








生物技术通报 ›› 2025, Vol. 41 ›› Issue (5): 62-69.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0955
高畅1( ), 庄添驰2, 李宁3, 刘云4, 顾鹏飞1, 赵昕怡5, 季明辉2(
), 庄添驰2, 李宁3, 刘云4, 顾鹏飞1, 赵昕怡5, 季明辉2( )
)
收稿日期:2024-09-30
出版日期:2025-05-26
发布日期:2025-06-05
通讯作者:
季明辉,男,博士,副教授,研究方向 :病原体检测;E-mail: jiminghui@njmu.edu.cn作者简介:高畅,女,研究方向 :病原体检测;E-mail: 1975480691@qq.com
基金资助:
GAO Chang1( ), ZHUANG Tian-chi2, LI Ning3, LIU Yun4, GU Peng-fei1, ZHAO Xin-yi5, JI Ming-hui2(
), ZHUANG Tian-chi2, LI Ning3, LIU Yun4, GU Peng-fei1, ZHAO Xin-yi5, JI Ming-hui2( )
)
Received:2024-09-30
Published:2025-05-26
Online:2025-06-05
摘要:
目的 开发一种快速高效的结核分枝杆菌(MTB)检测方法,给基层单位、偏远地区的结核病(Tuberculosis)快速筛查提供技术方案,更好地开展结核病的防治工作。 方法 设计RPA引物、crRNA,并引入重力驱动的微流控芯片,建立RPA-CRISPR/Cas12a的片上检测方法,进一步分析该方法的灵敏度与特异性,使用疑似结核病患者的样本进行片上检测和痰培养,比较建立的方法与痰培养法的一致性。 结果 建立的RPA-CRISPR/Cas12a片上检测方法可在30 min内实现MTB检测,LOD为1 copies/μL。以痰培养鉴定为参考标准,片上检测法的灵敏度为91.11%,特异度为94.34%,阳性预测值为93.19%,阴性预测值为92.59%,准确性为92.86%,Kappa值为0.856。 结论 建立的RPA-CRISPR/Cas12a的片上检测法的灵敏度、特异性高,并且快速、简单、便捷。
高畅, 庄添驰, 李宁, 刘云, 顾鹏飞, 赵昕怡, 季明辉. RPA-CRISPR/Cas12a结合重力驱动微流控芯片的MTB快检方法的建立[J]. 生物技术通报, 2025, 41(5): 62-69.
GAO Chang, ZHUANG Tian-chi, LI Ning, LIU Yun, GU Peng-fei, ZHAO Xin-yi, JI Ming-hui. Gravity-driven Microfluidic Chip Based on RPA-CRISPR/Cas12a for the Rapid Detection of Mycobacterium tuberculosis[J]. Biotechnology Bulletin, 2025, 41(5): 62-69.
| 名称 Primer | 序列 Primer sequence (5′-3′) | 长度 Length (bp) |
|---|---|---|
| F1 | GTCATGTCAGGTGGTTCATCGAGGAGGTACC | 31 |
| R1 | GCCCACTCCGAATCGTGCTGACCGCGGATCT | 31 |
| F2 | GTCATGTCAGGTGGTTCATCGAGGAGGTACC | 31 |
| R2 | TGCCCACTCCGAATCGTGCTGACCGCGGATC | 31 |
| F3 | GGTCATGTCAGGTGGTTCATCGAGGAGGTAC | 31 |
| R3 | TGCCCACTCCGAATCGTGCTGACCGCGGATC | 31 |
| F4 | GTCATGTCAGGTGGTTCATCGAGGAGGTACC | 31 |
| R4 | CCCACTCCGAATCGTGCTGACCGCGGATCTC | 31 |
| crRNA-1 | GATGAGCCCGCGGCCTATCAGCTTG | 25 |
| crRNA-2 | TGGTGGGGTGACGGCCTACCAAGGC | 25 |
| crRNA-3 | ACGACGGGTAGCCGGCCTGAGAGG | 24 |
