








生物技术通报 ›› 2025, Vol. 41 ›› Issue (6): 208-217.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0019
• 研究报告 • 上一篇
收稿日期:2025-01-08
出版日期:2025-06-26
发布日期:2025-06-30
通讯作者:
艾丽皎,女,博士,正高级工程师,研究方向 :园林植物应用与园林生态;E-mail: alj01461@foxmail.com作者简介:郭涛,男,博士,副研究员,研究方向 :园林植物遗传育种;E-mail: yushen0002008@126.com
基金资助:
GUO Tao( ), AI Li-jiao(
), AI Li-jiao( ), ZOU Shi-hui, ZHOU Ling, LI Xue-mei
), ZOU Shi-hui, ZHOU Ling, LI Xue-mei
Received:2025-01-08
Published:2025-06-26
Online:2025-06-30
摘要:
目的 探究山茶Related to ABI3 and VP1(CjRAV1)基因在开花调控中的功能及其分子机制,为四季山茶的分子育种提供理论依据。 方法 采用生物信息学分析、基因表达模式分析、转基因技术和DAP-seq等多种实验手段,系统研究CjRAV1的功能及其调控机制。生物信息学分析鉴定CjRAV1的基因结构、保守结构域及其系统进化关系。利用RT-qPCR技术分析CjRAV1在外源激素诱导下、不同组织以及花苞不同发育时期的表达模式。构建CjRAV1过表达转基因拟南芥植株,观察其表型变化,尤其是开花时间的改变。最后,采用DAP-seq技术筛选CjRAV1下游潜在的DNA结合位点及其调控基因,揭示CjRAV1的分子调控网络。 结果 生物信息学分析表明,CjRAV1的开放阅读框长度为1 101 bp,共编码366个氨基酸,具有AP2和B3保守结构域。系统进化分析显示,山茶CjRAV1蛋白与茶树CsRAV蛋白的亲缘关系最近,表明两者可能具有相似的功能。亚细胞定位分析证实,CjRAV1转录因子定位于细胞核,提示其可能在转录调控中发挥直接作用。表达模式分析显示,CjRAV1在山茶叶中表达量最高;在花苞成熟过程中,CjRAV1的表达量总体呈现逐步下降的趋势。CjRAV1的过表达转基因拟南芥表现出晚花的表型。通过DAP-seq筛选出潜在的下游调控基因CjERF。 结论 CjRAV1过表达导致转基因拟南芥植株表现出晚花的表型,且这一功能可能与潜在的调控基因CjERF协作完成。
郭涛, 艾丽皎, 邹世慧, 周玲, 李学梅. 山茶CjRAV1调控开花延迟的功能研究[J]. 生物技术通报, 2025, 41(6): 208-217.
GUO Tao, AI Li-jiao, ZOU Shi-hui, ZHOU Ling, LI Xue-mei. Functional Study of CjRAV1 from Camellia japonica in Regulating Flowering Delay[J]. Biotechnology Bulletin, 2025, 41(6): 208-217.
