








生物技术通报 ›› 2025, Vol. 41 ›› Issue (5): 119-128.doi: 10.13560/j.cnki.biotech.bull.1985.2024-1171
• 研究报告 • 上一篇
宋慧洋( ), 苏宝杰, 李京昊, 梅超, 宋倩娜, 崔福柱(
), 苏宝杰, 李京昊, 梅超, 宋倩娜, 崔福柱( ), 冯瑞云(
), 冯瑞云( )
)
收稿日期:2024-12-05
出版日期:2025-05-26
发布日期:2025-06-05
通讯作者:
冯瑞云,男,博士,研究员,研究方向 :农作物遗传改良;E-mail: fengruiyun1970@163.com作者简介:宋慧洋,男,硕士研究生,研究方向 :遗传育种与种质创新;E-mail: 1370274696@qq.com
基金资助:
SONG Hui-yang( ), SU Bao-jie, LI Jing-hao, MEI Chao, SONG Qian-na, CUI Fu-zhu(
), SU Bao-jie, LI Jing-hao, MEI Chao, SONG Qian-na, CUI Fu-zhu( ), FENG Rui-yun(
), FENG Rui-yun( )
)
Received:2024-12-05
Published:2025-05-26
Online:2025-06-05
摘要:
目的 ASYMMETRIC LEAVES2(AS2)基因家族是植物特有的转录家族,在植物生长发育和胁迫响应中发挥重要作用。研究AS2转录因子在马铃薯响应逆境胁迫特别是盐胁迫中的功能与机制,为培育抗盐马铃薯新品种提供理论和技术支撑。 方法 通过克隆野生型马铃薯品种底西芮(DES)叶片中的StAS2-15基因,并通过同源重组方法构建StAS2-15过表达载体,转化DES得到阳性植株,进行抗盐农艺性状分析及生理生化实验。 结果 盐胁迫表型实验表明,随着盐浓度的增加,过表达植株(OEs)和野生型(DES)的株高、鲜重、根数和根长显著降低,其中株高分别降低了2.42%-43.21%和28.77%-58.49%;同一盐浓度下,OEs的株高、鲜重、根数和根长较DES显著增加。不同盐浓度处理下,过表达StAS2-15基因阳性植株叶片中超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)活性和脯氨酸含量显著高于野生型,丙二醛(MDA)含量显著低于野生型。RT-qPCR结果显示,盐胁迫下马铃薯相关胁迫响应基因ABI3、MYB2、SnRK2s等在过表达植株中的表达量显著高于野生型。 结论 StAS2-15基因的过表达使马铃薯体内一些抗氧化酶活性、渗透调节物质含量和其他盐胁迫响应基因的表达水平发生改变,且盐胁迫下植株生长状态呈现脱敏感表型,影响了马铃薯的生长发育。
宋慧洋, 苏宝杰, 李京昊, 梅超, 宋倩娜, 崔福柱, 冯瑞云. 马铃薯StAS2-15基因的克隆及盐胁迫下功能分析[J]. 生物技术通报, 2025, 41(5): 119-128.
SONG Hui-yang, SU Bao-jie, LI Jing-hao, MEI Chao, SONG Qian-na, CUI Fu-zhu, FENG Rui-yun. Cloning and Functional Analysis of the StAS2-15 Gene in Potato under Salt Stress[J]. Biotechnology Bulletin, 2025, 41(5): 119-128.