| crRNA-4 | GGAGGTGGAGAAGAAGCACCGGCC | 24 |
| ssDNA报告探针 | 6-FAM-TTATT-BHQ-1 | 5 |
表 1 RPA 引物、crRNA和探针序列
Table 1 Primers, crRNA and ssDNA reporter used in this study
| 名称 Primer | 序列 Primer sequence (5′-3′) | 长度 Length (bp) |
|---|---|---|
| F1 | GTCATGTCAGGTGGTTCATCGAGGAGGTACC | 31 |
| R1 | GCCCACTCCGAATCGTGCTGACCGCGGATCT | 31 |
| F2 | GTCATGTCAGGTGGTTCATCGAGGAGGTACC | 31 |
| R2 | TGCCCACTCCGAATCGTGCTGACCGCGGATC | 31 |
| F3 | GGTCATGTCAGGTGGTTCATCGAGGAGGTAC | 31 |
| R3 | TGCCCACTCCGAATCGTGCTGACCGCGGATC | 31 |
| F4 | GTCATGTCAGGTGGTTCATCGAGGAGGTACC | 31 |
| R4 | CCCACTCCGAATCGTGCTGACCGCGGATCTC | 31 |
| crRNA-1 | GATGAGCCCGCGGCCTATCAGCTTG | 25 |
| crRNA-2 | TGGTGGGGTGACGGCCTACCAAGGC | 25 |
| crRNA-3 | ACGACGGGTAGCCGGCCTGAGAGG | 24 |
| crRNA-4 | GGAGGTGGAGAAGAAGCACCGGCC | 24 |
| ssDNA报告探针 | 6-FAM-TTATT-BHQ-1 | 5 |

图1 针对结核分枝杆菌IS6110基因的RPA引物、crRNA筛选A:琼脂糖凝胶电泳检测来自不同引物的RPA扩增产物;B:通过琼脂糖凝胶电泳检测RPA扩增的特异性(1:结核分枝杆菌;2:金黄色葡萄球菌;3:铜绿假单胞菌;4:肺炎克雷伯菌);C:不同crRNA的CRISPR/Cas12a反应的荧光强度;每次测试重复进行3次,荧光强度在图中显示为平均值±SD (NTC:阴性对照,ddH2O;* P<0.05,** P<0.01,**** P<0.000 1,下同)
Fig. 1 Screening of RPA primers and crRNA targeting the IS6110 gene of M. tuberculosisA: Detection of RPA amplification products from different primers by agarose gel electrophoresis. B: Detection of RPA amplification specificity by agarose gel electrophoresis (1: Mycobacterium tuberculosis; 2: Staphylococcus aureus; 3: Pseudomonas aeruginosa; 4: Klebsiella pneumoniae. C: Fluorescence intensity of CRISPR/Cas12a reactions with different crRNAs. Each test was repeated three times, and fluorescence intensity is shown in the figure as mean±SD (NTC: negative control, ddH2O; * P<0.05, ** P<0.01, **** P<0.000 1. The same below)
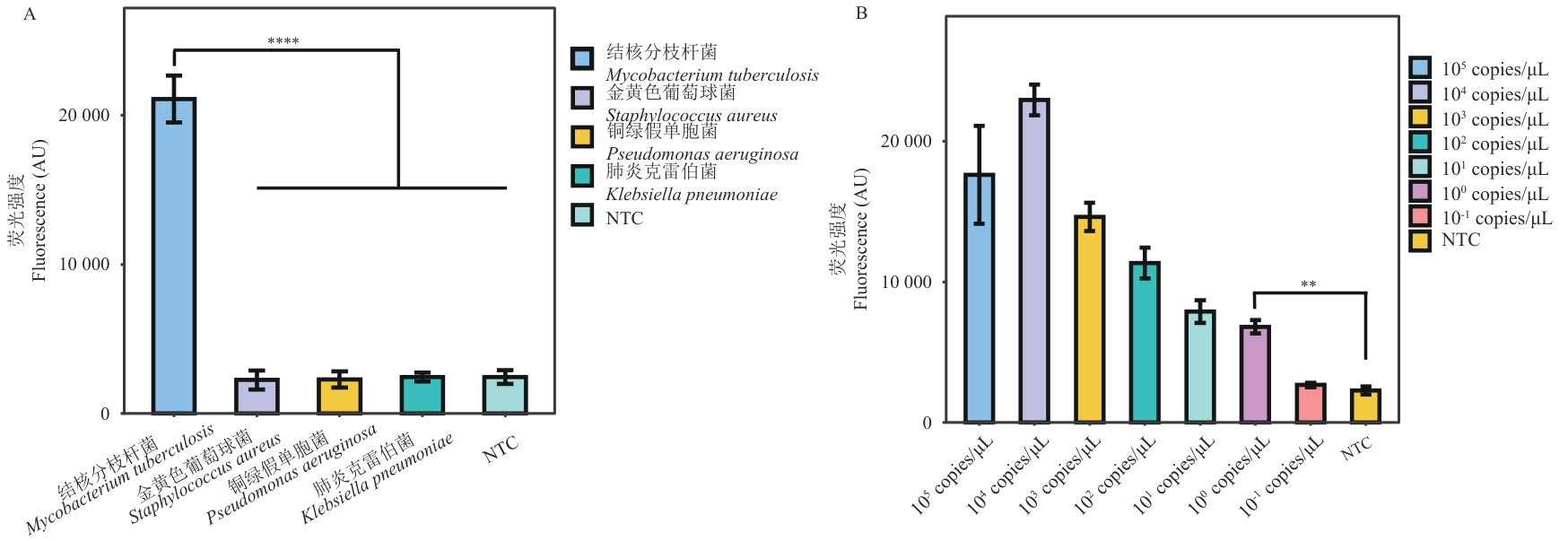
图2 RPA-CRISPR/Cas12a体系测试A:结核分枝杆菌的IS6110基因的RPA-CRISPR/Cas12a的特异性评估;B:结核分枝杆菌的IS6110基因的RPA-CRISPR/Cas12a的灵敏度评估;每次测试重复进行3次,荧光强度在图中显示为平均值±SD