| 名称 Primer name | 序列 Primer sequence (5′‒3′) |
|---|---|
| RAV1-f | ATGGATGGTAGTTACATAGATGAGA |
| RAV1-r | TTACAGAGCCCCAATTATCCTTTGT |
| 3302-R1-1 | GAACACGGGGGACTCTTGACATGGATGGTAGTTACATAGA |
| 3302-R1-2 | TTTACCCTCAGATCTACCATCAGAGCCCCAATTATCCTTT |
| RAV1-RT-1 | TAAAGGTTGGAGCCGATTTGTGA |
| RAV1-RT-2 | ACCCGTTTCTCGTATTCCAGTCA |
| CjGAPDH-1 | GGGAATCCTTGGTTACACTGAG |
| CjGAPDH-2 | ACCCCATTCGTTGTCATACC |
| Atactin-1 | ACCACTGTCCACTCTATCACTGC |
| Atactin-2 | TGAGGGATGGCAACACTTTCCC |
| AtFT-1 | ACCCTCACCTCCGAGAATATCTCCAT |
| AtFT-2 | CTAAAGTCTTCTTCCTCCGCAGCCAC |
| AtSOC-1 | AGGCATACTAAGGATCGAGTCAGCAC |
| AtSOC-2 | GAAGAACAAGGTAACCCAATGAACAA |
| p3302-f | TGACGCACAATCCCACTATCCTT |
| p3302-r | CCGTCCAGCTCGACCAGGAT |
表1 引物序列
Table 1 Sequences of primers
| 名称 Primer name | 序列 Primer sequence (5′‒3′) |
|---|---|
| RAV1-f | ATGGATGGTAGTTACATAGATGAGA |
| RAV1-r | TTACAGAGCCCCAATTATCCTTTGT |
| 3302-R1-1 | GAACACGGGGGACTCTTGACATGGATGGTAGTTACATAGA |
| 3302-R1-2 | TTTACCCTCAGATCTACCATCAGAGCCCCAATTATCCTTT |
| RAV1-RT-1 | TAAAGGTTGGAGCCGATTTGTGA |
| RAV1-RT-2 | ACCCGTTTCTCGTATTCCAGTCA |
| CjGAPDH-1 | GGGAATCCTTGGTTACACTGAG |
| CjGAPDH-2 | ACCCCATTCGTTGTCATACC |
| Atactin-1 | ACCACTGTCCACTCTATCACTGC |
| Atactin-2 | TGAGGGATGGCAACACTTTCCC |
| AtFT-1 | ACCCTCACCTCCGAGAATATCTCCAT |
| AtFT-2 | CTAAAGTCTTCTTCCTCCGCAGCCAC |
| AtSOC-1 | AGGCATACTAAGGATCGAGTCAGCAC |
| AtSOC-2 | GAAGAACAAGGTAACCCAATGAACAA |
| p3302-f | TGACGCACAATCCCACTATCCTT |
| p3302-r | CCGTCCAGCTCGACCAGGAT |

图1 不同物种RAVs蛋白的系统发育树及其同源关系分析A:CjRAV1的CDS区序列及编码蛋白;B:来自不同物种的15个RAVs蛋白的系统发育树(Cs:茶树;Cl:狭叶油茶;Cj:山茶;At:拟南芥;Os:水稻);C:指示物种中RAVs蛋白的序列同源矩阵
Fig. 1 Phylogenetic tree and homology analysis of RAVs proteins from different speciesA: CDS sequence and encoded protein of CjRAV1. B: A phylogenetic tree of 15 RAVs proteins from different species (Cs: Camellia sinensis. Cl: Camellia lanceoleosa. Cj: Camellia japonica. At: Arabidopsis thaliana; Os: Oryza sativa). C: The sequence homology matrix of RAVs proteins in the indicative species

图4 表达载体菌液PCR鉴定M:Marker;-:阴性对照;+:阳性对照;1:p3302-RAV1
Fig. 4 PCR identification of expression vector bacterial solutionM: Marker. -: Negative control. +: Positive control. 1: p3302-RAV1

图5 拟南芥转基因株系的阳性鉴定A:过表达CjRAV1拟南芥阳性株系DNA水平的PCR鉴定;B:实时荧光定量检测T1代转基因拟南芥中CjRAV1的相对表达水平;M:marker;-:阴性对照;+:阳性对照;1-8:p3302-RAV1-1至p3302-RAV1-8转基因拟南芥株系