引物名称 Primer name | 引物序列 Sequence(5'-3') |
|---|---|
| Actin-F | GGGATGGAGAAGTTTGGTGGTGG |
| Actin-R | CTTCGACCAAGGGATGGTGTAGC |
| StAS2-15-F | CGTCTCCGAGATCCAGTCTA |
| StAS2-15-R | GGTACTAGCAATGCCTCC |
| ABF4-F | CTTCTGCACATACAAGGATATTCAA |
| ABF4-R | TGCCTAGCCAAAGGGAAATCA |
| MYB2-F | ATGACATGGACCTCCGACGA |
| MYB2-R | CGTTCGCTTTAGACCAGCAC |
| SnRK2s-F | TTGCTGTAGAGAAGGTGGGT |
| SnRK2s-R | GTCCTACTGTCACTGCCGTC |
| DREB2A-F | CACAGAAGAAAAGAGAAATGGCT |
| DREB2A-R | TCCACTATAATCCAACGGCAGA |
| ABI3-F | TGCTGACATGAAGTGTGGCA |
| ABI3-R | TTTTGCCCTCCGACTTTGGT |
| ABI5-F | TAAAACAGGCCCTGGCGGAG |
| ABI5-R | TTGCGCCTTTGTTTGAGCTT |
表1 荧光定量引物序列
Table 1 Primer sequences for fluorescence quantification
引物名称 Primer name | 引物序列 Sequence(5'-3') |
|---|---|
| Actin-F | GGGATGGAGAAGTTTGGTGGTGG |
| Actin-R | CTTCGACCAAGGGATGGTGTAGC |
| StAS2-15-F | CGTCTCCGAGATCCAGTCTA |
| StAS2-15-R | GGTACTAGCAATGCCTCC |
| ABF4-F | CTTCTGCACATACAAGGATATTCAA |
| ABF4-R | TGCCTAGCCAAAGGGAAATCA |
| MYB2-F | ATGACATGGACCTCCGACGA |
| MYB2-R | CGTTCGCTTTAGACCAGCAC |
| SnRK2s-F | TTGCTGTAGAGAAGGTGGGT |
| SnRK2s-R | GTCCTACTGTCACTGCCGTC |
| DREB2A-F | CACAGAAGAAAAGAGAAATGGCT |
| DREB2A-R | TCCACTATAATCCAACGGCAGA |
| ABI3-F | TGCTGACATGAAGTGTGGCA |
| ABI3-R | TTTTGCCCTCCGACTTTGGT |
| ABI5-F | TAAAACAGGCCCTGGCGGAG |
| ABI5-R | TTGCGCCTTTGTTTGAGCTT |

图1 马铃薯StAS2-15过表达载体的构建和基因表达量分析A:1代表含目的基因StAS2-15的质粒;2代表Hind Ⅲ-Sac Ⅰ酶切质粒;M代表DNA marker。B:DES代表野生型;P代表含目的基因StAS2-15的质粒;1-3代表过表达阳性植株。C:OEs代表过表达阳性植株;显著性差异通过Duncan新复极差法确定;不同字母表示差异显著(P<0.05);误差线表示3个独立生物学实验获得的标准差;以Actin为内参对目的基因相对表达量进行标准化计算,下同
Fig. 1 Construction of potato StAS2-15 overexpression vector and gene expression analysisA: 1 refers to the plasmid containing the gene of interest StAS2-15; 2 refers to HindIII-Sac I digest plasmid; M refers to DNA marker. B: DES refers to wild type; P refers to the plasmid containing the gene of interest StAS2-15; 1-3 refers to overexpression-positive plants. C: OEs refer to overexpressed positive plants. Significant differences were determined by Duncan's new complex polarity difference method. Different letters showed significant differences (P<0.05). Error bars indicate standard deviations obtained from 3 independent biological experiments; Actin was used as the internal reference gene to standardize the relative expression of the target gene. The same below