Fig. 2 RPA-CRISPR/Cas12a system testingA: Specificity assessment of RPA-CRISPR/Cas12a targeting the IS6110 gene of M. tuberculosis. B: Sensitivity assessment of RPA-CRISPR/Cas12a targeting the IS6110 gene of M. tuberculosis. Each test was repeated three times, and fluorescence intensity is shown in the figure as mean±SD
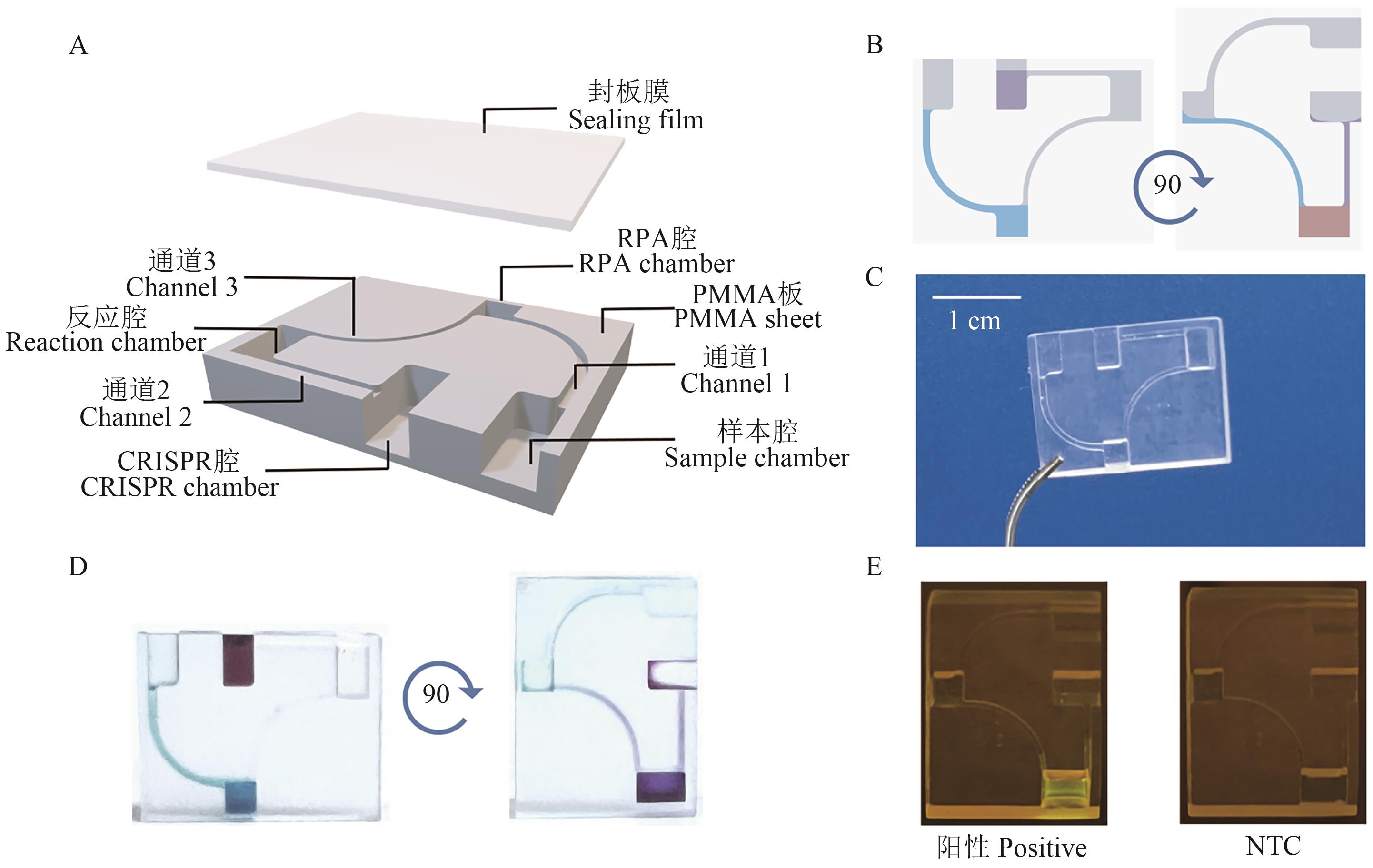
图3 RPA-CRISPR/Cas12a检测的重力驱动微流控芯片A:芯片结构示意图;B:芯片使用原理图;C:芯片实物图;D:使用染料进行芯片测试图;E:使用芯片进行RPA-CRISPR/Cas12a片上检测结果图
Fig. 3 Gravity-driven microfluidic chip for RPA-CRISPR/Cas12a detectionA: Schematic diagram of the chip structure. B: Schematic diagram of the chip working principle. C: Image of the chip. D: Chip testing image using dye. E: Detection results of RPA-CRISPR/Cas12a assay on the chip
RPA-CRISPR/Cas12a片上检测 RPA-CRISPR/Cas12a for on-chip detection | 痰培养 Sputum culture | 灵敏度 Sensitivity (%) | 特异性 Specificity (%) | 阳性预测值 Positive predictive value (%) | 阴性预测值 Negative predictive value (%) | 准确性 Accuracy (%) | Kappa值 | ||
|---|---|---|---|---|---|---|---|---|---|
阳性 Positive | 阴性 Negative | 合计 Total | |||||||
| 阳性 Positive | 123 | 9 | 132 | 91.11 | 94.34 | 93.19 | 92.59 | 92.86 | 0.856 |
| 阴性 Negative | 12 | 150 | 162 | ||||||
| 合计 Total | 135 | 159 | 294 | ||||||
表2 RPA-CRISPR/Cas12a片上检测和痰培养方法对痰液样本中的结核分枝杆菌检测结果
Table 2 Detection of M. tuberculosis in sputum samples using RPA-CRISPR/Cas12a and sputum culture methods
RPA-CRISPR/Cas12a片上检测 RPA-CRISPR/Cas12a for on-chip detection | 痰培养 Sputum culture | 灵敏度 Sensitivity (%) | 特异性 Specificity (%) | 阳性预测值 Positive predictive value (%) | 阴性预测值 Negative predictive value (%) | 准确性 Accuracy (%) | Kappa值 | ||
|---|---|---|---|---|---|---|---|---|---|
阳性 Positive | 阴性 Negative | 合计 Total | |||||||
| 阳性 Positive | 123 | 9 | 132 | 91.11 | 94.34 | 93.19 | 92.59 | 92.86 | 0.856 |
| 阴性 Negative | 12 | 150 | 162 | ||||||
| 合计 Total | 135 | 159 | 294 | ||||||
| 1 | Xiao J, Li JQ, Quan ST, et al. Development and preliminary assessment of a CRISPR-Cas12a-based multiplex detection of Mycobacterium tuberculosis complex [J]. Front Bioeng Biotechnol, 2023, 11: 1233353. |