Fig. 5 Positive identification of CjRAV1 transgenic Arabidopsis linesA: PCR identification of DNA for positive strains of CjRAV-overexpressed Arabidopsis. B: Relative expressions of CjRAV1 intransgenic Arabidopsis detected by RT-qPCR. M: Marker. -: Negative control. +: Positive control. 1-8: From p3302-RAV1-1 to p3302-RAV1-8 transgenic Arabidopsis lines

图6 野生型和CjRAV1转基因拟南芥花期比较A:野生型和CjRAV1转基因拟南芥植株的表型比较;B:野生型和CjRAV1转基因拟南芥植株的开花时间统计;C:过表达CjRAV1拟南芥中开花调控基因的表达水平;D:野生型和过表达CjRAV1拟南芥中总GA含量;WT:野生型拟南芥;1:p3302-RAV1-1转基因拟南芥株系;3:p3302-RAV1-3转基因拟南芥株系
Fig. 6 Comparative analysis of flowering time between wild type and CjRAV1 transgenic Arabidopsis plantsA: Phenotypic comparison between wild-type and CjRAV1 transgenic Arabidopsis plants. B: Flowering time statistics of wild-type and CjRAV1 transgenic Arabidopsis plants. C: Expressions of flowering regulatory genes in overexpressing CjRAV1 Arabidopsis. D: Total GAs content of wild type and CjRAV1 transgenic Arabidopsis plants. WT: Wild-type Arabidopsis; 1: p3302-RAV1-1 transgenic Arabidopsis line; 3: p3302-RAV1-3 transgenic Arabidopsis line
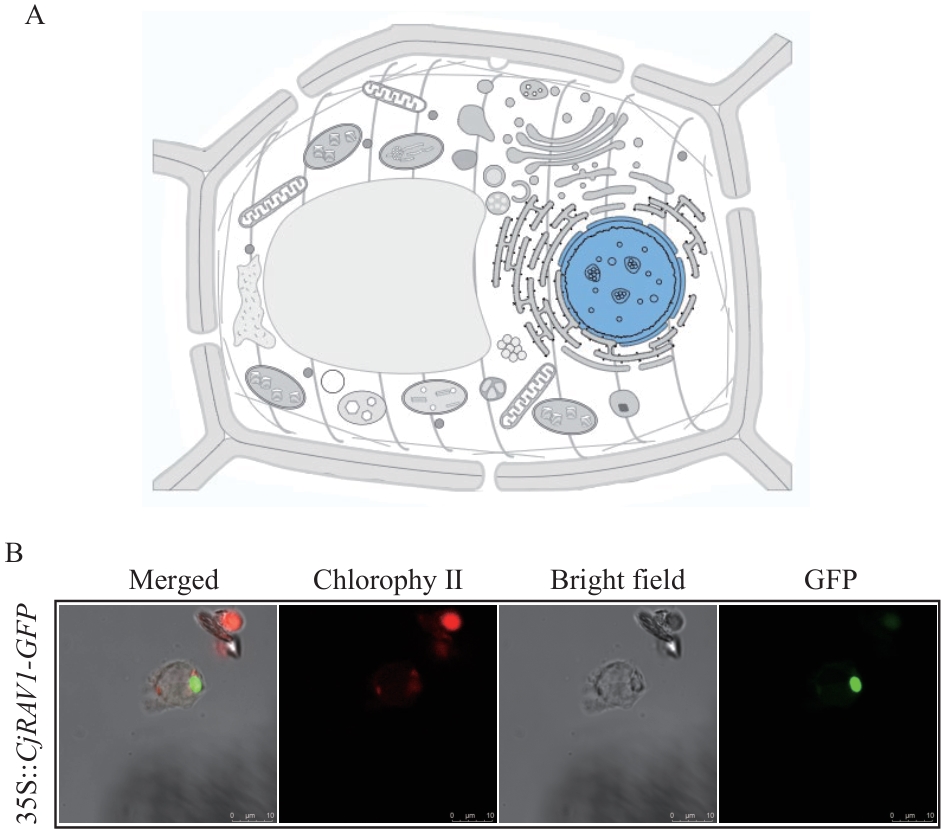
图7 CjRAV1的亚细胞定位分析A:CjRAV1在UniProt的定位预测结果;B:35S::CjRAV1-GFP的亚细胞定位分析
Fig. 7 Subcellular localization of the CjRAV1A: The localization prediction results of CjRAV1 in UniProt. B: Subcellular localization analysis of 35S::CjRAV1-GFP

图8 CjRAV1在山茶中的DAP-seq分析A:组内重复样本间的共有peak维恩图;B:Peak与距离相对基因TSS的位置分布图;C:在山茶中被CjRAV1结合的概率最大的DNA-motif;D:peak在基因功能元件上的分布比例图;E:所有位点对应基因的KEGG富集通路图
Fig. 8 DAP-seq analysis of CjRAV1in C. japonicaA: Common peak Venn plot between repeated samples within the group. B: Distribution map of peak distance relative to gene TSS position. C: The largest DNA-motif bound by CjRAV1 in C. japonica. D: Distribution ratio diagram of peak on gene functional elements. E: KEGG enrichment pathway of the genes corresponding to all sites
结合序列 Motif | 结合峰 Peak name | 配对序列 Matched sequence | 得分 Score | 富集倍数 Fold enrichment |
|---|---|---|---|---|
| RMAGCAACAGM | CjRAV1_peak_6710 | GCAGCAACAAC | 14.956 5 | 31.405 34 |
| RMAGCAACAGM | CjRAV1_peak_6710 | CCAGCAGCAGA | 14.318 8 | 31.405 34 |
| RMAGCAACAGM | CjRAV1_peak_6710 | AAAGCAGCAGC | 14.217 4 | 31.405 34 |
| RMAGCAACAGM | CjRAV1_peak_6710 | CCATCAACAGA | 11.826 1 | 31.405 34 |
| RMAGCAACAGM | CjRAV1_peak_6710 | AAAGCAGCAAC | 11.391 3 | 31.405 34 |
表2 CjRAV1潜在下游调控基因CjERF的结合位点
Table 2 Potential downstream regulatory gene CjERF binding site of CjRAV1
结合序列 Motif | 结合峰 Peak name | 配对序列 Matched sequence | 得分 Score | 富集倍数 Fold enrichment |
|---|---|---|---|---|
| RMAGCAACAGM | CjRAV1_peak_6710 | GCAGCAACAAC | 14.956 5 | 31.405 34 |