图2 马铃薯StAS2-15过表达阳性植株筛选流程A:茎段预培养;B:农杆菌侵染;C:愈伤组织形成;D:愈伤组织出苗;E:移苗入培养瓶
Fig. 2 Screening protocol of potato StAS2-15 overexpressing positive plantA: Pre-culture of stem segments. B: Agrobacterium infection. C: Callus formation. D: callus emergence. E: Transfer seedlings into culture flasks
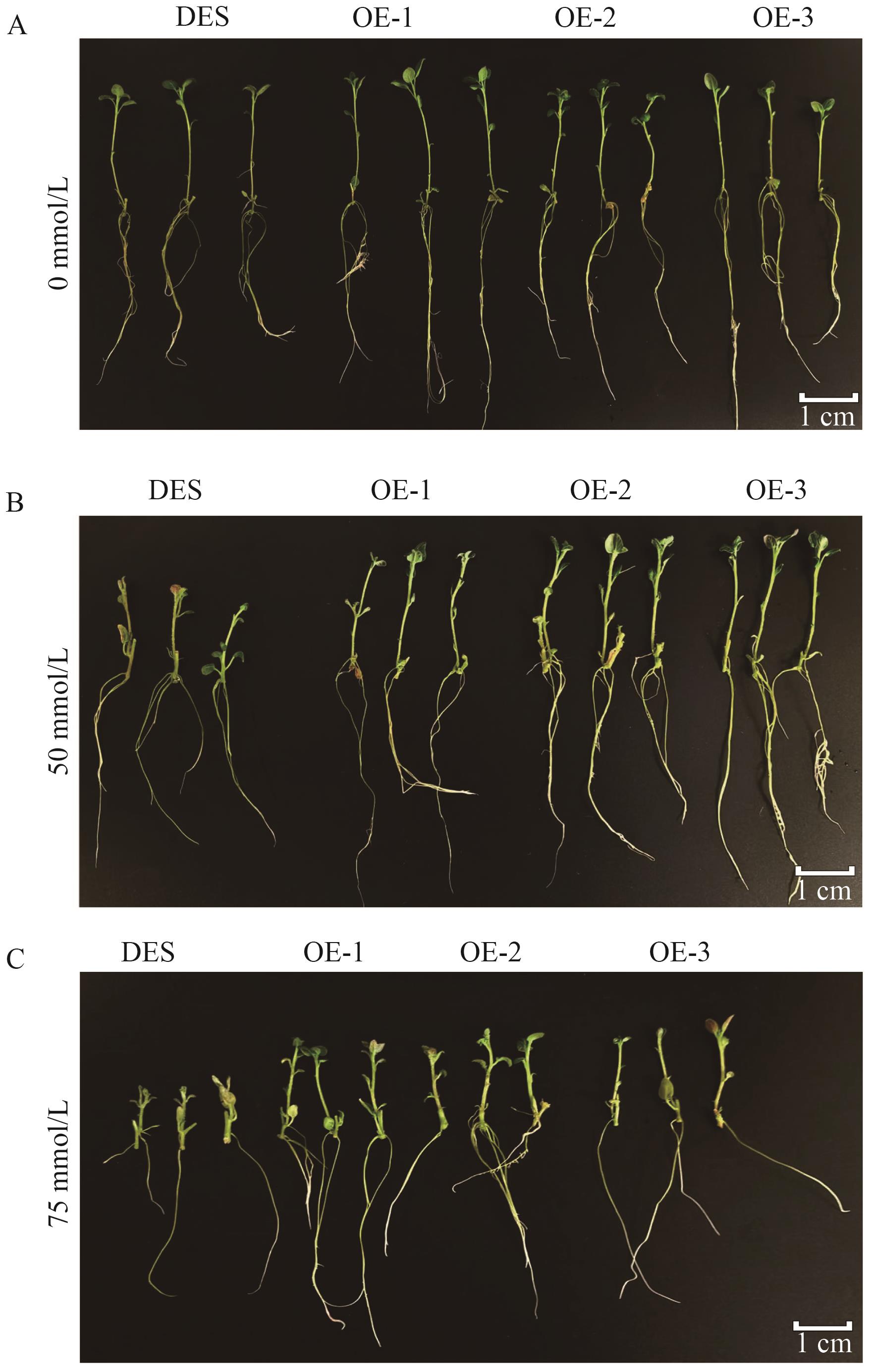
图4 盐胁迫20 d后组培苗生长状态DES:野生型;OEs:过表达阳性植株
Fig. 4 Growth state of tissue culture seedlings after 20 d of salt stressDES: Wild type. OEs: Overexpressed positive plants
| 1 | 黄凤玲, 张琳, 李先德, 等. 中国马铃薯产业发展现状及对策 [J]. 农业展望, 2017, 13(1): 25-31. |
| Huang FL, Zhang L, Li XD, et al. Development situation and countermeasures of China's potato industry [J]. Agric Outlook, 2017, 13(1): 25-31. | |
| 2 | 王希卓, 朱旭, 孙洁, 等. 我国马铃薯主粮化发展形势分析 [J]. 农产品加工, 2015(3): 52-55. |
| Wang XZ, Zhu X, Sun J, et al. The potato staple foods development situation analysis in China [J]. Farm Prod Process, 2015(3): 52-55. | |
| 3 | Hu C, He Y, Zhang WN, et al. Potato proteins for technical applications: nutrition, isolation, modification and functional properties-A review [J]. Innov Food Sci Emerg Technol, 2024, 91: 103533. |
| 4 | Liu XW, Sun HN, Mu TH, et al. Exploring the potential of potato products: puree and cellulose nanofibers, to improve the nutritional value of mayonnaise [J]. Food Chem, 2024, 437: 137864. |
| 5 | 谢从华, 柳俊. 中国马铃薯从济荒作物到主粮之变迁 [J]. 华中农业大学学报, 2021, 40(4): 8-15. |
| Xie CH, Liu J. Transition of potato from a famine relief crop to staple food in China [J]. J Huazhong Agric Univ, 2021, 40(4): 8-15. | |
| 6 | 赵映琴. 转拟南芥AtNHX1基因马铃薯耐盐性的田间鉴定 [D]. 兰州: 甘肃农业大学, 2009. |
| Zhao YQ. Field identification of salt tolerance of transgenic potato with Arabidopsis AtNHX1 gene [D]. Lanzhou: Gansu Agricultural University, 2009. | |
| 7 | Latchman DS. Transcription factors: an overview [J]. Int J Biochem Cell Biol, 1997, 29(12): 1305-1312. |
| 8 | Yoon Y, Seo DH, Shin H, et al. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants [J]. Agronomy, 2020, 10(6): 788. |
| 9 | 吴乃虎, 刁丰秋. 植物转录因子与发育调控 [J]. 科学通报, 1998, 43(20): 2133-2139. |
| Wu NH, Diao FQ. Plant transcription factors and development regulation [J]. Chin Sci Bull, 1998, 43(20): 2133-2139. | |
| 10 | 刘强, 张贵友, 陈受宜. 植物转录因子的结构与调控作用 [J]. 科学通报, 2000, 45(14): 1465-1474. |
| Liu Q, Zhang GY, Chen SY. Structure and regulation of plant transcription factors [J]. Chin Sci Bull, 2000, 45(14): 1465-1474. | |