| 2 | World Health O. Global tuberculosis report 2021 [R]. World Health Organization, 2021. |
| 3 | World Health O. Global tuberculosis report 2023 [R]. World Health Organization, 2023. |
| 4 | Zhang D, Yu F, Han DS, et al. ddPCR provides a sensitive test compared with GeneXpert MTB/RIF and mNGS for suspected Mycobacterium tuberculosis infection [J]. Front Cell Infect Microbiol, 2023, 13: 1216339. |
| 5 | Chen YJ, Zhu YY, Wang XF, et al. Gravity-driven and rotation-controlled microfluidic chip for point-of-care nucleic acid detection in the fully closed environment [J]. Talanta, 2024, 267: 125258. |
| 6 | Mosquera-Restrepo SF, Zuberogoïtia S, Gouxette L, et al. A Mycobacterium tuberculosis fingerprint in human breath allows tuberculosis detection [J]. Nat Commun, 2022, 13(1): 7751. |
| 7 | Maréchal A, Zou LE. RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response [J]. Cell Res, 2015, 25(1): 9-23. |
| 8 | Pike AM, Friend CM, Bell SP. Distinct RPA functions promote eukaryotic DNA replication initiation and elongation [J]. Nucleic Acids Res, 2023, 51(19): 10506-10518. |
| 9 | Hefel A, Honda M, Cronin N, et al. RPA complexes in Caenorhabditis elegans meiosis; unique roles in replication, meiotic recombination and apoptosis [J]. Nucleic Acids Res, 2021, 49(4): 2005-2026. |
| 10 | Nguyen LT, Gurijala J, Rananaware SR, et al. CRISPR-ENHANCE: an enhanced nucleic acid detection platform using Cas12a [J]. Methods, 2022, 203: 116-124. |
| 11 | Qiu XT, Xu S, Liu XP, et al. CRISPR/Cas12a-based diagnostic platform accurately detects Nocardia farcinica targeting a novel species-specific gene [J]. Front Cell Infect Microbiol, 2022, 12: 884411. |
| 12 | Phuphisut O, Poodeepiyasawat A, Yoonuan T, et al. Ov-RPA-CRISPR/Cas12a assay for the detection of Opisthorchis viverrini infection in field-collected human feces [J]. Parasit Vectors, 2024, 17(1): 80. |
