4): 139-147. |
| [7] | 殷亮, 王代玮, 刘悦莹, 刘海燕, 罗光宏. 蛋白酶SpP1基因克隆、表达及酶学性质的表征[J]. 生物技术通报, 2024, 40(4): 278-286. |
| [8] | 张玉, 石磊, 巩檑, 聂峰杰, 杨江伟, 刘璇, 杨文静, 张国辉, 颉瑞霞, 张丽. 马铃薯WOX基因家族的鉴定及在离体再生和非生物胁迫中的表达分析[J]. 生物技术通报, 2024, 40(3): 170-180. |
| [9] | 吴星星, 洪海波, 甘志承, 李瑞宁, 黄先忠. 辣椒CaPI的克隆与功能分析[J]. 生物技术通报, 2024, 40(3): 193-201. |
| [10] | 江林琪, 赵佳莹, 郑飞雄, 姚馨怡, 李效贤, 俞振明. 铁皮石斛14-3-3基因家族鉴定及表达分析[J]. 生物技术通报, 2024, 40(3): 229-241. |
| [11] | 郑菲, 杨俊钊, 牛羽丰, 李蕊麟, 赵国柱. 嗜热毁丝菌裂解性多糖单加氧酶TtLPMO9I的酶学性质及其功能研究[J]. 生物技术通报, 2024, 40(2): 289-299. |
| [12] | 王凤婷, 赵福顺, 乔凯彬, 徐珣, 刘金亮. 蔬菜嫁接砧穗互作分子机制研究进展[J]. 生物技术通报, 2024, 40(10): 149-159. |
| [13] | 吴圳, 张明英, 闫锋, 李依民, 高静, 颜永刚, 张岗. 掌叶大黄(Rheum palmatum L.)WRKY基因家族鉴定与分析[J]. 生物技术通报, 2024, 40(1): 250-261. |
| [14] | 杨志晓, 侯骞, 刘国权, 卢志刚, 曹毅, 芶剑渝, 王轶, 林英超. 不同抗性烟草品系Rubisco及其活化酶对赤星病胁迫的响应[J]. 生物技术通报, 2023, 39(9): 202-212. |
| [15] | 李帜奇, 袁月, 苗荣庆, 庞秋颖, 张爱琴. 盐胁迫盐芥和拟南芥褪黑素含量及合成相关基因表达模式分析[J]. 生物技术通报, 2023, 39(5): 142-151. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||