| RMAGCAACAGM | CjRAV1_peak_6710 | CCAGCAGCAGA | 14.318 8 | 31.405 34 |
| RMAGCAACAGM | CjRAV1_peak_6710 | AAAGCAGCAGC | 14.217 4 | 31.405 34 |
| RMAGCAACAGM | CjRAV1_peak_6710 | CCATCAACAGA | 11.826 1 | 31.405 34 |
| RMAGCAACAGM | CjRAV1_peak_6710 | AAAGCAGCAAC | 11.391 3 | 31.405 34 |
| 1 | 徐剑, 聂瑞敏, 耿芳, 等. 观赏山茶属植物育种现状与展望 [J/OL]. 分子植物育种, 2024. . |
| Xu J, Nie RM, Geng F, et al. Current achievements and prospects of ornamental Camellia plants breeding [J/OL]. Mol Plant Breed, 2024. . | |
| 2 | 游慕贤. 中国古茶树 [J]. 国土绿化, 2002(2): 28. |
| You MX. China ancient tea tree [J]. Land Green, 2002(2): 28. | |
| 3 | 周利, 李玲莉, 宋春艳, 等. 重庆地区川山茶古树资源调查及保护现状分析[C]//中国环境科学学会, 中国植物学会. 2021年中国植物园学术年会论文集. 北京: 中国林业出版社, 2021: 80-84. |
| Zhou L, Li LL, Song CY, et al. Investigation and protection of ancient Camellia tree resources in Chongqing area [C]// Chinese Society for Environmental Sciences, Botanical Society of China. Proceedings of the 2021 Annual Conference of Chinese Botanical Gardens. Beijing: China Forestry Publishing House, 2021: 80-84. | |
| 4 | Blümel M, Dally N, Jung C. Flowering time regulation in crops-what did we learn from Arabidopsis? [J]. Curr Opin Biotechnol, 2015, 32: 121-129. |
| 5 | Andrés F, Coupland G. The genetic basis of flowering responses to seasonal cues [J]. Nat Rev Genet, 2012, 13(9): 627-639. |
| 6 | Boyle PE, Wisdom MM, Richardson MD. Testing flowering perennial plants in a bermudagrass (Cynodon spp.) lawn [J]. HortScience, 2020, 55(10): 1642-1646. |
| 7 | Hyun Y, Vincent C, Tilmes V, et al. A regulatory circuit conferring varied flowering response to cold in annual and perennial plants [J]. Science, 2019, 363(6425): 409-412. |
| 8 | De La Torre AR, Piot A, Liu BB, et al. Functional and morphological evolution in gymnosperms: a portrait of implicated gene families [J]. Evol Appl, 2019, 13(1): 210-227. |
| 9 | Fletcher J, O’Connor-Moneley J, Frawley D, et al. Deletion of the Candida albicans TLO gene family using CRISPR-Cas9 mutagenesis allows characterisation of functional differences in α-, β- and γ- TLO gene function [J]. PLoS Genet, 2023, 19(12): e1011082. |
| 10 | Gu HJ, Pan ZJ, Jia MX, et al. Genome-wide identification and analysis of the cotton ALDH gene family [J]. BMC Genomics, 2024, 25(1): 513. |
| 11 | Osnato M, Castillejo C, Matías-Hernández L, et al. TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis [J]. Nat Commun, 2012, 3: 808. |