| 13 | 徐银, 刘婷, 徐慧, 等. PCR-CRISPR/Cas12a华支睾吸虫囊蚴检测方法的建立和应用 [J]. 中国寄生虫学与寄生虫病杂志, 2023, 41(4): 421-426, 433. |
| Xu Y, Liu T, Xu H, et al. Establishment and application of PCR-CRISPR/Cas12a-based detection method for Clonorchis sinensis metacercaria [J]. Chin J Parasitol Parasit Dis, 2023, 41(4): 421-426, 433. | |
| 14 | Wang T, Bai LL, Wang GL, et al. SATCAS: a CRISPR/Cas13a-based simultaneous amplification and testing platform for one-pot RNA detection and SNPs distinguish in clinical diagnosis [J]. Biosens Bioelectron, 2024, 263: 116636. |
| 15 | 侯雅超, 邢微微, 王亚楠, 等. 针对副溶血性弧菌toxR基因的RPA-CRISPR/Cas13a荧光快速检测方法的建立及应用 [J]. 中华医院感染学杂志, 2024, 34(14): 2081-2086. |
| Hou YC, Xing WW, Wang YN, et al. Establishment and application of RPA-CRISPR/Cas13a fluorescence rapid detection method for toxR gene of Vibrio parahaemolyticus [J]. China Ind Econ, 2024, 34(14): 2081-2086. | |
| 16 | 王文涛, 赵良, 侯立功, 等. RPA-CRISPR/Cas12a在病原微生物检测中的研究进展 [J]. 微生物学通报, 2024, 51(8): 2785-2796. |
| Wang WT, Zhao L, Hou LG, et al. Research progress of RPA-CRISPR/Cas12a in the detection of pathogenic microorganisms [J]. Microbiol China, 2024, 51(8): 2785-2796. | |
| 17 | 张徐俞, 黄俊, 杨稳, 等. 重组酶聚合酶扩增结合CRISPR-Cas12a快速检测十足目虹彩病毒1方法的建立 [J]. 微生物学通报, 2021, 48(12): 4980-4988. |
| Zhang XY, Huang J, Yang W, et al. Rapid detection of decapod iridescent virus 1 by recombinase polymerase amplification combined with CRISPR-Cas12a [J]. Microbiol China, 2021, 48(12): 4980-4988. | |
| 18 | Sun YY, Yu L, Liu CX, et al. One-tube SARS-CoV-2 detection platform based on RT-RPA and CRISPR/Cas12a [J]. J Transl Med, 2021, 19(1): 74. |
| 19 | Zhuang TC, Gao C, Zhao WW, et al. A minimal transcription template-based amplification-free CRISPR-Cas13a strategy for DNA detection [J]. Biosens Bioelectron, 2025, 270: 116918. |
| 20 | Hu ML, Qiu ZQ, Bi ZR, et al. Photocontrolled crRNA activation enables robust CRISPR-Cas12a diagnostics [J]. Proc Natl Acad Sci USA, 2022, 119(26): e2202034119. |