| 11 | Roy SJ, Negrão S, Tester M. Salt resistant crop plants [J]. Curr Opin Biotechnol, 2014, 26: 115-124. |
| 12 | Upadhyaya G, Sethi V, Modak A, et al. ALOG/LSHs, a novel class of transcription factors: Evolutionarily conserved regulators of plant growth and development [J]. J Exp Bot, 2024: erae409. |
| 13 | Iwakawa H, Ueno Y, Semiarti E, et al. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf Lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper [J]. Plant Cell Physiol, 2002, 43(5): 467-478. |
| 14 | Bortiri E, Chuck G, Vollbrecht E, et al. ramosa2 encodes a lateral organ boundary domain protein that determines the fate of stem cells in branch meristems of maize [J]. Plant Cell, 2006, 18(3): 574-585. |
| 15 | Guo B, Wang J, Lin S, et al. A genome-wide analysis of the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) gene family in barley (Hordeum vulgare L.)[J]. Journal of Zhejiang University-Science B, 2016, 17(10): 763-774. |
| 16 | Lu Q, Shao FJ, MacMillan C, et al. Genomewide analysis of the lateral organ boundaries domain gene family in Eucalyptus grandis reveals members that differentially impact secondary growth [J]. Plant Biotechnol J, 2018, 16(1): 124-136. |
| 17 | Liu JC, Sheng LH, Xu YQ, et al. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis [J]. Plant Cell, 2014, 26(3): 1081-1093. |
| 18 | Matsumura Y, Iwakawa H, Machida Y, et al. Characterization of genes in the asymmetric leaves2/lateral organ boundaries (As2/lob) family in Arabidopsis thaliana, and functional and molecular comparisons between As2 and other family members [J]. Plant J, 2009, 58(3): 525-537. |
| 19 | 熊静. 玉米LBD转录因子Ⅱ类成员ZmLBD5和ZmLBD33在干旱胁迫中的功能研究 [D]. 雅安: 四川农业大学, 2021. |
| Xiong J. Function of ZmLBD5 and ZmLBD33, members of maize LBD transcription factor Ⅱ, in drought stress [D]. Ya’an: Sichuan Agricultural University, 2021. | |
| 20 | 雷彪, 曲瑞芳, 任超, 等. 谷子LBD基因家族的鉴定及其对逆境胁迫的响应 [J]. 植物生理学报, 2023, 59(3): 527-542. |
| Lei B, Qu RF, Ren C, et al. Identification of SiLBDs gene family in foxtail millet and its participation in stress response [J]. Plant Physiol J, 2023, 59(3): 527-542. | |
| 21 | Lin WC, Shuai B, Springer PS. The Arabidopsis lateral organ boundaries-domain gene asymmetric leaves2 functions in the repression of Knox gene expression and in adaxial-abaxial patterning [J]. Plant Cell, 2003, 15(10): 2241-2252. |