| 12 | Matías-Hernández L, Aguilar-Jaramillo AE, Marín-González E, et al. RAV genes: regulation of floral induction and beyond [J]. Ann Bot, 2014, 114(7): 1459-1470. |
| 13 | Wang SM, Guo T, Shen YX, et al. Overexpression of MtRAV3 enhances osmotic and salt tolerance and inhibits growth of Medicago truncatula [J]. Plant Physiol Biochem, 2021, 163: 154-165. |
| 14 | Zhao L, Yang X, Du HL, et al. Reduced GA biosynthesis in GmRAV-transgenic tobacco causes the dwarf phenotype [J]. Russ J Plant Physiol, 2016, 63(5): 690-694. |
| 15 | Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis [J]. Nat Protoc, 2007, 2(7): 1565-1572. |
| 16 | Bartlett A, O'Malley RC, Huang SC, et al. Mapping genome-wide transcription-factor binding sites using DAP-seq [J]. Nat Protoc, 2017, 12(8): 1659-1672. |
| 17 | Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method [J]. Nat Protoc, 2008, 3(6): 1101-1108. |
| 18 | Shin HY, Nam KH. RAV1 negatively regulates seed development by directly repressing MINI3 and IKU2 in Arabidopsis [J]. Mol Cells, 2018, 41(12): 1072-1080. |
| 19 | Osnato M, Matias-Hernandez L, Aguilar-Jaramillo AE, et al. Genes of the RAV family control heading date and carpel development in rice [J]. Plant Physiol, 2020, 183(4): 1663-1680. |
| 20 | Wang SM, Guo T, Wang Z, et al. Expression of three Related to ABI3/VP1 genes in Medicago truncatula caused increased stress resistance and branch increase in Arabidopsis thaliana [J]. Front Plant Sci, 2020, 11: 611. |
| 21 | Yuan YC, Zhou NN, Bai SS, et al. Evolutionary and integrative analysis of the gibberellin 20-oxidase, 3-oxidase, and 2-oxidase gene family in Paeonia ostii: insight into their roles in flower senescence [J]. Agronomy, 2024, 14(3): 590. |
| 22 | Xue YG, Zhang YT, Shan JM, et al. Growth repressor GmRAV binds to the GmGA3ox promoter to negatively regulate plant height development in soybean [J]. Int J Mol Sci, 2022, 23(3): 1721. |
| 23 | Li JL, Song CY, Li HM, et al. Comprehensive analysis of cucumber RAV family genes and functional characterization of CsRAV1 in salt and ABA tolerance in cucumber [J]. Front Plant Sci, 2023, 14: 1115874. |
| 24 | Hu HM, Tian S, Xie GH, et al. TEM1 combinatorially binds to FLOWERING LOCUS T and recruits a Polycomb factor to repress the floral transition in Arabidopsis [J]. Proc Natl Acad Sci USA, 2021, 118(35): e2103895118. |
| 25 | Tao Z, Shen LS, Liu C, et al. Genome-wide identification of SOC1 and SVP targets during the floral transition in Arabidopsis [J]. Plant J, 2012, 70(4): 549-561. |