| 21 | Zhang WS, Pan JB, Li F, et al. Reverse transcription recombinase polymerase amplification coupled with CRISPR-Cas12a for facile and highly sensitive colorimetric SARS-CoV-2 detection [J]. Anal Chem, 2021, 93(8): 4126-4133. |
| 22 | Guo YF, Xia HM, Dai TT, et al. RPA-CRISPR/Cas12a mediated isothermal amplification for visual detection of Phytophthora sojae [J]. Front Cell Infect Microbiol, 2023, 13: 1208837. |
| 23 | Hajian R, Balderston S, Tran T, et al. Detection of unamplified target genes via CRISPR-Cas9 immobilized on a graphene field-effect transistor [J]. Nat Biomed Eng, 2019, 3(6): 427-437. |
| 24 | 林正龙, 张蓓英. 基因芯片技术快速检测结核分枝杆菌耐药基因的应用价值研究 [J]. 医学理论与实践, 2024, 37(17): 2893-2896. |
| Lin ZL, Zhang BY. Evaluation of gene chips for the rapid diagnosis of drug-resistance genes in Mycobacterium tuberculosis [J]. J Med Theory Pract, 2024, 37(17): 2893-2896. | |
| 25 | 陈墨岩, 祝诚. 基于CRISPR/Cas12a的生物传感平台的机制研究及应用 [J]. 生物技术通报, 2024, 40(7): 90-98. |
| Chen MY, Zhu C. Mechanism study and application of CRISPR/Cas12a-based biosensing platform [J]. Biotechnol Bull, 2024, 40(7): 90-98. | |
| 26 | Bai YM, Ji JC, Ji FD, et al. Recombinase polymerase amplification integrated with microfluidics for nucleic acid testing at point of care [J]. Talanta, 2022, 240: 123209. |
| 27 | Xia LP, Yin JX, Zhuang JJ, et al. Adsorption-free self-priming direct digital dual-crRNA CRISPR/Cas12a-assisted chip for ultrasensitive detection of pathogens [J]. Anal Chem, 2023, 95(10): 4744-4752. |
| 28 | Khan MS, Ning Y, Jinou C, et al. Are global tuberculosis control targets overlooking an essential indicator? Prolonged delays to diagnosis despite high case detection rates in Yunnan, China [J]. Health Policy Plan, 2017, 32(): i15-i21. |
| 29 | Liu Y, Chao Z, Ding W, et al. Correction: a multiplex RPA-CRISPR/Cas12a-based POCT technique and its application in human papillomavirus (HPV) typing assay [J]. Cell Mol Biol Lett, 2024, 29(1): 49. |