| 22 | Ori N, Eshed Y, Chuck G, et al. Mechanisms that control Knox gene expression in the Arabidopsis shoot [J]. Development, 2000, 127(24): 5523-5532. |
| 23 | Meng LS, Liu HL, Cui XH, et al. ASYMMETRIC LEAVES2-LIKE38 gene, a Member of AS2/LOB family of Arabidopsis, causes leaf dorsoventral alternation in transgenic cockscomb plants [J]. Acta Physiol Plant, 2009, 31(6): 1301-1306. |
| 24 | Feng XJ, Xiong J, Zhang WX, et al. ZmLBD5, a class-II LBD gene, negatively regulates drought tolerance by impairing abscisic acid synthesis [J]. Plant J, 2022, 112(6): 1364-1376. |
| 25 | 李爱宏, 张亚芳, 戴正元, 等. LBD基因家族在高等植物中的研究进展 [J]. 分子植物育种, 2006, 4(3): 301-308. |
| Li AH, Zhang YF, Dai ZY, et al. Progress of LBD gene family in higher plants [J]. Mol Plant Breed, 2006, 4(3): 301-308. | |
| 26 | Xu B, Li ZY, Zhu Y, et al. Arabidopsis genes AS1 AS2 and JAG negatively regulate boundary-specifying genes to promote sepal and petal development [J]. Plant Physiol, 2008, 146(2): 566-575. |
| 27 | Fan MZ, Xu CY, Xu K, et al. Lateral organ boundaries domain transcription factors direct callus formation in Arabidopsis regeneration [J]. Cell Res, 2012, 22(7): 1169-1180. |
| 28 | Okushima Y, Fukaki H, Onoda M, et al. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis [J]. Plant Cell, 2007, 19(1): 118-130. |
| 29 | Taramino G, Sauer M, Stauffer JL Jr, et al. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation [J]. Plant J, 2007, 50(4): 649-659. |
| 30 | 石玉, 沈诗雅, 张倩茹, 等. LBD基因家族研究进展 [J]. 中国细胞生物学学报, 2019, 41(4): 738-745. |
| Shi Y, Shen SY, Zhang QR, et al. The research progress of LBD gene family [J]. Chin J Cell Biol, 2019, 41(4): 738-745. | |
| 31 | Jiang QW, Wu XY, Zhang XY, et al. Genome-wide identification and expression analysis of AS2 genes in Brassica rapa reveal their potential roles in abiotic stress [J]. Int J Mol Sci, 2023, 24(13): 10534. |
| 32 | Machida Y, Suzuki T, Sasabe M, et al. Arabidopsis ASYMMETRIC LEAVES2 (AS2): roles in plant morphogenesis, cell division, and pathogenesis [J]. J Plant Res, 2022, 135(1): 3-14. |
| 33 | 张菊, 陈璨, 罗蕾, 等. AS1/AS2基因在叶形态建成中的作用研究进展 [J]. 植物遗传资源学报, 2022, 23(6): 1604-1612. |
| Zhang J, Chen C, Luo L, et al. Research progress in the role of AS1/AS2 genes in leaf morphogenesis [J]. J Plant Genet Resour, 2022, 23(6): 1604-1612. | |