| 26 | Peng Z, Wang M, Zhang L, et al. EjRAV1/2 delay flowering through transcriptional repression of EjFTs and EjSOC1s in loquat [J]. Front Plant Sci, 2021, 12: 816086. |
| 27 | Chandler JW, Werr W. A phylogenetically conserved APETALA2/ETHYLENE RESPONSE FACTOR, ERF12, regulates Arabidopsis floral development [J]. Plant Mol Biol, 2020, 102(1/2): 39-54. |
| 28 | Huang YY, Xing XJ, Jin JY, et al. CmbHLH110, a novel bHLH transcription factor, accelerates flowering in Chrysanthemum [J]. Hortic Plant J, 2024, 10(6): 1437-1448. |
| 29 | Huang JG, Yang M, Liu P, et al. GhDREB1 enhances abiotic stress tolerance, delays GA-mediated development and represses cytokinin signalling in transgenic Arabidopsis [J]. Plant Cell Environ, 2009, 32(8): 1132-1145. |
| 30 | Yamasaki Y, Randall SK. Functionality of soybean CBF/DREB1 transcription factors [J]. Plant Sci, 2016, 246: 80-90. |
| 31 | Magome H, Yamaguchi S, Hanada A, et al. The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis [J]. Plant J, 2008, 56(4): 613-626. |
| [1] | 刘源, 赵冉, 卢振芳, 李瑞丽. 植物类胡萝卜素生物代谢途径及其功能研究进展[J]. 生物技术通报, 2025, 41(5): 23-31. |
| [2] | 刘红利, 马一丹, 王婉茹, 杨娅茹, 贺丹, 刘艺平, 孔德政. 荷花NnCYP707A1的克隆及功能分析[J]. 生物技术通报, 2025, 41(5): 197-207. |
| [3] | 李文兰, 侯辛未, 李燕, 赵瑞君, 孟昭东, 岳润清. 转基因抗虫耐除草剂玉米LD05纯杂合植株的鉴定及抗性检测[J]. 生物技术通报, 2025, 41(4): 123-133. |
| [4] | 田琴, 刘奎, 吴翔纬, 纪媛媛, 曹一博, 张凌云. 转录因子VcMYB17调控蓝莓抗旱性的功能研究[J]. 生物技术通报, 2025, 41(4): 198-210. |
| [5] | 田栩瑞, 霍信屹, 郭云涵, 向林, 产祝龙, 王艳平. 百合LoSAUR10基因的表达特征及功能分析[J]. 生物技术通报, 2025, 41(1): 221-229. |
| [6] | 周麟, 黄顺满, 苏文坤, 姚响, 屈燕. 滇山茶bHLH基因家族鉴定及花色形成相关基因筛选[J]. 生物技术通报, 2024, 40(8): 142-151. |
| [7] | 吴丁洁, 陈盈盈, 徐静, 刘源, 张航, 李瑞丽. 植物赤霉素氧化酶及其功能研究进展[J]. 生物技术通报, 2024, 40(7): 43-54. |
| [8] | 李灿, 蒋湘宁, 盖颖. 日本落叶松LkF3H2基因克隆及调控类黄酮代谢功能研究[J]. 生物技术通报, 2024, 40(2): 245-252. |
| [9] | 陈应娥, 梁巧兰. 植物脱落酸及其受体基因PYL9的作用研究进展[J]. 生物技术通报, 2024, 40(12): 1-11. |
| [10] | 华炫, 田博雯, 周欣彤, 江梓涵, 王诗琦, 黄倩慧, 张健, 陈艳红. 旱柳SmERF B3-45的克隆及耐盐功能研究[J]. 生物技术通报, 2024, 40(12): 124-135. |
| [11] | 田锦, 张月秋, 张华, 陈子言, 田璐, 王颢潜, 高芳瑞, 梁晋刚, 陈红. 转基因玉米浙大瑞丰8特异性定性PCR检测方法研究[J]. 生物技术通报, 2024, 40(12): 45-52. |
| [12] | 刘奕君, 闫伟, 何禹璇, 董立明, 龙丽坤, 李飞武. 转基因大豆多靶标质粒DNA标准分子的研制及应用[J]. 生物技术通报, 2024, 40(11): 169-183. |
| [13] | 侯鹰翔, 费思恬, 宋松泉, 罗勇, 张超. 水稻MADS-box家族研究进展[J]. 生物技术通报, 2024, 40(11): 103-112. |
| [14] | 刘玉玲, 王梦瑶, 孙琦, 马利花, 朱新霞. 启动子RD29A对转雪莲SikCDPK1基因烟草抗逆性的影响[J]. 生物技术通报, 2023, 39(9): 168-175. |
| [15] | 王贵芳, 姚元涛, 许海峰, 相昆, 梁家慧, 张淑辉, 王文茹, 张明娟, 张美勇, 陈新. 核桃JrSnRK1α1.1调控种子油脂合成与积累[J]. 生物技术通报, 2023, 39(9): 183-191. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||