| 30 | Fozouni P, Son S, de León Derby MD, et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy [J]. Cell, 2021, 184(2): 323-333.e9. |
| 31 | Zhang XY, Tian Y, Xu L, et al. CRISPR/Cas13-assisted hepatitis B virus covalently closed circular DNA detection [J]. Hepatol Int, 2022, 16(2): 306-315. |
| 32 | Cheng M, Tan CW, Xiang B, et al. Chain hybridization-based CRISPR-lateral flow assay enables accurate gene visual detection [J]. Anal Chim Acta, 2023, 1270: 341437. |
| [1] | 田兴苗, 王健霖, 郭磊, 司朵朵, 龚振兴, 李继东. 鸡毒支原体和滑液囊支原体双重LFD-RPA快速检测方法的建立[J]. 生物技术通报, 2024, 40(7): 117-124. |
| [2] | 陈墨岩, 祝诚. 基于CRISPR/Cas12a的生物传感平台的机制研究及应用[J]. 生物技术通报, 2024, 40(7): 90-98. |
| [3] | 陈晓琳, 刘洋儿, 许文涛, 郭明璋, 刘慧琳. 合成生物学细胞传感技术在食品安全快速检测中的应用[J]. 生物技术通报, 2023, 39(1): 137-149. |
| [4] | 罗雪琮, 安梦楠, 吴元华, 夏子豪. 重组酶聚合酶扩增技术在植物病毒检测中的应用[J]. 生物技术通报, 2022, 38(2): 269-280. |
| [5] | 付志强, 熊艳. 便携式生物光学传感器的研究进展[J]. 生物技术通报, 2021, 37(3): 219-226. |
| [6] | 李信申, 黄小梅, 吴淑秀, 黄瑞荣, 魏林根, 华菊玲. 植物青枯病菌环介导等温扩增快速检测技术研究[J]. 生物技术通报, 2021, 37(1): 272-281. |
| [7] | 赵颖, 王楠, 陆安祥, 冯晓元, 郭晓军, 栾云霞. 核酸适配体侧流层析分析技术在真菌毒素检测中的应用[J]. 生物技术通报, 2020, 36(8): 217-227. |
| [8] | 高威芳, 章礼平, 朱鹏. 等温扩增技术及其结合CRISPR在微生物快速检测中的研究进展[J]. 生物技术通报, 2020, 36(5): 22-31. |
| [9] | 王琦, 颜春蕾, 高洪伟, 吴薇, 杨庆利. 基于核酸适配体传感器检测食品致病菌的研究进展[J]. 生物技术通报, 2020, 36(11): 245-258. |
| [10] | 林惠娇, 杨华卫, 古恒森, 蒋湘, 张海磊, 刘昱辰, 周而勋. 三种重要水果褐腐病菌快速检测试纸的研制[J]. 生物技术通报, 2019, 35(6): 205-212. |
| [11] | 窦雯, 李尤, 逯欣宇, 钱雪梅, 沈丹宇. 转基因大豆RPA检测技术的建立及应用[J]. 生物技术通报, 2019, 35(5): 170-175. |
| [12] | 郭沛, 赵龙, 胡翮. 公共场所水环境中活嗜肺军团菌的快速检测方法[J]. 生物技术通报, 2019, 35(3): 203-209. |
| [13] | 李子微, 邓仲良. 土拉弗朗西斯氏菌LAMP快速检测方法的应用[J]. 生物技术通报, 2019, 35(2): 212-217. |
| [14] | 张微, 李志新, 付春江, 刘卫平. 马铃薯S病毒胶体金免疫层析试纸条的研制[J]. 生物技术通报, 2019, 35(12): 184-188. |
| [15] | 付加芳, 张佩佩, 宗工理, 王新圆, 刘萌, 曹广祥. 结核分枝杆菌拟核结合蛋白Lsr2阻遏调控rv1057基因转录的研究[J]. 生物技术通报, 2018, 34(11): 191-197. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||