| 34 | Dong RR, Yuan YQ, Liu ZQ, et al. ASYMMETRIC LEAVES 2 and ASYMMETRIC LEAVES 2-LIKE are partially redundant genes and essential for fruit development in tomato [J]. Plant J, 2023, 114(6): 1285-1300. |
| 35 | Meena RP, Ghosh G, Vishwakarma H, et al. Expression of a Pennisetum glaucum gene DREB2A confers enhanced heat, drought and salinity tolerance in transgenic Arabidopsis [J]. Mol Biol Rep, 2022, 49(8): 7347-7358. |
| 36 | Chen HL, Liu LP, Wang LX, et al. VrDREB2A, a DREB-binding transcription factor from Vigna radiata, increased drought and high-salt tolerance in transgenic Arabidopsis thaliana [J]. J Plant Res, 2016, 129(2): 263-273. |
| 37 | Liu HX, Wang X, Zhu XL, et al. Meta-analysis of SnRK2 gene overexpression in response to drought and salt stress [J]. Physiol Plant, 2024, 176(6): e14578. |
| 38 | Yang A, Dai XY, Zhang WH. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice [J]. J Exp Bot, 2012, 63(7): 2541-2556. |
| 39 | Shkolnik-Inbar D, Adler G, Bar-Zvi D. ABI4 downregulates expression of the sodium transporter HKT1;1 in Arabidopsis roots and affects salt tolerance [J]. Plant J, 2013, 73(6): 993-1005. |
| 40 | Chang HC, Tsai MC, Wu SS, et al. Regulation of ABI5 expression by ABF3 during salt stress responses in Arabidopsis thaliana [J]. Bot Stud, 2019, 60(1): 16. |
| 41 | Fu MJ, Kang HK, Son SH, et al. A subset of Arabidopsis RAV transcription factors modulates drought and salt stress responses independent of ABA [J]. Plant Cell Physiol, 2014, 55(11): 1892-1904. |
| 42 | Yi J, Zhao DM, Chu JF, et al. AtDPG1 is involved in the salt stress response of Arabidopsis seedling through ABI4 [J]. Plant Sci, 2019, 287: 110180. |
| 43 | Han ZL, Shang XW, Shao LX, et al. Meta-analysis of the effect of expression of MYB transcription factor genes on abiotic stress [J]. PeerJ, 2021, 9: e11268. |
| 44 | Bai JP, Mao J, Yang HY, et al. Sucrose non-ferment 1 related protein kinase 2 (SnRK2) genes could mediate the stress responses in potato (Solanum tuberosum L.) [J]. BMC Genet, 2017, 18(1): 41. |
| 45 | Sengupta S, Ray A, Mandal D, et al. ABI3 mediated repression of RAV1 gene expression promotes efficient dehydration stress response in Arabidopsis thaliana [J]. Biochim Biophys Acta Gene Regul Mech, 2020, 1863(9): 194582. |
| 46 | 刘洋, 郑洋洋, 宫超, 等. 番茄AS2基因家族的系统进化分析 [J]. 基因组学与应用生物学, 2018, 37(9): 3958-3965. |
| Liu Y, Zheng YY, Gong C, et al. Phylogenetic analysis of the AS2 gene family in tomato [J]. Genom Appl Biol, 2018, 37(9): 3958-3965. | |
| 47 | 梅超, 刘玉卫, 孙蕾, 等. 马铃薯AS2基因家族的鉴定与逆境胁迫表达 [J]. 应用与环境生物学报, 2020, 26(6): 1498-1507. |
| Mei C, Liu YW, Sun L, et al. Identification and expression analysis of the AS2 gene family under abiotic stress in Solanum tuberosum L [J]. Chin J Appl Environ Biol, 2020, 26(6): 1498-1507. | |
| 48 | 杨阳, 唐宁, 李正国. 番茄SlSnRK2s基因表达在促进叶片衰老中的作用 [J]. 热带作物学报, 2014, 35(5): 950-956. |
| Yang Y, Tang N, Li ZG. The role of SlSnRK2s expression in promoting the leaf senescence in tomato [J]. Chin J Trop Crops, 2014, 35(5): 950-956. | |
| 49 | 关淑艳, 焦鹏, 蒋振忠, 等. MYB转录因子在植物非生物胁迫中的研究进展 [J]. 吉林农业大学学报, 2019, 41(3): 253-260. |
| Guan SY, Jiao P, Jiang ZZ, et al. Research progress of MYB transcription factors in plant abiotic stress [J]. J Jilin Agric Univ, 2019, 41(3): 253-260. |
| [1] | 文博霖, 万敏, 胡建军, 王克秀, 景晟林, 王心悦, 朱博, 唐铭霞, 李兵, 何卫, 曾子贤. 马铃薯川芋50遗传转化及基因编辑体系的建立[J]. 生物技术通报, 2025, 41(4): 88-97. |
| [2] | 刘涛, 王志淇, 吴文博, 石文婷, 王超楠, 杜崇, 杨中敏. 马铃薯GRAM基因家族鉴定与表达分析[J]. 生物技术通报, 2025, 41(4): 145-155. |
| [3] | 张益瑄, 马宇, 王童童, 盛苏奥, 宋家凤, 吕钊彦, 朱晓彪, 侯华兰. 马铃薯DIR家族全基因组鉴定及表达模式分析[J]. 生物技术通报, 2025, 41(3): 123-136. |
| [4] | 俞婷, 黄丹丹, 朱炎辉, 杨梅宏, 艾菊, 高冬丽. 马铃薯Stpatatin 05基因转录调控因子筛选及互作验证[J]. 生物技术通报, 2025, 41(3): 137-145. |
| [5] | 覃悦, 杨妍, 张磊, 卢丽丽, 李先平, 蒋伟. 二倍体和四倍体马铃薯StGAox基因鉴定与比较分析[J]. 生物技术通报, 2025, 41(3): 146-160. |
| [6] | 李彩霞, 李艺, 穆宏秀, 林俊轩, 白龙强, 孙美华, 苗妍秀. 中国南瓜bHLH转录因子家族的鉴定与生物信息学分析[J]. 生物技术通报, 2025, 41(1): 186-197. |
| [7] | 袁柳娇, 黄文琳, 陈崇志, 梁敏, 黄梓淇, 陈雪雪, 陈日檬, 王锂韫. 盐胁迫对广藿香叶片生理特性、超微结构及药效成分的影响[J]. 生物技术通报, 2025, 41(1): 230-239. |
| [8] | 申鹏, 高雅彬, 丁红. 马铃薯SAT基因家族的鉴定和表达分析[J]. 生物技术通报, 2024, 40(9): 64-73. |
| [9] | 宋兵芳, 柳宁, 程新艳, 徐晓斌, 田文茂, 高悦, 毕阳, 王毅. 马铃薯G6PDH基因家族鉴定及其在损伤块茎的表达分析[J]. 生物技术通报, 2024, 40(9): 104-112. |
| [10] | 王超, 白如仟, 管俊梅, 罗稷林, 何雪姣, 迟绍轶, 马玲. 马铃薯块茎变绿中StHY5对龙葵素合成的促进作用[J]. 生物技术通报, 2024, 40(9): 113-122. |
| [11] | 夏士轩, 耿泽栋, 祝光涛, 张春芝, 李大伟. 基于深度学习的马铃薯花粉活力快速检测[J]. 生物技术通报, 2024, 40(9): 123-130. |
| [12] | 毛向红, 卢瑶, 范向斌, 杜培兵, 白小东. 基于SSR荧光标记毛细管电泳的马铃薯品种遗传多样性分析及分子身份证构建[J]. 生物技术通报, 2024, 40(9): 131-140. |
| [13] | 袁兰, 黄娅楠, 张贝妮, 熊雨萌, 王洪洋. 基于流式细胞仪鉴定马铃薯倍性的高通量样品制备方法[J]. 生物技术通报, 2024, 40(9): 141-147. |
| [14] | 宋倩娜, 段永红, 冯瑞云. CRISPR/Cas9介导的高效四倍体马铃薯试管薯基因编辑体系的建立[J]. 生物技术通报, 2024, 40(9): 33-41. |
| [15] | 王柯然, 闫俊杰, 刘建凤, 高玉林. RNAi技术在马铃薯害虫防控中的应用和风险[J]. 生物技术通报, 2024, 40(9): 4-10. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||